Abstract
In controlled organ donation after circulatory determination of death (cDCDD), accurate and timely death determination is critical, yet knowledge gaps persist. Further research to improve the science of defining and determining death by circulatory criteria is therefore warranted. In a workshop sponsored by the National Heart, Lung, and Blood Institute, experts identified research opportunities pertaining to scientific, conceptual, and ethical understandings of DCDD and associated technologies. This article identifies a research strategy to inform the biomedical definition of death, the criteria for its determination, and circulatory death determination in cDCDD. Highlighting knowledge gaps, we propose that further research is needed to inform the observation period following cessation of circulation in pediatric and neonatal populations, the temporal relationship between the cessation of brain and circulatory function after the withdrawal of life-sustaining measures in all patient populations, and the minimal pulse pressures that sustain brain blood flow, perfusion, activity, and function. Additionally, accurate predictive tools to estimate time to asystole following the withdrawal of treatment and alternative monitoring modalities to establish the cessation of circulatory, brainstem, and brain function are needed. The physiologic and conceptual implications of postmortem interventions that resume circulation in cDCDD donors likewise demand attention to inform organ recovery practices. Finally, because jurisdictionally variable definitions of death and the criteria for its determination may impede collaborative research efforts, further work is required to achieve consensus on the physiologic and conceptual rationale for defining and determining death after circulatory arrest.
INTRODUCTION
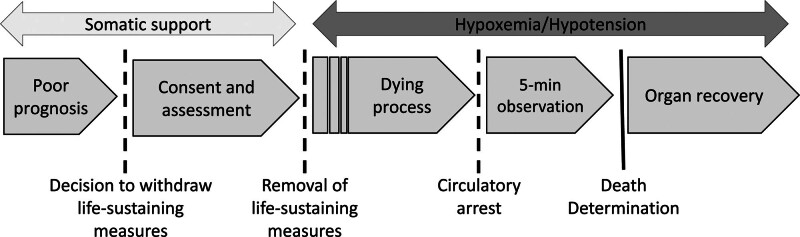
In 2021, >20% of all deceased organ donors donated via the controlled donation after circulatory determination of death (cDCDD) pathway.1 In cDCDD, patients with a poor prognosis who do not meet the criteria for death determination by neurologic criteria but wish to donate organs undergo controlled withdrawal of treatment and progress to asystole. Following an observation period to exclude the possibility of autoresuscitation, surgical teams recover viable organs for transplant as rapidly as possible to minimize ischemic damage (Figure 1).2-4 Although early experience of cDCDD showed recipient outcomes markedly inferior to those observed in donation after neurologic determination of death (DNDD), ongoing advances have resulted in acceptable outcomes across organ types,4,5 including kidneys,6,7 livers,8,9 lungs,10,11 and, more recently, hearts.12,13 cDCDD is practiced at a growing number of centers internationally and is an effective means of expanding the pool of eligible organ donors.2,4
FIGURE 1.
Conventional cDCDD protocol. The process of cDCDD begins with a consensual decision to withdraw life-sustaining measures and ends with organ recovery following death determination by circulatory criteria. cDCDD, controlled donation after circulatory determination of death.
Despite the growing proportion of organ donors who donate via the cDCDD pathway, important knowledge gaps regarding the science of defining and determining death by circulatory criteria remain.14 A biomedical definition of death describes the jointly necessary conditions for death determination in clinical contexts, while the criteria detail the measurable and observable biological indicators used to determine whether these conditions are met.15 In cDCDD, clarity of definition and accurate and timely death determination are paramount. However, recommendations concerning death determination in cDCDD guidelines internationally are often based on low to moderate certainty of evidence or expert opinion.16,17 Moreover, jurisdictional variation in the biomedical definition of death and the criteria for its determination complicates agreement on best practices.18,19 Given the importance of exactitude in death determination in deceased donation, further research to improve the science of defining and determining death by circulatory criteria is warranted.2,14
Although recently published observational research has reassured stakeholders of the integrity of cDCDD protocols,20 empirical uncertainties persist. The dead donor rule is the ethical and legal injunction governing deceased donation stipulating that organs may only be recovered after a donor has died.21 If current practices have the potential to produce false positives (ie, determining a living patient to be deceased), both donor welfare and stakeholder trust in donation systems would be jeopardized. Furthermore, successful transplantation of cDCDD-derived organs is contingent on minimizing organ injury by limiting warm ischemic time (WIT). If current practices have the potential to produce false negatives (ie, determining a deceased patient to be living), organ viability and transplant outcomes―to say nothing of honoring the patient’s donation wishes―would likewise be threatened. Research to inform circulatory death determination practices is therefore in the interest of protecting donors who wish to leave a legacy, families who find meaning in the success of a loved one’s donation, transplant recipients who benefit from optimally viable organs, and donation systems built on stakeholder trust.14,22
Advancing the science of death determination faces significant obstacles. The shortage of high-quality evidence is partly explained by ethical issues, regulatory uncertainties, and logistical challenges facing research with imminently dying or recently deceased patient-participants.23,24 Additionally, international and intranational variation in the biomedical definition of death and the criteria for its determination impedes collaborative efforts. Finally, the emergence of in situ organ-preserving technologies that restore circulation postmortem poses conceptual, ethical, and legal challenges, stimulating debates regarding the physiologic rationale for death determination in cDCDD and its associated conceptual apparatus.25,26 These obstacles must be overcome to strengthen the science of death determination by circulatory criteria in cDCDD.
This article identifies research opportunities to inform the biomedical definition of death, the criteria for its determination, and circulatory death determination practices in cDCDD. We outline what is known and what is unknown about the science of circulatory death determination while indicating how resolving uncertainties will help to ensure donor welfare is not jeopardized and lead to improved organ supply and transplant outcomes. We conclude that, because jurisdiction-specific biomedical definitions of death and frameworks for its determination bear on the relevance of some of the questions we highlight, any research agenda should be aligned with the medicolegal definition of death and the criteria for its determination in a given jurisdiction. International agreement on the physiologic and conceptual basis of death in cDCDD would therefore be conducive to advancing the science of circulatory death determination.18,19 In the absence of such harmonization, we propose a “death concept–dependent” research agenda to inform the priorities of researchers, regulators, and funders internationally. To ensure research directions are responsive to the needs of stakeholders, patients, donor families, and healthcare providers who care for patients at the end of life should be included in the design and conduct of any resulting research studies.
The scope of this article is limited to empirical issues in cDCDD and does not explore associated ethical, legal, and policy questions addressed elsewhere.27 Nor does it explore empirical questions specific to uncontrolled DCDD.28 Investigators and funding bodies should be cognizant that advances in ethics, policy, and practice relating to cDCDD may have unforeseen consequences for uncontrolled DCDD, a donation pathway with tremendous potential for increasing the supply of transplantable organs.28-30
MATERIALS AND METHODS
In March 2023, the National Heart Lung and Blood Institute invited experts to participate in a 2-d workshop titled “Heart & Lung Transplantation: Science & Ethics of DCDD and Xenotransplants.” The workshop brought together clinicians, ethicists, legal scholars, and scientists to discuss the state of science and current knowledge around DCDD and xenotransplantation and to identify research priorities.31 Our group was tasked with addressing the question: when is a potential DCDD donor dead, and how can we be sure? The working group members are experts in the fields of bioethics, critical care, death determination, emergency medicine, organ donation, and perfusion technologies. The overview that follows is based on content expertise as well as on recently conducted systematic reviews of existing scientific evidence bearing on DCDD.32-36 In each section, we address an area in need of further research and conclude with a list of priority research questions agreed on by the working group. These are recapitulated for ease of reference at the end of the article.
RESULTS
Debated Physiologic and Conceptual Rationale for Circulatory Death Determination
Death determination in conventional cDCDD relies on confirmation that circulation has ceased permanently, meaning that the period during which autoresuscitation can occur has elapsed and that no intervention will be undertaken to restore circulation.37,38 Although at odds with statutory language in some jurisdictions stipulating that the loss of cardiorespiratory function must be irreversible (meaning “cannot be reversed”), the permanence principle is widely accepted internationally among practitioners of cDCDD,2-4,32,39 despite some lingering disagreement in the academic literature over its validity.37,40 Indeed, the practice of cDCDD is predicated on the notion that death can legitimately be determined when circulatory function will not be restored when it could be, based on an ethically and legally valid decision made because the patient and/or their surrogate decision-makers rejected further treatment or would not benefit from it.37,41
The development of postmortem techniques such as normothermic regional perfusion (NRP) to preserve and repair organs in DCDD challenges the traditional medicolegal framework for circulatory death determination, particularly in jurisdictions where this framework is embedded in legislation, such as Australia and the United States. NRP causes circulation to resume, arguably invalidating a death determination based on the donor’s circulation having ceased permanently.26,42,43 These and other developments―including an evolving evidence base and recently published international death determination guidelines―have prompted renewed attention to the physiologic and conceptual rationale underpinning circulatory death determination.44,45 Although many jurisdictional frameworks continue to rely on the cessation of circulation without elucidating why the permanent loss of circulation indicates death, others have sought to clarify this rationale by demonstrating that the cessation of circulation is significant only because it is a valid proxy for the cessation of brain function, which cannot persist in the absence of intracranial circulation.19,35 For example, recently published Canadian death determination guidelines define death as the permanent cessation of brain function but allow that this can be determined using either neurologic or circulatory criteria.32 In contrast, the US Uniform Determination of Death Act offers a framework wherein an individual can be declared dead when they have sustained either irreversible cessation of circulatory and respiratory functions (where “irreversible” is interpreted as “permanent”) or the irreversible cessation of all functions of the entire brain (where “irreversible” is interpreted strictly; Table 1). The statute treats these as 2, equivalent bases for determining death but does not elaborate any physiologic criterion that unites the 2 standards. In 1980, when the Uniform Determination of Death Act was proposed, brain-based determinations were the focus, and a connection between the 2 standards was drawn in the opposite direction, namely, that the irreversible loss of brain functions meant that spontaneous circulatory and respiratory functions could not resume.47 Debate therefore persists over whether this legislation legally forecloses treating cDCDD organ donors as dead when mechanical support reestablishes circulation in their bodies.43,48
TABLE 1.
Glossary of terms
| Term | Definition19,32,46 |
|---|---|
| Permanent cessation | Ceases in perpetuity because it will not resume on its own and will not be resumed through medical intervention, based on a legally and ethically valid decision |
| Irreversible cessation | Ceases in perpetuity and cannot be resumed with available technology |
| Brain function | The brain’s vital functions include consciousness and behavior, coordination and control of movement, and the ability to breathe. |
| Brainstem function | Refers to brainstem reflexes including the capacity to breathe without mechanical support and the generation of consciousness arising from the reticular activating system |
| Brain activity | Physiologic or electrophysiologic properties of cells and groups of cells that can be measured by laboratory means. Not necessarily indicative of function |
| Brain blood flow | Steady movement of blood through the intracranial and/or brainstem vessels |
| Brain/brainstem perfusion | Capillary-level flow in target tissue |
| Consciousness | Awareness, including capacity for sensory perception or coordinated responsiveness to the external environment, and wakefulness |
This dispute makes clear the need for research to inform DCDD practices. International medical consensus appears to be emerging that the irreversible cessation of brain functions is the terminus in the dying process, but conceptual, ethical, legal, and scientific uncertainties remain.2,44,49,50 For example, in the US context, even if it were accepted that the cessation of brain function is the physiologic basis of human death, the implications for cDCDD are debated. Must the cessation of brain function be irreversible, as previously stipulated in the American Academy of Neurology guidelines for neurologic determination of death,51 or may it be merely permanent, as suggested by the World Brain Death Project?44,52,53 In DCDD-NRP, may death be declared based on neurologic criteria when autoresuscitation is impossible and brain blood flow is permanently excluded—just as in conventional DCDD, but with reference to brain as opposed to bodily circulation?18,43,54 If cessation of brain functions provides the unifying concept of death, ought death be determined based on a single operational criterion (eg, the irreversible cessation of brain functions) or with reference to its observable indicators (eg, the permanent cessation of intracranial circulation)? In cDCDD, death is now confirmed by observing the permanent cessation of bodily circulation. Under the unity concept, is that sufficient, or must circulation specifically to the brain be measured?
International agreement on the definition of death and the physiologic and conceptual rationale for circulatory death determination would encourage collaborative efforts to resolve outstanding research questions (Table 2). Nevertheless, acknowledging ongoing debates, and to encourage research in all jurisdictions with cDCDD programs, we have derived an inclusive set of research questions, some of which will not be applicable in the context of jurisdiction-specific death determination frameworks. Although debate regarding irreversibility and permanence persists in the academic literature,40,41 we use the language of permanence because while irreversibility entails permanence, permanence does not entail irreversibility, meaning the language accommodates both positions. Furthermore, independent of whether cessation of brain function must be permanent or irreversible in cDCDD, international medical consensus is that a person is not dead when they retain brain activity that can support brainstem reflexes (eg, spontaneous respiration) and/or consciousness.49,54,55 Therefore, following the widely endorsed World Brain Death Project,44 we assume that, minimally, death can only be determined following the permanent loss of the capacity for consciousness and brainstem reflexes, regardless of whether this is ascertained by neurologic criteria or inferred by circulatory criteria. That is, even if a cDCDD death determination framework makes no reference to brain function, brain function still matters (Table 3). Although the application of the permanence principle in relation to brain function is debated,52,53 acknowledging the importance of brain function for death determination in cDCDD has important implications for a research agenda to advance the science of circulatory death determination.14
TABLE 2.
Candidate biomedical definitions of death applicable to cDCDD
| Death in DCDD based on… | Criteria met under current cDCDD protocols |
|---|---|
| Cessation of circulation | |
| Permanent | Yes |
| Irreversible | No |
| Cessation of intracranial circulation | |
| Permanent | Yes |
| Irreversible | No |
| Cessation of brain function | |
| Permanent | Yesa |
| Irreversible | No |
Under condition that brain function has ceased at the time of organ recovery.
cDCDD, controlled donation after circulatory determination of death; DCDD, donation after circulatory determination of death.
TABLE 3.
Physiologic pathways to death
| Physiologic pathways to death50 |
|---|
| Primary or secondary brain event with cessation of brain function, most often associated with intracranial hypertension and cessation of brain blood flow, leading to apnea, hypoxemia, cardiac arrest, and cessation of circulation |
| Primary or secondary respiratory event causing hypoxemia resulting in cardiac arrest and cessation of circulation to all organs including the brain, resulting in the permanent loss of the capacity for consciousness and brainstem reflexes |
| Primary or secondary cardiac event resulting in cardiac arrest and cessation of circulation to all organs including the brain, resulting in the permanent loss of the capacity for consciousness and brainstem reflexes |
Q1 : What is the physiologic rationale for defining and determining death in cDCDD, and what are the conceptual implications for circulatory death determination frameworks?
Prediction of Time to Death in cDCDD
In cDCDD, following WLSM, organs are subject to variable periods of hypoxemia and hypotension resulting in ischemic damage that may render organs unsuitable for transplantation. Although the maximum allowable periods of hypotension and hypoxia by organ are uncertain, institutions set limits to the length of time from the onset of warm ischemia to circulatory arrest (specified by organ type), generally ranging from 1 to 2 h (up to 4 h for kidneys) outside of which they do not attempt recovery.3,56 Time zero for defining the onset of WIT varies, and WLSM is thus a commonly used (though arbitrary) time point. Some centers advocate the start of WIT based on thresholds of hypoxemia/hypotension, such as oxygen saturation of <80% or systolic blood pressure of <50 mm Hg in adults.56-59
Depending on the institution, roughly 20%–40% of consenting cDCDD candidates do not die within the (institutionally variable) WIT limit.3,60 Unsuccessful donation prevents donors from leaving a legacy, consumes scarce healthcare resources (eg, operating room time, personnel costs), and can be difficult for families who may seek meaning in organ donation.22,61 Accurate predictive tools to estimate time to death based on patient characteristics or other factors (eg, withdrawal practices, end-of-life care medications) are therefore needed to support selectivity when considering approaches for cDCDD authorization.3
Predicting the time to death after WLSM is difficult and physician estimates can be unreliable.61,62 Although some predictive tools have been validated, these generally consider only time to death ≤60 mins, and none have seen widespread acceptance.60,63-65 A 2022 retrospective analysis (n = 429) resulted in a predictive model incorporating vital sign variability, clinical features, and physicians’ prediction of rapid death, with area under the receiver operating characteristic curve values of 0.78, 0.79, and 0.80 for a prediction of time to death within 30 min, 1 h, or 2 h, respectively.56 A limitation of this study was the variability of withdrawal practices across participating institutions, as time to death is likely influenced by the manner of WLSM. For example, a recent study found that terminal extubation results in shorter agonal phases, at least at institutions where lower levels of respiratory and/or vasopressor support are employed.66
Although predictive tools for adults have been developed based on a reasonably large body of observational research, their accuracy could be improved.60 Future research should consider other relevant patient characteristics and compare institutional withdrawal practices against time to death.67 Machine learning may be a valuable means to increase positive predictive values in this domain.65,66 Consideration could also be given to research to inform cDCDD-specific approaches to end-of-life management, although these must not intentionally hasten death. Indeed, whether any departures from usual withdrawal practices for the purposes of facilitating organ donation are ethically and legally permissible requires further debate and discussion. Nonetheless, objective guidelines on end-of-life management for cDCDD candidates would be welcomed by the critical care community due to wide variability in practice.68,69
Subgroup Considerations
There are few available tools for predicting time to death in neonates and pediatric patients, and all are incompletely validated.60,70 Research to develop accurate prediction tools in pediatrics is important for supporting families and enabling successful pediatric cDCDD.
Q2 : What measurements are optimal triggers for defining WIT onset?
Q3 : What are the allowable time limits from WIT onset to circulatory arrest for each transplantable organ among adult and pediatric populations?
Q4 : Can WIT limits be extended when recovery includes in situ and/or ex situ perfusion techniques?
Q4b: Is there a difference in graft function between organs subjected to ex situ versus in situ perfusion?
Q5 : How can the accuracy of predictive tools for time to death be improved in adult, neonate, and pediatric populations?
Q5b : Which patient characteristics are relevant to predictive accuracy?
Q6 : How do withdrawal practices impact patient management and treatment of suffering during end-of-life care and time to death after WLSM?
Q6b : Is there an optimal set of practices for facilitating cDCDD without intentionally hastening death?
Q6 c: How do withdrawal practices impact the quality of donor organs?
Temporal Relationship Between the Cessation of Circulation and Brain Function
Current DCDD practice assumes―but does not confirm―the permanent cessation of brain function following a mandated observation period of circulatory arrest.71 Although this assumption is rooted in a sound physiologic rationale, it has yet to be confirmed in prospective studies of sufficient power. Lack of confirmatory evidence leads to concern that donors could be exposed to harm should they retain any degree of sentience and that retention of any brain activity could contravene medicolegal death determination frameworks.40,72,73 Emerging evidence showing surges of cerebral electrical activity following cardiac arrest in some clinical contexts likewise gives stakeholders pause, although the significance of these is unknown.34,74-76 Similarly, an inability to directly confirm the absence of sub-cortical and brainstem activity (which could play a role in sentience) raises questions regarding the functional state of the brain at the time of organ recovery.71
Much of the research concerning brain function following circulatory arrest stems from studies in resuscitative medicine and continuous intraoperative monitoring of patients with electroencephalogram (EEG) during unexpected cardiac arrest, and neuromonitoring during WLSM.34,77,78 In the latter context, studies with small cohorts suggest cessation of brain function―as defined by absence of consciousness, isoelectric EEG, and brainstem-based apnea―may reliably occur before the cessation of circulation due to progressive hypotension and hypoxia.71,75-80 However, the overall body of evidence supporting this conclusion is limited, most evidence is indirect, and pediatric patients are underrepresented in the studies that have been undertaken.34
Although available data suggest that it is highly unlikely that brain function persists at the time of organ retrieval in cDCDD, direct confirmatory data from large studies using neuromonitoring in conjunction with invasive arterial monitoring throughout the dying process and observation period may provide reassurance to stakeholders. A prospective multicenter observational study in Canada is currently underway to address this issue by documenting the temporal relationship between the cessation of brain and circulatory function during the dying process using multimodal neuromonitoring, including EEG, transcranial Doppler, brainstem auditory evoked potentials, and somatosensory event-related potentials.71
For those jurisdictions that do not support the permanence principle in death determination, or for those that require the irreversible as opposed to permanent cessation of brain function before initiation of NRP (see Postmortem in situ organ preservation interventions in cDCDD), it is important to determine the length of time that must elapse following cessation of circulation before irreversibility of the loss of brain function occurs.2
Subgroup Considerations
The temporal relationship between the cessation of circulation and brain function may be sensitive to practice- and patient-level factors (eg, institutional withdrawal practices, pediatric patients or patients previously supported by extracorporeal membrane oxygenation). Current evidence supporting circulatory death determination practice in cDCDD derives largely from research performed with adult participants undergoing controlled WLSM, meaning external validity is uncertain.34,78,80 Large-scale multicenter studies with participants across age ranges and patient populations are desirable to enhance the generalizability of findings and to fill knowledge gaps relating to subpopulations. Basic science studies could also inform how monitoring correlates with lack of perfusion.
Q7 : What is the temporal relationship between the cessation of circulatory and neurologic function (including brainstem function) in adult, pediatric, and neonatal patients, and patients previously supported with mechanical circulatory assistance?
Q8 : Are there feasible monitoring modalities that can reliably confirm the absence of brain (including brainstem) function following circulatory arrest within the time constraints of cDCDD?
Q9 : What is the lower limit of time following cardiac arrest past which brain activity and function is irreversible?
Q9b : How is this impacted by pre-existing brain injury?
Q10 : To what extent do practice-level factors (e.g., withdrawal practices) influence the temporal relationship between the cessation of circulatory and neurologic function?
Minimum Pulse Pressure to Sustain Brain Blood Flow, Perfusion, and Function
Closely related to questions regarding the temporal relationship between the cessation of circulatory and brain function is uncertainly surrounding the minimum arterial pulse pressure and frequency of pulse beats that can sustain brain blood flow, perfusion, and function. For there to be brain function there must be brain perfusion, and for there to be brain perfusion there must be brain blood flow. Yet just as brain activity is not necessarily indicative of function, brain blood flow is not necessarily indicative of perfusion, and perfusion is not necessarily indicative of function.46,57 Currently, the lower limits of regional brain blood flow and perfusion required to generate brain activity and function are unknown in the DCDD setting, the lower pulsatile blood pressure limits to generate regional brain blood flow are likewise unknown, and there is no agreed definition of pulselessness for circulatory death determination.14,34 Uncertainty regarding the significance of low arterial pulse pressures for brain perfusion and function during the dying process is problematic because circulatory death determination is reliant on the observed cessation of circulation which, under the permanence model, is used as a proxy for the cessation of brain function in some jurisdictions.19,32 Determining the minimum arterial pulse pressure required to sustain brain blood flow, perfusion, and function is therefore critical for defining the point at which the observation period should begin.
Although other methods are possible, cDCDD typically involves invasive arterial monitoring to measure pulse pressure.2,33,57 The minimum cerebral perfusion pressure and blood flow required to sustain organized neuronal function in the cDCDD process has not been studied. Because at very low pulse pressures it is currently not possible to distinguish with any certainty between brain blood flow that is meaningful (ie, supports brain function) and meaningless (ie, is insufficient to support brain function), physicians must rely on no flow, or pulselessness, as a reliable surrogate. A recent systematic review including studies of arterial pulse pressure as monitored by an indwelling arterial pressure transducer recommended operationalizing “cessation of circulation” (ie, pulselessness) using a conservative blood pressure threshold of ≤5 mm Hg to rule out the possibility of cerebral blood flow (and therefore perfusion and function).34 A threshold lower than 5 mm Hg was ruled out as the accuracy of clinical monitoring in detecting arterial pulse pressures below 5 mm Hg is not known, and a higher threshold was ruled out based on evidence from a small cohort substudy that cerebral electrical activity may persist at 8 mm Hg, meaning false positives may occur at >5 mm Hg.77,81 That said, this activity is unlikely to represent function. Given the small number of included studies (most with small cohorts or no comparison groups) and a dearth of direct evidence specific to the cDCDD context, this recommendation is based on very low certainty of evidence.32,34 Further research is indicated to buttress the evidence base supporting this threshold.
Subgroup Considerations
Cerebral perfusion pressure is measured as mean arterial pressure minus intracranial pressure. Evidence supporting ≤5 mm Hg as a marker of pulselessness is derived mostly from studies with adults who underwent controlled WLSM and thus its external validity is not known. Based on physiologic principles, for brain blood flow to be generated with a pulse pressure of ≤5 mm Hg would infer a brain tissue pressure or brain venous pressure of <5 mm Hg (ie, to perfuse the brain, the mean arterial pressure must exceed brain tissue or brain venous pressures). It is unknown whether the suggested ≤5 mm Hg threshold should apply to neonates, pediatric patients, and patients previously supported by mechanical circulatory assistance. Large-scale studies should quantify cerebral electrical activity, brainstem neuronal activity, and levels of consciousness (through behavioral assessments) in relation to arterial pulse pressure in a range of clinical contexts to determine with higher accuracy the thresholds for determining cessation of circulation in different populations. Research to determine the accuracy of clinical monitoring at very low pulse pressures is also recommended.34
Q11 : What is the minimum pulse pressure and pulse frequency that generates (a) cerebral blood flow, (b) perfusion, and (c) function across all patient populations?
Q12 : Are there modalities to accurately evaluate brainstem flow, perfusion, and function within the time constraints of cDCDD?
Optimal Observation Period
All cDCDD protocols include an observation period after circulatory arrest to confirm the permanent cessation of circulation.2,4,82 The purpose of the observation period is to ensure adherence to the dead donor rule by excluding the possibility of autoresuscitation, or the unassisted return of spontaneous circulation.17 Mandated observation periods vary by protocol, but all introduce a tension between the need to ensure accuracy in death determination and the need to minimize WIT. Waiting longer than necessary may lead to poorer outcomes in organ recipients, whereas not waiting long enough could lead to violations of the dead donor rule.
Autoresuscitation is known to occur most frequently in the wake of failed cardiopulmonary resuscitation, but also occurs following WLSM.17,35,83 Several observational studies seeking to identify the optimal (ie, minimum) observation period have been undertaken in the context of controlled WLSM, meaning that evidence regarding the observation period in adult cDCDD is comparatively robust.35 A recent international multicenter prospective observational study of autoresuscitation in adult patients following WLSM (n = 631) found that resumption of circulatory activity occurred in 14% of patients who had complete waveform data (n = 480).20 Reassuringly, no resumption of cardiac electrical activity and pulsatile activity (≥5 mm Hg) occurred beyond 4 m 20 s following pulselessness (defined as ≤5 mm Hg for 60 s). All resumptions were transient, and no patients survived. Combined with evidence from other observational studies of controlled WLSM identified in a recent systematic review, these data suggest that the 5-min observation period in cDCDD supported by many jurisdictions internationally can be said with moderate certainty to preclude the possibility of false positives.32,35
Subgroup Considerations
No direct or indirect evidence regarding optimal observation periods exists for neonates.35 Case report have documented autoresuscitation in pediatric patients, 1 following WLSM in the cDCDD setting.84,85 Two other observational studies with pediatric patients (n = 12) reported no events.86,87 Further research specific to neonates and pediatric populations is needed.
Q13 : What is the optimal observation period for neonates and pediatric patients?
Noninvasive Monitoring Techniques
Although often used in conjunction with other modalities, invasive arterial blood pressure monitoring is the preferred method for determining cessation of circulation in cDCDD.33,36 However, as noted in “Minimum pulse pressure to sustain brain blood flow, perfusion, and function,” arterial blood pressure is an imperfect surrogate for cerebral (and brainstem) blood flow, perfusion, and function. Some have called for research into noninvasive monitoring techniques to observe the cessation of brain (and brainstem) function either directly or by other indirect means.14 Moreover, although invasive arterial monitoring is the gold standard, there are instances in which it may be difficult or impossible (eg, technical challenges; neonates or pediatric patients). Noninvasive means to determine the cessation of circulation, brain perfusion, and brain function are therefore needed to accommodate circulatory death determination in instances where invasive arterial monitoring is not available.
A recent systematic review explored the sensitivity and specificity of alternative methods for determining the cessation of circulation in potential cDCDD donors, including electrocardiogram (ECG), palpable pulse, point-of-care ultrasound (POCUS) pulse, cerebral near-infrared spectroscopy, and echocardiography/POCUS cardiac motion.33 The authors found no studies comparing noninvasive methods to invasive arterial monitoring, although they did find 21 assessing the modalities of interest in various contexts. These were mostly small and observational, and many offer only indirect evidence. Ultimately, the review found that there is insufficient evidence to conclude that any of the alternative modalities of interest are equal or superior to invasive arterial blood pressure monitoring in terms of specificity/sensitivity, at least within the time constraints of cDCDD.
Two observational studies found that the use of ECG as a surrogate for cessation of circulation carries a very low risk of false positives.20,87 However, the shortcoming of this modality when used in isolation is that it can prolong time to death determination due to the persistence of cardiac electrical activity beyond cessation of circulation in many patients for considerable periods of time.20,78,87 ECG could therefore be considered in those instances when arterial blood pressure monitoring is not possible or desirable, but further research into alternative modalities (especially POCUS) is needed to identify monitoring that does not have the disadvantage of prolonging WIT.33
Subgroup Considerations
Both neonates and pediatric patients are underrepresented in existing data. Future research should compare arterial line monitoring and ECG and increase the representation of pediatric patients in any study exploring alternative monitoring modalities.33
Q14 : Are there alternative monitoring modalities that directly or indirectly confirm the absence of (a) circulation, (b) brain perfusion, and (c) brain function that do not have the disadvantage of prolonging WIT?
Q15 : Is the accuracy of alternative monitoring modalities generalizable among patient populations (eg, neonates and pediatric patients)?
Postmortem In Situ Organ Preservation Interventions in cDCDD
Postmortem measures that seek to improve the viability and number of organs in situ include normothermic regional perfusion (NRP)25,88 and tidal ventilation,2,89 whereas others are likely to arise in future.90 As noted, these interventions are controversial because they may intentionally or unintentionally restore circulation, potentially contravening medicolegal death determination frameworks.19,26,42 Moreover, because they could conceivably result in the return of intracranial blood flow and hence brain function, each could have implications for death determination even in those jurisdictions that support a unified, brain-based definition of death.90,91 Research is warranted to assess the potential impact of these preservation techniques on postmortem brain oxygenation and compatibility with the dead donor rule.92,93
Normothermic Regional Perfusion
NRP harnesses extracorporeal membrane oxygenation technology to restore regional circulation postmortem to improve organ viability, enable DCDD heart donation, and allow organ viability assessment in situ.88,94,95 Thoraco-abdominal NRP (TA-NRP) restores circulation to organs in the abdominal and thoracic cavities, whereas abdominal NRP (A-NRP) restores circulation only to the abdominal organs.88 The optimal length of perfusion time is still unknown, and periods vary by center from 1 to 6 h.94-97 In both TA-NRP and A-NRP, surgical safeguards are typically used to preclude the resumption of intracranial blood flow by blocking the major vessels to the brain through transection, ligation, or occlusion.88 However, it is unknown whether these measures are effective in all instances. For example, intracranial flow could resume via equipment failure, collateral vessels, or in cases where the donor has atypical vasculature.91-93 The absence of reports in the literature of brain activity resuming is not probative because neuromonitoring is not standard of care in NRP. A reassuring study was undertaken in a porcine model and one retrospective study (n = 2) in humans found no intracranial blood flow during TA-NRP.98,99 Additionally, a recent prospective study (n = 12) enrolling donors undergoing A-NRP or TA-NRP using invasive monitoring at the circle of Willis reported no brain perfusion.100 However, to date, only 4 donors undergoing TA-NRP have been studied. Because the risk of resumption of intracranial blood flow during TA-NRP is thought to be higher than in A-NRP, further prospective studies in humans using multimodal neuromonitoring observed in real-time during NRP would reassure stakeholders of the absence of flow.19,93
A further complication facing NRP arises from contrasting perspectives on the requirements for death determination in cDCDD-NRP. In conventional cDCDD, death is determined based on the permanent cessation of circulation. Yet, because circulation is restored in cDCDD-NRP, the basis for determining the donor’s death arguably transitions from “circulatory death” to “brain death” (as understood with reference to neurologic criteria for its determination).101 Some argue that because the traditional means of determining death by neurologic criteria are based on the irreversible loss of brain functions, cDCDD-NRP ought to adhere to irreversibility rather than permanence. The permissibility of NRP under this view would turn on establishing that the loss of brain functions is irreversible, necessitating clinical diagnostic tests that may be impracticable within the time constraints of cDCDD.43 (A further difficulty with this model is that while the length of time following cessation of circulation that must elapse before the restoration of brain functions becomes impossible has not been established, it surely exceeds allowable WIT limits for most organs.)18 Alternatively, under a unifying concept of death, DCDD-NRP may be permissible provided the permanence principle applies to the cessation of intracranial circulation and therefore brain function.19,90 Resolution of this debate will have significant implications for NRP’s uptake (Table 4).
TABLE 4.
NRP permissibility under variable death determination frameworks
| Death in DCDD based on | Criteria met under current DCDD protocols | Criteria met under current DCDD-NRP protocols |
|---|---|---|
| Cessation of circulation | ||
| Permanent | Yes | No |
| Irreversible | No | No |
| Cessation of intracranial circulation | ||
| Permanent | Yes | Yesa |
| Irreversible | No | No |
| Cessation of brain function | ||
| Permanent | Yes | Yesa |
| Irreversible | No | Nob |
Under condition that brain function has ceased at the time NRP is initiated and that surgical safeguards are effective.
Could be permissible provided brain function is irreversibly lost, but not permissible under current protocols because NRP is initiated prior.
DCDD, donation after circulatory determination of death; NRP, normothermic regional perfusion.
Q16: What is the optimal length of time DCDD donors should undergo NRP (according to organ type)?
Q16b : Does NRP negatively impact the viability of organs not targeted by the intervention (eg, lungs)?
Q17 : Are in situ postmortem reperfusion NRP methods (whether coupled with ex situ perfusion or not) superior to ex situ perfusion techniques in terms of cost and improving organ viability, graft outcomes, and the number of organs recovered per donor?
Q18 : Are surgical safeguards to preclude the resumption of intracranial blood flow effective in A-NRP and TA-NRP?
Q18b : What are the optimal methods?
Q9 : What is the lower limit of time following cardiac arrest past which brain activity and function is irreversible?
Tidal Ventilation in Lung Donation
Postmortem resumption of tidal ventilation in DCDD lung donors during organ recovery can limit WIT, mitigate injury, enable distribution of flushing solutions, and increase the number of lungs recovered for transplant.89,102-104 However, tidal ventilation could lead to anterograde circulation due to cardiopulmonary interactions, resumption of coronary blood flow, myocardial contraction and spontaneous circulation, which could in turn conceivably restore intracranial blood flow.3,90 To our knowledge, there are no validated surgical safeguards to preclude the resumption of circulation, though different methods are possible and in use.2 Due to concerns regarding resumption of circulation and intracranial flow, closed chest postmortem tidal ventilation is currently not practiced in jurisdictions such as ON, Canada; static inflation of lungs is favored.105 Given the uncertain potential of postmortem cyclic ventilation, further research to determine optimal safeguards is needed.
Q19: In comparison with tidal ventilation, is static ventilation superior as an organ-preserving measure?
Q20 : What are the optimal methods to preclude resumption of circulation and the possibility of the restoration of intracranial flow in postmortem tidal ventilation?
DISCUSSION
The relevance to funders and investigators of several of the research questions described earlier is contingent on jurisdictionally variable (and contested) definitions of death and medicolegal frameworks for death determination. For example, in many jurisdictions the definition of death relevant to cDCDD is the “irreversible loss of circulatory and respiratory function.” If interpreted literally, an implication is that questions concerning the functional state of the brain after the possibility of autoresuscitation has passed are (technically) moot because donors are legally dead following the observation period and brain function is assumed to be permanently lost. The permissibility of postmortem interventions that may restore circulation or intracranial blood flow is likewise contingent on legal and ethical frameworks pertaining to definitions of death and practices for its determination. Here again, many of the empirical unknowns described earlier will not be relevant for jurisdictions that rely on the cessation of circulation without elaborating on its relation to brain function and, hence, ought not be priority research questions to local funders. Similarly, if strict irreversibility of the cessation of brain function is required for cDCDD-NRP, unknowns relating to the minimal pulse pressures required to generate brain blood flow are irrelevant because initiation of NRP could only happen after the time at which restoration of brain function is impossible.
Given the contingent relevance of some of the research questions described earlier, international agreement on the biomedical definition of death and the physiologic and conceptual rationale underpinning circulatory death determination in DCDD would encourage collaborative efforts to resolve them. Yet, recognizing that such agreement has not yet been achieved, and to encourage research in all jurisdictions where cDCDD is practiced, we list all empirical unknowns described in this article in Table 5 and indicate which are of relevance to 5 possible concepts of death that may be applicable in the cDCDD setting depending on jurisdictional death determination frameworks.
TABLE 5.
Death concept dependent research agenda
| Death concept | |||||||
|---|---|---|---|---|---|---|---|
| # | Empirical question | PCC | ICC | PCIC | ICIC | PCBF | ICBF |
| 1 | What is the physiologic rationale for defining and determining death in cDCDD, and what are the conceptual implications for circulatory death determination frameworks? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2 | What measurements are optimal triggers for defining WIT onset? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3 | What are the allowable time limits from WIT onset to circulatory arrest for each transplantable organ among adult and pediatric populations? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4 | Can WIT limits be extended when recovery includes in situ and/or ex situ perfusion techniques? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5 | How can the accuracy of predictive tools for time to death be improved in adult, neonate, and pediatric populations? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 6 | How do withdrawal practices impact management of suffering during end-of-life care and time to death after WLSM? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 7 | What is the temporal relationship between the cessation of circulatory and neurologic function (including brainstem function) in adult, pediatric, and neonatal patients, and patients previously supported with mechanical circulatory assistance? | ✓ | ✓ | ||||
| 8 | Are there feasible monitoring modalities that can reliably confirm the absence of brain (including brainstem) function following circulatory arrest within the time constraints of cDCDD? | ✓ | ✓ | ||||
| 9 | What is the lower limit of time following cardiac arrest past which brain activity and function is irreversible? | ✓ | |||||
| 10 | To what extent do practice-level factors (eg, withdrawal practices) influence the temporal relationship between the cessation of circulatory and neurologic function? | ✓ | ✓ | ||||
| 11 | What is the minimum pulse pressure and pulse frequency that generates (a) cerebral blood flow, (b) perfusion, and (c) function across all patient populations? | ✓ | ✓ | ✓ | ✓ | ||
| 12 | Are there modalities to accurately evaluate brainstem flow, perfusion, and function within the time constraints of cDCDD? | ✓ | ✓ | ✓ | ✓ | ||
| 13 | What is the optimal observation period for neonates and pediatric patients? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 14 | Are there alternative monitoring modalities that directly or indirectly confirm the absence of (a) circulation, (b) brain perfusion, and (c) brain function that do not have the disadvantage of prolonging WIT? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 15 | Is the accuracy of alternative monitoring modalities generalizable among patient populations (eg, neonates and pediatric patients)? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 16 | What is the optimal length of time DCDD donors should undergo NRP (according to organ type)? | ✓ | ✓ | ✓a | |||
| 17 | Are in situ postmortem reperfusion NRP methods (whether coupled with ex situ perfusion or not) superior to ex situ perfusion techniques in terms of cost and improving organ viability, graft outcomes, and the number of organs recovered per donor? | ✓ | ✓ | ✓a | |||
| 18 | Are surgical safeguards to preclude the resumption of intracranial blood flow effective in A-NRP and TA-NRP? | ✓ | ✓ | ||||
| 19 | In comparison with tidal ventilation, is static ventilation superior as an organ-preserving measure? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 20 | What are the optimal methods to preclude resumption of circulation and the possibility of the restoration of intracranial flow in postmortem tidal ventilation? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Provided restoration of brain function is impossible with current technologies.
A-NRP, abdominal normothermic regional perfusion; cDCDD, controlled donation after circulatory determination of death; DCDD, donation after circulatory determination of death; ICBF, irreversible cessation of brain function; ICC, irreversible cessation of circulation; ICIC, irreversible cessation of intracranial circulation; PCBF, permanent cessation of brain function; PCC, permanent cessation of circulation; PCIC, permanent cessation of intracranial circulation NRP, normothermic regional perfusion; TA-NRP, thoraco-abdominal normothermic regional perfusion; WIT, warm ischemic time; WLSM, withdrawal of life-sustaining measures.
CONCLUSION
Ensuring donors are protected from welfare harms, minimizing WIT, and adhering to the dead donor rule demand accurate and timely death determination. However, empirical knowledge gaps and conceptual debates pertaining to circulatory death determination remain. As the proportion of donors who donate via the cDCDD pathway continues to grow, and as the practice of cDCDD continues to expand internationally with evolving strategies to improve organ function, strengthening the science of defining and determining death by circulatory criteria should be a focus of research. To ensure research directions align with stakeholder priorities, investigators should include patients, donor families, and healthcare providers who care for patients at the end of life in the design and conduct of research studies. This article highlights empirical knowns and unknowns relating to circulatory death determination in the interest of advancing a research agenda that will benefit patients, donors, their families, and deceased donation systems.
ACKNOWLEDGMENTS
The authors thank the organizers of the National Heart, Lung, and Blood Institute’s workshop “Heart & Lung Transplantation: Science & Ethics of DCDD and Xenotransplants.”
Footnotes
S.D.S. conceived and led the project. N.B.M. authored the first and all subsequent versions of the article. S.D.S., J.L.B., A.C., K.F., T.G., A.H., K.K.K., T.N., R.D.T., B.S., and S.P.W. participated in the writing of this article and revised it for important intellectual content. All authors approved the final version of the article.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Global Observatory on Donation and Transplantation. International report on organ donation and transplantation activities 2021. 2022. Available at https://www.transplant-observatory.org/2021-global-report-5/. Accessed November 21, 2023.
- 2.Dominguez-Gil B, Ascher N, Capron AM, et al. Expanding controlled donation after the circulatory determination of death: statement from an international collaborative. Intensive Care Med. 2021;47:265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manara AR, Murphy PG, O’Callaghan G. Donation after circulatory death. Br J Anaesth. 2012;108(Suppl 1):ii108–ii121. [DOI] [PubMed] [Google Scholar]
- 4.Smith M, Dominguez-Gil B, Greer DM, et al. Organ donation after circulatory death: current status and future potential. Intensive Care Med. 2019;45:310–321. [DOI] [PubMed] [Google Scholar]
- 5.Kwon JH, Blanding WM, Shorbaji K, et al. Waitlist and transplant outcomes in organ donation after circulatory death: trends in the United States. Ann Surg. 2023;278:609–620. [DOI] [PubMed] [Google Scholar]
- 6.Phillips B, Asgari E, Berry M, et al. British Transplantation Society guidelines on abdominal organ transplantation from deceased donors after circulatory death. Transplant Rev. 2023;38:100801. [DOI] [PubMed] [Google Scholar]
- 7.Rijkse E, Ceuppens S, Qi H, et al. Implementation of donation after circulatory death kidney transplantation can safely enlarge the donor pool: a systematic review and meta-analysis. Int J Surg. 2021;92:106021. [DOI] [PubMed] [Google Scholar]
- 8.Hessheimer AJ, Coll E, Torres F, et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol. 2019;70:658–665. [DOI] [PubMed] [Google Scholar]
- 9.Tang JX, Na N, Li JJ, et al. Outcomes of controlled donation after cardiac death compared with donation after brain death in liver transplantation: a systematic review and meta-analysis. Transplant Proc. 2018;50:33–41. [DOI] [PubMed] [Google Scholar]
- 10.Cantador B, Moreno P, González FJ, et al. Influence of donor type-donation after brain death versus donation after circulatory death-on lung transplant outcomes. Transplant Proc. 2023;55:2292–2294. [DOI] [PubMed] [Google Scholar]
- 11.Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015;34:675–684. [DOI] [PubMed] [Google Scholar]
- 12.Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2017;36:1311–1318. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed HF, Kulshrestha K, Kennedy JT, et al. Donation after circulatory death significantly reduces waitlist times while not changing post-heart transplant outcomes: a United Network for Organ Sharing analysis. J Heart Lung Transplant. 2024;43:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maitre G, Shemie SD, Baker A, et al. Knowledge gaps in the definition and determination of death. Can J Anaesth. 2023;70:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernat JL. Challenges to brain death in revising the Uniform Determination of Death Act: the UDDA Revision Series. Neurology. 2023;101:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhanani S, Hornby L, Ward R, et al. Variability in the determination of death after cardiac arrest: a review of guidelines and statements. J Intensive Care Med. 2012;27:238–252. [DOI] [PubMed] [Google Scholar]
- 17.Hornby L, Dhanani S, Shemie SD. Update of a systematic review of autoresuscitation after cardiac arrest. Crit Care Med. 2018;46:e268–e272. [DOI] [PubMed] [Google Scholar]
- 18.Gardiner D, McGee A, Bernat JL. Permanent brain arrest as the sole criterion of death in systemic circulatory arrest. Anaesthesia. 2020;75:1223–1228. [DOI] [PubMed] [Google Scholar]
- 19.Bernat JL, Domínguez-Gil B, Glazier AK, et al. Understanding the brain-based determination of death when organ recovery is performed with DCDD in situ normothermic regional perfusion. Transplantation. 2023. 1;107:1650–1654. [DOI] [PubMed] [Google Scholar]
- 20.Dhanani S, Hornby L, van Beinum A, et al. ; Canadian Critical Care Trials Group. Resumption of cardiac activity after withdrawal of life-sustaining measures. N Engl J Med. 2021;384:345–352. [DOI] [PubMed] [Google Scholar]
- 21.Robertson JA. The dead donor rule. Hastings Cent Rep. 1999;29:6–14. [PubMed] [Google Scholar]
- 22.Taylor LJ, Buffington A, Scalea JR, et al. Harms of unsuccessful donation after circulatory death: an exploratory study. Am J Transplant. 2018;18:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy NB, Weijer C, Lalgudi Ganesan S, et al. Nontherapeutic research with imminently dying and recently deceased study populations: addressing practical and ethical challenges. Can J Anaesth. 2023;70:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin DE, Cronin AJ, Dalle Ave A, et al. Addressing ethical confusion in deceased donation and transplantation research: the need for dedicated guidance. Transpl Int. 2021;34:2459–2468. [DOI] [PubMed] [Google Scholar]
- 25.Parent B, Moazami N, Wall S, et al. Ethical and logistical concerns for establishing NRP-cDCD heart transplantation in the United States. Am J Transplant. 2020;20:1508–1512. [DOI] [PubMed] [Google Scholar]
- 26.Entwistle JW, Drake DH, Fenton KN, et al. ; Cardiothoracic Ethics Forum. Normothermic regional perfusion: ethical issues in thoracic organ donation. J Thorac Cardiovasc Surg. 2022;164:147–154. [DOI] [PubMed] [Google Scholar]
- 27.Bernat JL, Khush KK, Shemie SD, et al. Knowledge gaps in heart and lung donation following the circulatory determination of death: report of a workshop of the National Heart, Lung, and Blood Institute. J Heart Lung. [Epub ahead of print March 2, 2024]. doi: 10.1016/j.healun.2024.02.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison LJ, Sandroni C, Grunau B, et al. Organ donation after out-of-hospital cardiac arrest: a scientific statement from the International Liaison Committee on Resuscitation. Circulation. 2023;148:e120–e146. [DOI] [PubMed] [Google Scholar]
- 29.Schiff T, Koziatek C, Pomerantz E, et al. Extracorporeal cardiopulmonary resuscitation dissemination and integration with organ preservation in the USA: ethical and logistical considerations. Crit Care. 2023;27:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coll E, Miñambres E, Sánchez-Fructuoso A, et al. Uncontrolled donation after circulatory death: a unique opportunity. Transplantation. 2020;4:1542–1552. [DOI] [PubMed] [Google Scholar]
- 31.National Heart, Lung, and Blood Institute. Heart and lung transplantation: science and ethics of DCDD and xenotransplants hybrid workshop. 2023. Available at https://www.nhlbi.nih.gov/events/2023/heart-and-lung-transplantation-science-and-ethics-dcdd-and-xenotransplants-hybrid. Accessed August 21, 2023.
- 32.Shemie SD, Wilson LC, Hornby L, et al. A brain-based definition of death and criteria for its determination after arrest of circulation or neurologic function in Canada: a 2023 clinical practice guideline. Can J Anaesth. 2023;70:483–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klowak JA, Nguyen AV, Malik A, et al. Diagnostic test accuracy for cessation of circulation during death determination: a systematic review. Can J Anaesth. 2023;70:671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalgudi Ganesan S, Hornby L, Weiss M, et al. Brain-based arterial pulse pressure threshold for death determination: a systematic review. Can J Anaesth. 2023;70:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zorko DJ, Shemie J, Hornby L, et al. Autoresuscitation after circulatory arrest: an updated systematic review. Can J Anaesth. 2023;70:699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shemie J, Scales NB, Sucha E, et al. Variability in criteria for death determination in the intensive care unit. Can J Anaesth. 2023;70:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernat JL. How the distinction between “irreversible” and “permanent” illuminates circulatory-respiratory death determination. J Med Philos. 2010;35:242–255. [DOI] [PubMed] [Google Scholar]
- 38.McGee A, Gardiner D. Permanence can be defended. Bioethics. 2017;31:220–230. [DOI] [PubMed] [Google Scholar]
- 39.Lomero M, Gardiner D, Coll E, et al. ; European Committee on Organ Transplantation of the Council of Europe (CD-P-TO). Donation after circulatory death today: an updated overview of the European landscape. Transpl Int. 2020;33:76–88. [DOI] [PubMed] [Google Scholar]
- 40.Joffe AR, Carcillo J, Anton N, et al. Donation after cardiocirculatory death: a call for a moratorium pending full public disclosure and fully informed consent. Philos Ethics Humanit Med. 2011;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernat JL, Capron AM, Bleck TP, et al. The circulatory-respiratory determination of death in organ donation. Crit Care Med. 2010;38:963–970. [DOI] [PubMed] [Google Scholar]
- 42.Peled H, Mathews S, Rhodes D, et al. Normothermic regional perfusion requires careful ethical analysis before adoption into DCD. Crit Care Med. 2022;50:1644–1648. [DOI] [PubMed] [Google Scholar]
- 43.Glazier AK, Capron AM. Normothermic regional perfusion and US legal standards for determining death are not aligned. Am J Transplant. 2022;22:1289–1290. [DOI] [PubMed] [Google Scholar]
- 44.Greer DM, Shemie SD, Lewis A, et al. Determination of brain death/death by neurologic criteria: The World Brain Death Project. JAMA. 2020;324:1078–1097. [DOI] [PubMed] [Google Scholar]
- 45.Murphy NB, Hartwick M, Wilson LC, et al. Rationale for revisions to the definition of death and criteria for its determination in Canada. Can J Anaesth. 2023;70:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plourde G, Briard JN, Shemie SD, et al. Flow is not perfusion, and perfusion is not function: ancillary testing for the diagnosis of brain death. Can J Anaesth. 2021;68:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Defining Death: A Report on the Medical, Legal and Ethical Issues in the Determination of Death. President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research; 1981. [Google Scholar]
- 48.Wall AE, Fiedler A, Karp S, et al. Applying the ethical framework for donation after circulatory death to thoracic normothermic regional perfusion procedures. Am J Transplant. 2022;22:1311–1315. [DOI] [PubMed] [Google Scholar]
- 49.Shemie SD, Hornby L, Baker A, et al. ; The International Guidelines for Determination of Death Phase 1 Participants, in Collaboration With the World Health Organization. International guideline development for the determination of death. Intensive Care Med. 2014;40:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shemie SD, Gardiner D. Circulatory arrest, brain arrest and death determination. Front Cardiovasc Med. 2018;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell JA, Epstein LG, Greer DM, et al. Brain death, the determination of brain death, and member guidance for brain death accommodation requests: AAN position statement. Neurology. 2019;92:228–232. [DOI] [PubMed] [Google Scholar]
- 52.Joffe AR. Should the criterion for brain death require irreversible or permanent cessation of function? Irreversible: the UDDA revision series. Neurology. 2023;101:181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGee A, Gardiner D. S Should the criterion for brain death require irreversible or permanent cessation of function? Permanent: the UDDA revision series. Neurology. 2023;101:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalle Ave AL, Bernat JL. Using the brain criterion in organ donation after the circulatory determination of death. J Crit Care. 2016;33:114–118. [DOI] [PubMed] [Google Scholar]
- 55.Zheng K, Sutherland S, Hornby L, et al. Healthcare professionals’ understandings of the definition and determination of death: a scoping review. Transplant Direct. 2022;8:e1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scales NB, Herry CL, van Beinum A, et al. Predicting time to death after withdrawal of life-sustaining measures using vital sign variability: derivation and validation. Crit Care Explor. 2022;4:e0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Neill S, Asgari E, Callaghan C, et al. The British transplantation society guidelines on organ donation from deceased donors after circulatory death. Transplant Rev. 2023;37:100791. [DOI] [PubMed] [Google Scholar]
- 58.Perera MT, Bramhall SR. Current status and recent advances of liver transplantation from donation after cardiac death. World J Gastrointest Surg. 2011;3:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee DD, Joyce C, Duehren S, et al. Oxygen saturation during donor warm ischemia time and outcome of donation after circulatory death (DCD) liver transplantation with static cold storage: a review of 1114 cases. Liver Transpl. 2023;29:1192–1198. [DOI] [PubMed] [Google Scholar]
- 60.Munshi L, Dhanani S, Shemie SD, et al. Predicting time to death after withdrawal of life-sustaining therapy. Intensive Care Med. 2015;41:1014–1028. [DOI] [PubMed] [Google Scholar]
- 61.Lindemann J, Dageforde LA, Vachharajani N, et al. Cost evaluation of a donation after cardiac death program: how cost per organ compares to other donor types. J Am Coll Surg. 2018;226:909–916. [DOI] [PubMed] [Google Scholar]
- 62.Brieva J, Coleman N, Lacey J, et al. Prediction of death in less than 60 minutes after withdrawal of cardiorespiratory support in potential organ donors after circulatory death. Transplantation. 2014;98:1112–1118. [DOI] [PubMed] [Google Scholar]
- 63.Wind J, Snoeijs MG, Brugman CA, et al. Prediction of time of death after withdrawal of life-sustaining treatment in potential donors after cardiac death. Crit Care Med. 2012;40:766–769. [DOI] [PubMed] [Google Scholar]
- 64.Lewis J, Peltier J, Nelson H, et al. Development of the University of Wisconsin donation after cardiac death evaluation tool. Prog Transplant. 2003;13:265–273. [DOI] [PubMed] [Google Scholar]
- 65.Winter MC, Day TE, Ledbetter DR, et al. Machine learning to predict cardiac death within 1 hour after terminal extubation. Pediatr Crit Care Med. 2021;22:161–171. [DOI] [PubMed] [Google Scholar]
- 66.Waldauf P, Scales N, Shahin J, et al. Machine learning determination of motivators of terminal extubation during the transition to end-of-life care in intensive care unit. Sci Rep. 2023;13:2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sprung CL, Ricou B, Hartog CS, et al. Changes in end-of-life practices in European Intensive Care Units from 1999 to 2016. JAMA. 2019;322:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cappucci SP, Smith WS, Schwartzstein R, et al. End-of-life care in the potential donor after circulatory dead: a systematic review. Neurohospitalist. 2023;13:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Beinum A, Hornby L, Ward R, et al. Variations in the operational process of withdrawal of life-sustaining therapy. Crit Care Med. 2015;43:e450–e457. [DOI] [PubMed] [Google Scholar]
- 70.Shore PM, Huang R, Roy L, et al. Development of a bedside tool to predict time to death after withdrawal of life-sustaining therapies in infants and children. Pediatr Crit Care Med. 2012;13:415–422. [DOI] [PubMed] [Google Scholar]
- 71.Gofton T, Dhanani S, Meade M, et al. Neurologic Physiology after Removal of Therapy (NeuPaRT) study: study protocol of a multicentre, prospective, observational, pilot feasibility study of neurophysiology after withdrawal of life-sustaining measures. BMJ Open. 2023;13:e073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rady MY, Verheijde JL. General anesthesia for surgical procurement in non-heart-beating organ donation: why we should care. Anesth Analg. 2010;111:1562; author reply 1563. [DOI] [PubMed] [Google Scholar]
- 73.Bastami S, Matthes O, Krones T, et al. Systematic review of attitudes toward donation after cardiac death among healthcare providers and the general public. Crit Care Med. 2013;41:897–905. [DOI] [PubMed] [Google Scholar]
- 74.Matory AL, Alkhachroum A, Chiu WT, et al. Electrocerebral signature of cardiac death. Neurocrit Care. 2021;35:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chawla LS, Akst S, Junker C, et al. Surges of electroencephalogram activity at the time of death: a case series. J Palliat Med. 2009;12:1095–1100. [DOI] [PubMed] [Google Scholar]
- 76.Auyong DB, Klein SM, Gan TG, et al. Processed electroencephalogram during donation after cardiac death. Anesth Analg. 2010;110:1428–1432. [DOI] [PubMed] [Google Scholar]
- 77.Park E, Liu E, Shemie SD, et al. Relating clinical and electrophysiological parameters in death determination in a laboratory model of progressive hypoxemia. Neurocrit Care. 2018;28:133–141. [DOI] [PubMed] [Google Scholar]
- 78.Gofton TE, Norton L, Laforge G, et al. Cerebral cortical activity after withdrawal of life-sustaining measures in critically ill patients. Am J Transplant. 2022;22:3120–3129. [DOI] [PubMed] [Google Scholar]
- 79.Pana R, Hornby L, Shemie SD, et al. Time to loss of brain function and activity during circulatory arrest. J Crit Care. 2016;34:77–83. [DOI] [PubMed] [Google Scholar]
- 80.Norton L, Gibson RM, Gofton T, et al. Electroencephalographic recordings during withdrawal of life-sustaining therapy until 30 minutes after declaration of death. Can J Neurol Sci. 2017;44:139–145. [DOI] [PubMed] [Google Scholar]
- 81.Romagnoli S, Ricci Z, Quattrone D, et al. Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care. 2014;18:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernat JL. Conceptual issues in DCDD donor death determination. Hastings Cent Rep. 2018;48(Suppl 4):S26–S28. [DOI] [PubMed] [Google Scholar]
- 83.Gordon L, Pasquier M, Brugger H, et al. Autoresuscitation (Lazarus phenomenon) after termination of cardiopulmonary resuscitation—a scoping review. Scand J Trauma Resusc Emerg Med. 2020;28:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steinhorn D, Calligan AL. Lazarus syndrome in pediatric hospice care: does it occur and what home hospice providers should know? Pediatrics. 2021;147:538–539. [Google Scholar]
- 85.Zier JL, Newman NA. Unassisted return of spontaneous circulation following withdrawal of life sustaining therapy during donation after circulatory determination of death in a child. Crit Care Med. 2021;50:06. [DOI] [PubMed] [Google Scholar]
- 86.Sheth KN, Nutter T, Stein DM, et al. Autoresuscitation after asystole in patients being considered for organ donation. Crit Care Med. 2012;40:158–161. [DOI] [PubMed] [Google Scholar]
- 87.Dhanani S, Hornby L, Ward R, et al. ; Canadian Critical Care Trials Group and in Collaboration With the Bertram Loeb Chair and Research Consortium in Organ and Tissue Donation. Vital signs after cardiac arrest following withdrawal of life-sustaining therapy: a multicenter prospective observational study. Crit Care Med. 2014;42:2358–2369. [DOI] [PubMed] [Google Scholar]
- 88.Manara A, Shemie SD, Large S, et al. Maintaining the permanence principle for death during in situ normothermic regional perfusion for donation after circulatory death organ recovery: A United Kingdom and Canadian proposal. Am J Transplant. 2020;20:2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santos PARD, Teixeira PJZ, Moraes Neto DM, et al. Donation after circulatory death and lung transplantation. J Bras Pneumol. 2022;48:e20210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy NB, Weijer C, Slessarev M, et al. Implications of the updated Canadian death determination guidelines for organ donation interventions that restore circulation after determination of death by circulatory criteria. Can J Anaesth. 2023;70:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Truog RD, Flescher A, Ladin K. Normothermic regional perfusion—the next frontier in organ transplants? JAMA. 2023;329:2123–2124. [DOI] [PubMed] [Google Scholar]
- 92.Basmaji J, Weijer C, Skaro A, et al. Paving the road for the adoption of normothermic regional perfusion in Canada. Crit Care Explor. 2021;3:e0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slessarev M, Gofton T, Shemie SD. Ensuring the permanent cessation of brain function during normothermic regional perfusion. Transplantation. 2022;106:1726–1727. [DOI] [PubMed] [Google Scholar]
- 94.Hessheimer AJ, de la Rosa G, Gastaca M, et al. Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: outcomes and risk factors for graft loss. Am J Transplant. 2022;22:1169–1181. [DOI] [PubMed] [Google Scholar]
- 95.Miñambres E, Royo-Villanova M, Domínguez-Gil B. Normothermic regional perfusion provides a great opportunity to maximize organ procurement in donation after the circulatory determination of death. Crit Care Med. 2022;50:1649–1653. [DOI] [PubMed] [Google Scholar]
- 96.Jochmans I, Hessheimer AJ, Neyrinck AP, et al. ; ESOT Workstream 04 of the TLJ (Transplant Learning Journey) Project. Consensus statement on normothermic regional perfusion in donation after circulatory death: Report from the European Society for Organ Transplantation’s Transplant Learning Journey. Transpl Int. 2021;34:2019–2030. [DOI] [PubMed] [Google Scholar]
- 97.Ortega-Deballon I, Hornby L, Shemie SD. Protocols for uncontrolled donation after circulatory death: a systematic review of international guidelines, practices and transplant outcomes. Crit Care. 2015;19:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalsgaard FF, Moeslund N, Zhang ZL, et al. Clamping of the aortic arch vessels during normothermic regional perfusion after circulatory death prevents the return of brain activity in a porcine model. Transplantation. 2022;106:1763–1769. [DOI] [PubMed] [Google Scholar]
- 99.Frontera JA, Lewis A, James L, et al. Thoracoabdominal normothermic regional perfusion in donation after circulatory death does not restore brain blood flow. J Heart Lung Transplant. 2023;42:1161–1165. [DOI] [PubMed] [Google Scholar]
- 100.Royo-Villanova M, Miñambres E, Sánchez JM, et al. Maintaining the permanence principle of death during normothermic regional perfusion in controlled donation after the circulatory determination of death: Results of a prospective clinical study. Am J Transplant. 2024;24:213–221. [DOI] [PubMed] [Google Scholar]
- 101.American College of Physicians. Ethics, determination of death, and organ transplantation in normothermic regional perfusion (NRP) with controlled donation after circulatory determination of death (cDCD): American College of Physicians statement of concern. 2021. Available at https://www.acponline.org/sites/default/files/documents/clinical_information/resources/end_of_life_care/ethics_determination_of_death_and_organ_transplantation_in_nrp_2021.pdf. Accessed August 23, 2023.
- 102.Egan TM, Haithcock BE, Lobo J, et al. Donation after circulatory death donors in lung transplantation. J Thorac Dis. 2021;13:6536–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saxena P, Zimmet AD, Snell G, et al. Procurement of lungs for transplantation following donation after circulatory death: the Alfred technique. J Surg Res. 2014;192:642–646. [DOI] [PubMed] [Google Scholar]
- 104.Egan TM, Requard JJ. Afterlife for lungs: a way to increase donor lungs for transplant. Am J Transplant. 2020;20:2954–2955. [DOI] [PubMed] [Google Scholar]
- 105.Healey A, Watanabe Y, Mills C, et al. Initial lung transplantation experience with uncontrolled donation after cardiac death in North America. Am J Transplant. 2020;20:1574–1581. [DOI] [PubMed] [Google Scholar]