Abstract
BACKGROUND:
Remote ischemic preconditioning (RIPC) has 2 time windows for organ protection: acute and delayed. Previous studies have mainly focused on the organoprotective effects of acute RIPC. We aimed to determine whether delayed RIPC can reduce the occurrence of acute kidney injury (AKI) and postoperative complications in patients undergoing cardiac surgery.
METHODS:
This prospective, single-center, double-blind, randomized controlled trial involved 509 patients at high risk for AKI who were scheduled for elective cardiac surgery requiring cardiopulmonary bypass. Patients were randomized to receive RIPC (4 cycles of 5-minute inflation and 5-minute deflation on 1 upper arm with a blood pressure cuff) 24 hours before surgery or a sham condition (control group) that was induced by 4 cycles of 5-minute inflation to a pressure of 20 mm Hg followed by 5-minute cuff deflation. The primary end point was the incidence of AKI within the prior 7 days after cardiac surgery. The secondary end points included renal replacement therapy during hospitalization, change in urinary biomarkers of AKI and markers of myocardial injury, duration of intensive care unit stay and mechanical ventilation, and occurrence of nonfatal myocardial infarction, stroke, and all-cause mortality by day 90.
RESULTS:
A total of 509 patients (mean age, 65.2±8.2 years; 348 men [68.4%]) were randomly assigned to the RIPC group (n=254) or control group (n=255). AKI was significantly reduced in the RIPC group compared with the control group (69/254 [27.2%] versus 90/255 [35.3%]; odds ratio, 0.68 [95% CI, 0.47–1.00]; P=0.048). There were no significant between-group differences in the secondary end points of perioperative myocardial injury (assessed by the concentrations of cardiac troponin T, creatine kinase myocardial isoenzyme, and NT-proBNP [N-terminal pro-brain natriuretic peptide]), duration of stay in the intensive care unit and hospital, and occurrence of nonfatal myocardial infarction, stroke, and all-cause mortality by day 90.
CONCLUSIONS:
Among high-risk patients undergoing cardiac surgery, delayed RIPC significantly reduced the occurrence of AKI.
REGISTRATION:
URL: https://www.chictr.org.cn; Unique identifier: ChiCTR2000035568.
Keywords: acute kidney injury, biomarkers, cardiac surgical procedures, ischemic preconditioning
Clinical Perspective.
What Is New?
With 509 patients, this is the largest randomized trial of delayed remote ischemic preconditioning for cardiac surgery to report renoprotective effects to date.
The occurrence of acute kidney injury was significantly reduced in the remote ischemic preconditioning group compared with the control group (27.2% versus 35.3%; odds ratio, 0.68 [95% CI, 0.47–1.00]; P=0.048).
No differences were observed in perioperative myocardial injury, the occurrence of nonfatal myocardial infarction, stroke, and all-cause mortality by day 90 between the 2 groups.
What Are the Clinical Implications?
The simple, noninvasive, and well-tolerated application of delayed remote ischemic preconditioning in high-risk patients undergoing cardiac surgery reduced the incidence of acute kidney injury. However, the efficacy and safety of delayed remote ischemic preconditioning as a renoprotective strategy should be investigated further in additional adequately powered clinical trials.
Acute kidney injury (AKI) is the most common and serious complication in patients undergoing cardiac surgery. The incidence of cardiac surgery–associated AKI (CS-AKI) is 22% to 43%,1–3 which is associated with increased morbidity and mortality, prolonged intensive care unit and hospital stay, and increased cost of care.4,5 CS-AKI requiring renal replacement therapy occurs in ≈6% of patients after cardiac surgery, with a mortality rate of up to 50%.2,6–10 Even mild and transient renal dysfunctions are associated with the subsequent chronic kidney disease and cardiovascular events.2,11 In addition, patient outcomes relate to the duration of AKI, with prolonged AKI (>48 hours) associated with worse outcomes independently of stage.12 No pharmacological and nonpharmacological therapies have demonstrated clear efficacy in the prevention of CS-AKI.
Remote ischemic preconditioning (RIPC) is elicited by brief episodes of ischemia and reperfusion in the distal tissues, which may provide protection from subsequent organ or tissue injury.13 Zarbock et al14 demonstrated that RIPC significantly reduced the occurrence of AKI and the need for renal replacement therapy in high-risk cardiac surgery patients in a multicenter trial. However, 2 large-scale trials of RIPC in cardiac surgery performed by Meybohm et al15 and Hausenloy et al16 presented contrasting results, with both studies showing that RIPC did not reduce AKI and perioperative major adverse cardiac and cerebral events. We previously conducted a systematic review of 30 randomized controlled trials, including the Zarbock et al,14 Hausenloy et al,16 and Meybohm et al15 studies, which included a total of 7244 patients, to investigate the effects of RIPC on the incidence and outcomes of AKI and concluded that RIPC significantly decreased the incidence of AKI from 23.3% to 11.5% (risk ratio, 0.834 [95% CI, 0.728–0.955]; P=0.009). In the subgroup analysis, we found that RIPC significantly reduced the incidence of AKI in the contrast-induced AKI subgroup (P=0.000) but not in the CS-AKI subgroup (P=0.173), and RIPC had no significant effect on the incidence of stage 1 to 3 AKI or renal replacement therapy.17
RIPC has acute and delayed time windows for organ protection, including an early phase (<4 hours after RIPC) and a late phase (24–72 hours after RIPC).18,19 Previous studies focused on the acute time window to evaluate organ protection by RIPC, which is mostly performed after anesthesia and immediately before surgical incision, called acute RIPC.14–16 However, these studies have demonstrated conflicting results. Few studies have evaluated renoprotection by delayed RIPC. Kim et al20 evaluated myocardial and renal protection by RIPC that was performed 24 to 48 hours before surgery in adult patients undergoing cardiac surgery, and the results showed that delayed RIPC did not provide cardioprotective effects. Moreover, the incidence of AKI, defined by the Acute Kidney Injury Network staging system, was lower in the delayed RIPC group compared with the control group, whereas changes in serum creatinine were not significantly different between the 2 groups. The present study was a randomized controlled clinical trial conducted to assess whether delayed RIPC (ie, conducted 24 hours before cardiac surgery) reduces the occurrence of AKI and other clinical outcomes in high-risk patients undergoing cardiac surgery.
METHODS
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure until all planned, related studies, including a long-term follow-up study based on the initial data, are completed and published. On request with a proposal and approval by the corresponding authors and steering committee, data will be made available for secondary analysis.
Study Design and Participants
This was a prospective, single-center, double-blind, randomized, sham-controlled clinical trial. The trial protocol was approved by the ethics committee of Zhongshan Hospital Fudan University. All patients provided written informed consent. Eligible patients were adults at high risk for AKI who underwent elective cardiac surgery requiring cardiopulmonary bypass (CPB) during the period from May 2021 to November 2022. A score of ≥4 was used to define patients at high risk for AKI, according to our previous study.21 We developed a scoring system for predicting the occurrence of CS-AKI with the regression coefficient data from the multivariable regression analyses of the derivative cohort patients, and the area under receiver-operating characteristics curve value for CS-AKI prediction was up to 0.82.21 The derivation cohort consisted of 6081 patients; the validation cohort consisted of 1152 patients. Hosmer-Lemeshow goodness-of-fit tests revealed a good agreement between the expected and observed CS-AKI rates. The scoring system contained various risk factors, including patient characteristics, comorbidities, and operative procedures (Table S1). The predictive risks of CS-AKI occurrence were low with 0 to 1 point, medium with 2 to 3 points, and high with ≥4 points. Exclusion criteria were peripheral arterial disease affecting upper limbs, Raynaud syndrome, end-stage kidney disease (estimated glomerular filtration rate, <15 mL/min per 1.73 m2 of body surface area), preexisting AKI, pregnancy, and kidney transplantation.
Randomization and Blinding
Patients were randomized to the RIPC treatment group or the control group on a 1:1 basis. Codes were computer generated and kept in sealed envelopes. Before RIPC treatment or sham procedure, the corresponding envelope was opened by an independent investigator who was the only person aware of the patient’s treatment assignment; this investigator performed the RIPC but was not involved in further study procedures. Patients, cardiac surgeons, critical care staff, and statisticians were unaware of the treatment assignment.
Procedures
RIPC or sham procedure was conducted 24 hours before cardiac surgery. The RIPC consists of 4 cycles of 5-minute inflation of a standard blood pressure cuff to 200 mm Hg on one upper arm or to a pressure of 50 mm Hg greater than the systolic arterial pressure if the patient’s systolic arterial pressure was ≤150 mm Hg followed by 5-minute cuff deflation. If the systolic blood pressure was >180 mm Hg, the treatment was suspended and the patients received extra antihypertensive drugs until the systolic blood pressure returned to <180 mm Hg. For patients assigned to the control group, sham preconditioning was induced by 4 cycles of 5-minute blood pressure cuff inflation to a pressure of 20 mm Hg followed by 5-minute cuff deflation.
Anesthesia was induced by propofol and maintained with sevoflurane and propofol. We standardized the management of CPB as described in our previous study.22 Briefly, the circuit was primed with 1000 mL of Plasma-Lyte A (Baxter Healthcare, Deerfield, IL) and 1000 mL of Gelofusine (4%; B/Braun). Anticoagulation was established before the initiation of CPB. The arterial inflow was drawn by femoral artery cannula through the femoral artery. Venous drainage was gained by a femoral vein drainage tube with 40–mm Hg negative pressure. The pump flow was maintained at 2.0–2.4 L·min−1·m−2 on the bypass; the mean arterial blood pressure was maintained at 60 to 70 mm Hg; and carbon dioxide field flooding was routinely used.
Outcomes
The primary end point was the occurrence of AKI, defined as an increase in serum creatinine to ≥1.5× baseline within the 7 days after surgery or a urine volume <0.5 mL/kg per hour for 6 hours, according to the Kidney Disease: Improving Global Outcomes criteria.23 The secondary end points were the concentrations of urinary biomarkers of AKI at 6, 12, and 24 hours after cardiac surgery; serum concentrations of cardiac troponin T (cTnT), creatine kinase isoenzyme MB (CK-MB), and N-terminal probrain natriuretic peptide (NT-proBNP) at 24, 48, and 72 hours after cardiac surgery; the need for renal replacement therapy during hospital stay; length of stay in the intensive care unit and hospital; duration of ventilator support; and 90-day rate of death resulting from any cause, nonfatal myocardial infarction, and stroke. Nonfatal myocardial infarction was defined as an increase in cTnT concentration from baseline to at least twice the upper limit of normal reference range together with one or more indications of myocardial ischemia: electrocardiographic changes such as new pathological Q waves or persistent ST-segment depression or shift, a new finding of ischemia by echocardiography (imaging evidence of new loss of viable myocardium or new regional wall motion abnormality) or angiography, or angina symptoms.14 Stroke was defined by any new, temporary or permanent, focal or global neurological deficit or evidence of cerebral lesion seen on imaging.
Blood and Urine Sampling and Analysis
Blood samples were drawn before surgery and then daily for 7 days postoperatively unless the patient was discharged within this period for measurement of serum creatinine concentration. Serum cTnT, CK-MB, and NT-proBNP were detected before surgery and at 24, 48, and 72 hours after surgery. Serum interleukin-6, malondialdehyde, thromboxane B2, and HSP70 (heat shock protein 70) were detected before surgery and 6 hours after surgery with a commercially available ELISA kit (Elabscience, Wuhan, China), according to manufacturer protocol. Urine samples were collected after RIPC; before surgery; and at 6, 12, and 24 hours after surgery for measurement of AKI biomarkers, tissue inhibitor of metalloproteinase 2 (TIMP-2), and IGFBP7 (insulin like growth factor–binding protein 7), which were measured with commercially available ELISA kits (R&D) according to manufacturer protocol.
Statistical Analysis
The sample size was determined according to the expected incidence of AKI on the basis of our previous data.21 In an observational study that we performed in a similar patient population, the incidence of CS-AKI was 35.5%.21 To detect an ≈33% lower risk of the primary end point with RIPC than with sham RIPC (from 35% to 23%) with a power of 80% and a significance level of 5%, assuming a 5% dropout rate, we calculated that 254 subjects per randomization group would be required.
The collection and management of trial data were achieved with the Investigator Initiated Trial-Electronic Data Capture version 1.5.3 (Zhejiang Taimei Medical Technology Co, Ltd). Analyses were performed with the intention-to-treat principle. Categorical variables are presented as percentages and counts and were compared by use of the χ2 test or Fisher exact test (if the produced matrixes contained cells with an expected count <5). Continuous variables that were distributed normally are reported as mean±SD and are compared between groups with the unpaired Student t test. Nonnormally distributed continuous data are presented as median (interquartile range) and are analyzed with nonparametric tests (Mann-Whitney U and Wilcoxon test). For analysis of the repeated measurement data of urinary TIMP-2×IGFBP7, serum cTnT, CK-MB, and NT-proBNP over time between the RIPC group and the control group, 2-way repeated-measures ANOVA was used. We estimated the odds ratio, including 95% CIs, for the occurrence of AKI. To compare subgroups with respect to the effect of RIPC on the incidence of CS-AKI, we analyzed the subgroup variable in the logistic regression model. We stratified the analyses by age (40–60 or >60 years), hypertension (yes or no), coronary artery bypass graft surgery (yes or no), left ventricular ejection fraction (≥50% or < 50%), bypass time (30–90 minutes or >90 minutes), risk score (4 or >4), baseline serum creatinine (≤115 or >115 μmol/L), and baseline urine TIMP-2×IGFBP7 (<0.2 or ≥0.2 ng·mL−2·1000−1). The interactions of intervention type and the stratified factors on the incidence of CS-AKI were tested with the likelihood ratio test by including interaction terms in logistic regression model. In addition, the logistic regression model was used to evaluate dialysis during hospitalization and 90-day nonfatal myocardial infarction, stroke, and all-cause mortality. All statistical tests were 2 tailed, and the statistical significance was set at P<0.05. Statistical analyses were performed with SPSS17.0 software.
RESULTS
Study Population
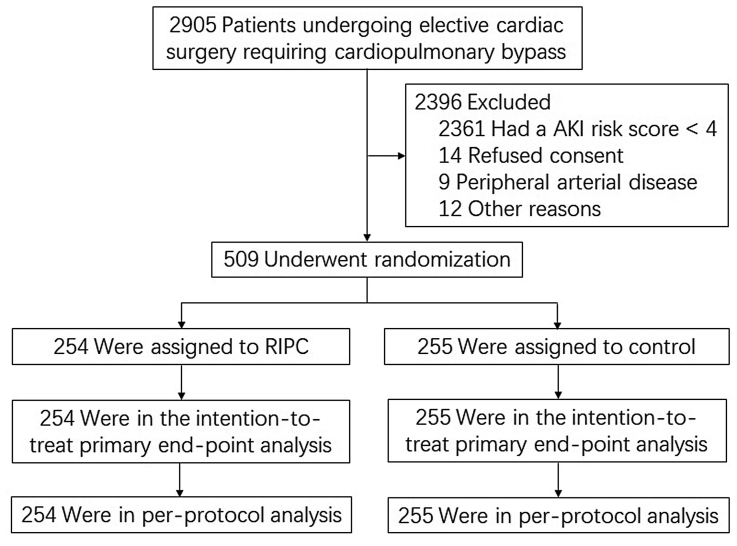
Of 2905 patients screened for the trial, 509 patients were enrolled and randomized to RIPC (n=254) or to sham conditioning (control; n=255) and included in the intention-to-treat analysis and per-protocol analysis (Figure 1). Baseline and intraoperative characteristics were similar between the 2 groups (Table 1).
Figure 1.
Flowchart of screening, randomization, and follow-up of the patients. AKI, indicates acute kidney injury; and RIPC, remote ischemic preconditioning.
Table 1.
Baseline and Operative Characteristics

Primary Outcome
The incidence of AKI within the 7 days after cardiac surgery was significantly reduced in the RIPC group compared with the control group (27.2% versus 35.3%; odds ratio, 0.68 [95% CI, 0.47–1.00]; P=0.048; Table 2). Fifty patients (19.7%) in the RIPC group and 76 patients (29.8%) in the control group developed Kidney Disease: Improving Global Outcomes stage 1 AKI; 19 patients (7.5%) in the RIPC group and 16 patients (6.3%) in the control group developed stage 2 or 3 AKI (P=0.191). AKI incidence was compared among the various subgroups (Figure 2). There was a significant interaction between hypertension and RIPC (P=0.019). Logistic regression analysis revealed that RIPC was associated with a decreased incidence of AKI in patients with hypertension (odds ratio, 0.46 [95% CI, 0.28–0.75]; P=0.002). No significant interactions were observed between the RIPC and other stratified factors on the incidence of CS-AKI (Figure 2).
Table 2.
Clinical Outcomes
Figure 2.
Incidence of acute kidney injury in prespecified subgroups. CABG indicates coronary artery bypass graft surgery; IGFBP7, insulin-like growth factor–binding protein 7; LVEF, left ventricular ejection fraction; RIPC, remote ischemic preconditioning; and TIMP-2, tissue inhibitor of metalloproteinase 2.
Secondary Outcomes
There were no significant differences between the RIPC group and the control group with respect to the secondary end points, including the median [interquartile range] length of hospital stay (6 [5–9] days versus 7 [5–9] days; P=0.339), intensive care unit stay (1 [1–3] day versus 1 [1–3] day; P=0.399), time of mechanical ventilation (24 [10–24] hours versus 24 [12-24] hours; P=0.710), and number of patients with hemodialysis during hospitalization (2 [0.8%] versus 4 [1.6%]; P=0.685) or with 90-day nonfatal myocardial infarction (2 [0.8%] versus 0 [0 %]; P=0.156), stroke (4 [1.6%] versus 1 [0.4%]; P=0.176), and mortality (3 [1.2%] versus 3 [1.2%]; P=0.996; Table 2).
Biomarkers
The baseline urine concentrations of TIMP-2 and IGFBP7 were detected after RIPC before cardiopulmonary bypass. The results showed that the urinary TIMP-2×IGFBP7 level was higher in the RIPC group than in the control group (P=0.013). Furthermore, the urinary TIMP-2×IGFBP7 level 6 hours after CPB was also higher in the RIPC group (P=0.031), although there were no significant differences in the urinary TIMP-2×IGFBP7 level at 12 and 24 hours after CPB between the RIPC group and the control group (Figure 3). An intriguing finding was that patients with a urinary TIMP-2×IGFBP7 level ≥0.2 ng·mL−2·1000−1 before CPB initiation had a significantly reduced rate of AKI in the RIPC group compared with the control group (Figure 2).
Figure 3.
Analysis of urine TIMP-2×IGFBP7 before and after CPB. The urinary tissue inhibitor of metalloproteinase 2 (TIMP-2)×insulin-like growth factor–binding protein 7 (IGFBP7) level at baseline and 6 hours after cardiopulmonary bypass (CPB) was higher in the remote ischemic preconditioning (RIPC) group compared with the control group (baseline, P=0.013; 6 hours after CPB, P=0.031).
We detected the concentrations of the myocardial injury biomarkers cTnT and CK-MB in serum before surgery and 24, 48, and 72 hours after CPB and found that the release of cTnT and CK-MB was markedly increased after surgery, but this did not differ significantly between the 2 groups (Figure 4A and 4B). In addition, the baseline and postoperative concentrations of serum NT-proBNP were similar between the RIPC and control groups (Figure 4C).
Figure 4.
Analysis of serum myocardial injury markers before and after CPB. Levels of cardiac troponin T (cTnT; A), creatine kinase isoenzyme MB (CK-MB; B); and N-terminal probrain natriuretic peptide (Pro-BNP; C). CPB indicates cardiopulmonary bypass; and RIPC, remote ischemic preconditioning.
We measured the concentrations of interleukin-6, malondialdehyde, thromboxane B2, and HSP70 in serum at baseline and at 6 hours after cardiac surgery. The results revealed that the levels of interleukin-6, malondialdehyde, and thromboxane B2 were not significantly different between the 2 groups at baseline or at 6 hours after cardiac surgery. The concentration of serum HSP70 was not significantly different between the 2 groups at baseline; however, serum HSP70 was significantly increased 6 hours after cardiac surgery in the RIPC group compared with the control group (P=0.026; Table 3), suggesting that RIPC induced the upregulation of HSP70.
Table 3.
Analysis of Serum Interleukin-6, Malondialdehyde, Thromboxane B2, and HSP70 in the Control and RIPC Groups

DISCUSSION
This large, randomized, sham-controlled trial was conducted to assess the effects of delayed RIPC on AKI and outcomes in patients undergoing cardiac surgery. Our data demonstrated that delayed RIPC significantly reduced the incidence of AKI within 7 days after surgery and did not provide cardioprotective effects compared with sham conditioning.
Recently, Hariri et al24 evaluated the effects of 10 nonpharmacological interventions on the incidence of CS-AKI (including 86 trials; 25 855 patients) and found that 2 interventions, RIPC and goal-directed perfusion, were associated with a significant reduction in CS-AKI with a moderate quality of evidence. A meta-analysis evaluating the renoprotective effect of RIPC in adult patients undergoing cardiac surgery, including 21 randomized controlled trials with a total of 6302 patients, demonstrated that RIPC significantly reduced the incidence of AKI compared with controls.25 However, another meta-analysis assessing the effects of RIPC on organ function in children and adults undergoing cardiac surgery found no evidence of renal protection.26 These inconsistent findings are probably related to differences in the study protocols, definitions of AKI, anesthetic regimens, surgical procedures and techniques, and timing of RIPC. It is noteworthy that in previous clinical trials, most cases of RIPC were performed after anesthesia induction and before surgical incision or CPB (acute RIPC). As mentioned, RIPC has acute and delayed time windows for organ protection. Acute preconditioning establishes a transient protective cellular state lasting for 2 to 3 hours; delayed preconditioning is onset at 12 to 24 hours after the initial preconditioning stimulus and is effective for 3 to 4 days.19,27 In this study, our results demonstrated that delayed preconditioning reduced the incidence of CS-AKI, confirming those of the study conducted by Kim et al.20 The mechanisms of acute and delayed preconditioning are different. Acute preconditioning is associated with immediate posttranslational modifications of preexisting proteins and activation of relevant signal transduction pathways, including phosphatidylinositol 3-kinase-protein kinase B/Akt and extracellular signal-regulated kinases 1 and 2,28 which disappear within a few hours. Delayed RIPC occurs as a result of the increased synthesis of protective proteins such as superoxide dismutase, heat stress protein, and inducible nitric oxide synthase,29 as well as regulation of antiapoptotic molecules,30 which takes several hours to occur. Given the 2 time windows of protection and their different mechanisms, RIPC may have different protective effects on organ injury, depending on whether the preconditioning is acute or delayed. It is noted that studies on delayed RIPC have shown conflicting results and have been limited by small sample sizes. Wagner et al31 conducted a single-center, randomized controlled trial to test whether RIPC performed 18 hours before surgery improved myocardial protection in coronary artery bypass graft surgery with cold-crystalloid cardioplegia. They found that delayed RIPC significantly reduced the postoperative troponin I level after 8 hours. However, Guerra et al32 evaluated the effects of early and delayed RIPC on myocardial dysfunction in young infants undergoing surgery for congenital heart disease and identified no differences in biomarkers or clinical markers of myocardial dysfunction between the RIPC group and the control group. As we know, the pathophysiology of AKI after cardiac surgery is complex and involves multiple factors, including ischemia-reperfusion injury, hypoperfusion, inflammation, oxidative stress, and neurohumoral activation,3 which occur mainly during the surgical procedure. Early transient protection elicited by acute preconditioning may not be sufficient to resist these stresses. In a systematic review and meta-analysis of RIPC in animal studies, delayed RIPC showed better efficacy than acute RIPC in preventing ischemia-reperfusion–induced AKI.33 We previously demonstrated that microRNA-21, an important antiapoptosis small RNA, was involved in the renal protection provided by RIPC and that RIPC induced systemic upregulation of microRNA-21 (including in the kidneys, lungs, liver, and skeletal muscle) in a time-dependent manner, which peaked 24 hours after RIPC.34 This suggests that 24 hours before cardiac surgery may be the optimal timing for preconditioning.
In the present study, we found an early increase in urinary TIMP-2×IGFBP-7 level after RIPC and that a TIMP-2×IGFBP-7 level higher than 0.2 ng·mL−2·1000−1 was associated with a significantly lower risk for CS-AKI. IGFBP-7 and TIMP-2 are considered markers of G1 cell-cycle arrest, a natural self-defense mechanism that allows cells to stop duplicating and dividing under stress. Our data imply that RIPC might induce G1 cell-cycle arrest in the kidneys, as indicated by the increased urinary TIMP-2×IGFBP7, which is protective from subsequent AKI induced by cardiac surgery. Furthermore, in the early response to ischemia-reperfusion injury, renal tubular epithelial cells enter G1 cell-cycle arrest to allow repair and avoid further damage.35 IGFBP7 and TIMP-2 are promising biomarker candidates for AKI, predicting the development of AKI in patients undergoing cardiac surgery, and have been validated in our previous studies and other reports.36–39 In fact, IGFBP7 and TIMP-2 are actively involved in a wide variety of cellular responses and processes.40,41 Their exact biological roles should be further explored beyond their utility as predictors of CS-AKI.
In this study, we found that serum HSP70 was significantly increased 6 hours after cardiac surgery in the RIPC group compared with the control group. HSP70 is a chaperone protein that binds to other proteins to chaperone them across membranes, prevents protein misfolding, and inhibits aggregation.42 HSP70 can inhibit apoptosis by preventing both apoptosome formation and cytochrome c release from the mitochondria, suppressing caspase-9 and caspase-3 activity.43 Previous studies have demonstrated that RIPC increased intracellular HSP70, which had a cytoprotective effect, and was released into the extracellular environment and peripheral blood circulation during stress.44 It has been suggested that HSP70 may contribute to the underlying mechanism of ischemic preconditioning. Further studies are warranted to determine the relationship between the RIPC and HSP70 production in kidney and whether HSP70 mediates the renal protective effects of RIPC.
To date, studies on RIPC performed in patients undergoing cardiovascular surgery have shown conflicting results in terms of myocardial injury and perioperative major adverse cardiac events, with some studies showing benefits45 and others failing to demonstrate any benefit.15,16 Consistent with these acute RIPC studies in patients undergoing cardiac surgery,15,16 our data also showed that delayed RIPC did not provide cardioprotective effects in this study, as evidenced by the concentrations of serum cTnT, CK-MB, and NT-proBNP compared with sham conditioning. It is likely that hypothermia and cardioplegia, as well as CPB itself, are protective, and further protection is perhaps not possible to achieve.46 It is noted that enhanced organ protection could not be obtained when 2 different types of preconditioning were combined with similar cytoprotective signal transduction pathways. Concomitant medications, especially anesthetics, may produce a pharmacological mimicking conditioning effect, described as anesthetic preconditioning, that interferes with acute RIPC when administered concomitantly. For example, propofol and volatile anesthetics have been shown to diminish or even abrogate the cardioprotective effects of RIPC.47–49 It has also been documented that anesthetic preconditioning shares the similar protective signal transduction pathways, including protein kinase B/Akt and extracellular signal-regulated kinases 1 and 2, with acute RIPC but not delayed RIPC.28 Furthermore, many anesthetic agents are short-acting and do not produce a second window of preconditioning.50 In such cases, delayed RIPC should not be affected by the induction of anesthesia. In this study, both propofol and the volatile anesthetic sevoflurane were used for anesthetic induction and maintenance. Whether the maintenance of anesthesia during cardiac surgery affects the second window of RIPC (delayed RIPC) requires further investigation. In addition, our findings raise the possibility that the cardioprotective effects of RIPC might be insufficient in complicated cardiac surgeries in patients with severe myocardial damage.
Study Limitations
Our study has certain limitations. First, although this is a large study, it is a single-center study, as we wanted to facilitate the standardization of preoperative and perioperative anesthesia and medication, maintaining homogeneity of the surgical procedures as much as possible. Second, the number of outcomes for the secondary end points, including dialysis during hospitalization and 90-day nonfatal myocardial infarction, stroke, and mortality, in our study was small, so the analysis produced limited information. Furthermore, the follow-up time was relatively short, and the long-term effects of RIPC were not evaluated. Third, although the rate of CS-AKI was significantly lower in the RIPC group compared with the control group, we did not observe a significant difference in the urinary TIMP-2×IGFBP7 level (biomarker of AKI) at 12 and 24 hours after surgery between the 2 groups. Urinary TIMP-2×IGFBP7 is an established marker of AKI predicting the development of CS-AKI. RIPC-induced rise in the urinary TIMP-2×IGFBP7 level before prolonged ischemic insult (surgery) was associated with a lower risk of AKI, as previously reported by Zarbock et al14 and shown in this study. Thus, the predictive value of TIMP-2×IGFBP7 varies in the context of RIPC. Fourth, all participants in this study are Chinese patients from a single race, which could limit the generalizability of our findings. In addition, for patients with an estimated glomerular filtration rate <60 mL·min−1·1.73 m−2, we observed a reduction of AKI in the RIPC group compared with the control group (28.4% versus 42.3%), although it did not reach statistical difference (P=0.112). Finally, although we sought to prevent any bias by blinding the patients, cardiac surgeons, critical care staff, and data analysis team, the study design could not prevent all potential biasing factors. We cannot exclude the possibility that some patients achieved additional protection by concomitant medications that may have produced a pharmacological mimicking conditioning effect, in turn influencing the effects of delayed RIPC.
Conclusions
This study demonstrated that the simple, noninvasive application of delayed RIPC in high-risk patients undergoing cardiac surgery with CPB significantly reduced the incidence of AKI. However, the efficacy and safety of delayed RIPC as a renoprotective strategy should be further investigated in future adequately powered clinical trials. More sufficiently designed trials with multicenter, multirace participants are needed to assess the effects of delayed RIPC on AKI, longer-term clinical outcomes, and mortality in patients undergoing cardiac surgery.
ARTICLE INFORMATION
Acknowledgments
The authors thank all the patients who participated in the study and the clinical and research staff at all the trial sites for their assistance.
Sources of Funding
This research was supported by a clinical research plan of Shanghai ShenKang Hospital Development Center (No. SHDC2020CR2022B); special fund for clinical research of Zhongshan Hospital, Fudan University, 2018; Science and Technology Commission of Shanghai (14DZ2260200); Shanghai municipal key clinical specialty grant (shslczdzk02501); and Shanghai “science and technology innovation plan” Yangtze River Delta scientific and technological innovation community project (No. 21002411500).
Disclosures
None.
Supplemental Material
Table S1
Updated Protocol
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AKI
- acute kidney injury
- CK-MB
- creatine kinase isoenzyme MB
- CPB
- cardiopulmonary bypass
- CS-AKI
- cardiac surgery-associated acute kidney injury
- cTnT
- cardiac troponin T
- HSP70
- heat shock protein 70
- IGFBP7
- insulin like growth factor–binding protein 7
- NT-proBNP
- N-terminal probrain natriuretic peptide
- RIPC
- remote ischemic preconditioning
- TIMP-2
- tissue inhibitor of metalloproteinase 2
P. Jia and Q. Ji contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.124.071408.
For Sources of Funding and Disclosures, see pages 1374–1375.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Qiang Ji, Email: ji.qiang@zs-hospital.sh.cn.
Zhouping Zou, Email: zouzhouping520@163.com.
Qi Zeng, Email: jpwxl999@163.com.
Ting Ren, Email: renting_97@163.com.
Weize Chen, Email: 20111210015@fudan.edu.cn.
Zhixin Yan, Email: zxyan21@m.fudan.edu.cn.
Daoqi Shen, Email: boshen1980@126.com.
Yang Li, Email: li.yang1@zs-hospital.sh.cn.
Fangyuan Peng, Email: 22211210013@m.fudan.edu.cn.
Ying Su, Email: su.ying@zs-hospital.sh.cn.
Jiarui Xu, Email: xu.jiarui@zs-hospital.sh.cn.
Bo Shen, Email: boshen1980@126.com.
Zhe Luo, Email: luo.zhe@zs-hospital.sh.cn.
Chunsheng Wang, Email: wangchunsheng_999@163.com.
Xiaoqiang Ding, Email: ding_xiaoqiang999@163.com.
REFERENCES
- 1.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011 [DOI] [PubMed] [Google Scholar]
- 2.Hu J, Chen R, Liu S, Yu X, Zou J, Ding X. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2016;30:82–89. doi: 10.1053/j.jvca.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711. doi: 10.1038/nrneph.2017.119 [DOI] [PubMed] [Google Scholar]
- 4.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, Danesi TH, Kim JC, Nayak A, Neri M, et al. Cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2013;3:178–199. doi: 10.1159/000353134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortega-Loubon C, Fernández-Molina M, Carrascal-Hinojal Y, Fulquet-Carreras E. Cardiac surgery-associated acute kidney injury. Ann Card Anaesth. 2016;19:687–698. doi: 10.4103/0971-9784.191578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milne B, Gilbey T, Kunst G. Perioperative management of the patient at high-risk for cardiac surgery-associated acute kidney injury. J Cardiothorac Vasc Anesth. 2022;36:4460–4482. doi: 10.1053/j.jvca.2022.08.016 [DOI] [PubMed] [Google Scholar]
- 7.Petäjä L, Vaara S, Liuhanen S, Suojaranta-Ylinen R, Mildh L, Nisula S, Korhonen AM, Kaukonen KM, Salmenperä M, Pettilä V. Acute kidney injury after cardiac surgery by complete KDIGO criteria predicts increased mortality. J Cardiothorac Vasc Anesth. 2017;31:827–836. doi: 10.1053/j.jvca.2016.08.026 [DOI] [PubMed] [Google Scholar]
- 8.Howitt SH, Grant SW, Caiado C, Carlson E, Kwon D, Dimarakis I, Malagon I, McCollum C. The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: a validation study. BMC Nephrol. 2018;19:149. doi: 10.1186/s12882-018-0946-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10:500–514. doi: 10.2215/CJN.07830814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187. doi: 10.1186/s13054-016-1352-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vives M, Hernandez A, Parramon F, Estanyol N, Pardina B, Muñoz A, Alvarez P, Hernandez C. Acute kidney injury after cardiac surgery: prevalence, impact and management challenges. Int J Nephrol Renovasc Dis. 2019;12:153–166. doi: 10.2147/IJNRD.S167477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe SH, Cho H, Bae J, Ji SH, Yoon HK, Lee HJ, Lee JH, Kim JT, Kim WH. Severity and duration of acute kidney injury and chronic kidney disease after cardiac surgery. J Clin Med. 2021;10:1556. doi: 10.3390/jcm10081556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M, et al. Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723 [DOI] [PubMed] [Google Scholar]
- 14.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, et al. ; RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189 [DOI] [PubMed] [Google Scholar]
- 15.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Böning A, Niemann B, et al. ; RIPHeart Study Collaborators. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579 [DOI] [PubMed] [Google Scholar]
- 16.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, et al. ; ERICCA Trial Investigators. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534 [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Liu S, Jia P, Xu X, Song N, Zhang T, Chen R, Ding X. Protection of remote ischemic preconditioning against acute kidney injury: a systematic review and meta-analysis. Crit Care. 2016;20:111. doi: 10.1186/s13054-016-1272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H19–H27. doi: 10.1152/ajpheart.00712.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972 [DOI] [PubMed] [Google Scholar]
- 20.Kim TK, Min JJ, Cho YJ, Hausenloy DJ, Ahn H, Kim KH, Hwang HY, Hong DM, Jeon Y. Effects of delayed remote ischemic preconditioning on peri-operative myocardial injury in patients undergoing cardiac surgery: a randomized controlled trial. Int J Cardiol. 2017;227:511–515. doi: 10.1016/j.ijcard.2016.10.111 [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Teng J, Xu J, Shen B, Wang Y, Fang Y, Zou Z, Jin J, Zhuang Y, Liu L, et al. Dynamic predictive scores for cardiac surgery-associated acute kidney injury. J Am Heart Assoc. 2016;5:e003754. doi: 10.1161/JAHA.116.003754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H, Qi X, Shi H, Zhao H, Liu C, Chen H, Peng R, Yu Z, Hu K, Wang C, et al. Single-dose del Nido cardioplegia used in adult minimally invasive valve surgery. J Thorac Dis. 2019;11:2373–2382. doi: 10.21037/jtd.2019.05.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 24.Hariri G, Collet L, Duarte L, Martin GL, Resche-Rigon M, Lebreton G, Bouglé A, Dechartres A. Prevention of cardiac surgery-associated acute kidney injury: a systematic review and meta-analysis of non-pharmacological interventions. Crit Care. 2023;27:354. doi: 10.1186/s13054-023-04640-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou C, Bulluck H, Fang N, Li L, Hausenloy DJ. Age and surgical complexity impact on renoprotection by remote ischemic preconditioning during adult cardiac surgery: a meta analysis. Sci Rep. 2017;7:215. doi: 10.1038/s41598-017-00308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haji Mohd Yasin NA, Herbison P, Saxena P, Praporski S, Konstantinov IE. The role of remote ischemic preconditioning in organ protection after cardiac surgery: a meta-analysis. J Surg Res. 2014;186:207–216. doi: 10.1016/j.jss.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy DJ, Yellon DM. The second window of preconditioning (SWOP) where are we now? Cardiovasc Drugs Ther. 2010;24:235–254. doi: 10.1007/s10557-010-6237-9 [DOI] [PubMed] [Google Scholar]
- 28.Pei H, Zhou C. Cardiac or renal protection by delayed remote ischemic preconditioning in the clinical practice: potential additive effect from concurrent medications with pharmacological mimicking conditioning. Int J Cardiol. 2017;234:105–106. doi: 10.1016/j.ijcard.2016.12.157 [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol. 2002;34:5–15. doi: 10.1006/jmcc.2001.1482 [DOI] [PubMed] [Google Scholar]
- 30.Lang JA, Kim J. Remote ischaemic preconditioning: translating cardiovascular benefits to humans. J Physiol. 2022;600:3053–3067. doi: 10.1113/JP282568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg. 2010;11:758–762. doi: 10.1510/icvts.2010.243600 [DOI] [PubMed] [Google Scholar]
- 32.Guerra GG, Joffe AR, Seal R, Phillipos E, Wong M, Moez EK, Dinu IA, Duff JP, Ross D, Rebeyka I, et al. Pilot randomized controlled trial on early and late remote ischemic preconditioning prior to complex cardiac surgery in young infants. Paediatr Anaesth. 2017;27:433–441. doi: 10.1111/pan.13125 [DOI] [PubMed] [Google Scholar]
- 33.Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, Ritskes-Hoitinga M, Hooijmans CR, Warlé M. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS One. 2012;7:e32296. doi: 10.1371/journal.pone.0032296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan T, Jia P, Chen N, Fang Y, Liang Y, Guo M, Ding X. Delayed remote ischemic preconditioning confers renoprotection against septic acute kidney injury via exosomal miR-21. Theranostics. 2019;9:405–423. doi: 10.7150/thno.29832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kellum JA, Chawla LS. Cell-cycle arrest and acute kidney injury: the light and the dark sides. Nephrol Dial Transplant. 2016;31:16–22. doi: 10.1093/ndt/gfv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Zou Z, Jin J, Teng J, Xu J, Shen B, Jiang W, Zhuang Y, Liu L, Luo Z, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017;18:177. doi: 10.1186/s12882-017-0592-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Shen B, Cao X, Lu Z, Zhang Y, Zhu B, Zhang W, Shi Y, Wang J, Fang Y, et al. Serum IGFBP7 deriving from spleen and lung could be used for early recognition of cardiac surgery-associated acute kidney injury. Cardiorenal Med. 2023;13:221–231. doi: 10.1159/000531489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wetz AJ, Richardt EM, Wand S, Kunze N, Schotola H, Quintel M, Bräuer A, Moerer O. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015;19:3. doi: 10.1186/s13054-014-0717-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irqsusi M, Beckers J, Wiesmann T, Talipov I, Ramzan R, Rastan AJ, Vogt S. Urinary TIMP-2 and IGFBP-7 protein levels as early predictors of acute kidney injury after cardiac surgery. J Card Surg. 2022;37:717–724. doi: 10.1111/jocs.16200 [DOI] [PubMed] [Google Scholar]
- 40.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, et al. ; American Society of Nephrology Acute Kidney Injury Advisory Group. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19–28. doi: 10.1053/j.ajkd.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honore PM, Jacobs R, Spapen HD. Remote ischemic preconditioning to prevent cardiac surgery-related acute kidney injury: how far away from a breakthrough? Ann Transl Med. 2016;4:314. doi: 10.21037/atm.2016.08.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiber A, Ravid T. Chaperoning proteins for destruction: diverse roles of Hsp70 chaperones and their co-chaperones in targeting misfolded proteins to the proteasome. Biomolecules. 2014;4:704–724. doi: 10.3390/biom4030704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200 [DOI] [PubMed] [Google Scholar]
- 44.Zhong Z, Dong H, Wu Y, Zhou S, Li H, Huang P, Tian H, Li X, Xiao H, Yang T, et al. Remote ischemic preconditioning enhances aerobic performance by accelerating regional oxygenation and improving cardiac function during acute hypobaric hypoxia exposure. Front Physiol. 2022;13:950086. doi: 10.3389/fphys.2022.950086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6 [DOI] [PubMed] [Google Scholar]
- 46.Zaugg M, Lucchinetti E. Remote ischemic preconditioning in cardiac surgery: ineffective and risky? N Engl J Med. 2015;373:1470–1472. doi: 10.1056/NEJMe1510338 [DOI] [PubMed] [Google Scholar]
- 47.Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;147:376–382. doi: 10.1016/j.jtcvs.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 48.Zaugg M, Lucchinetti E, Clanachan A, Finegan B. Remote ischemic preconditioning is redundant in patients undergoing coronary artery bypass graft surgery who are already protected by volatile anesthetics. Circ Res. 2012;110:e42–e43. doi: 10.1161/CIRCRESAHA.112.265116 [DOI] [PubMed] [Google Scholar]
- 49.Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, Zaugg M. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012;116:296–310. doi: 10.1097/ALN.0b013e318242349a [DOI] [PubMed] [Google Scholar]
- 50.Kehl F, Pagel PS, Krolikowski JG, Gu W, Toller W, Wartltier DC, Kersten JR. Isoflurane does not produce a second window of preconditioning against myocardial infarction in vivo. Anest Analg. 2002;95:1162–1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.