Abstract
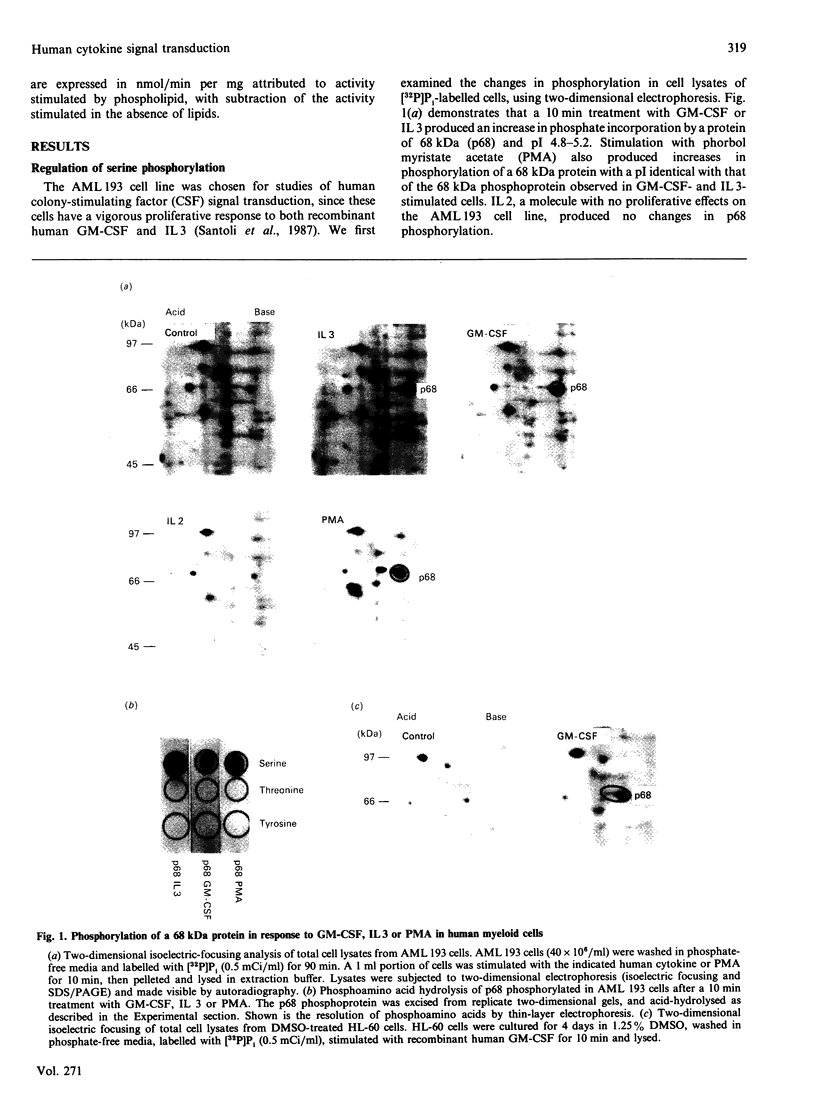
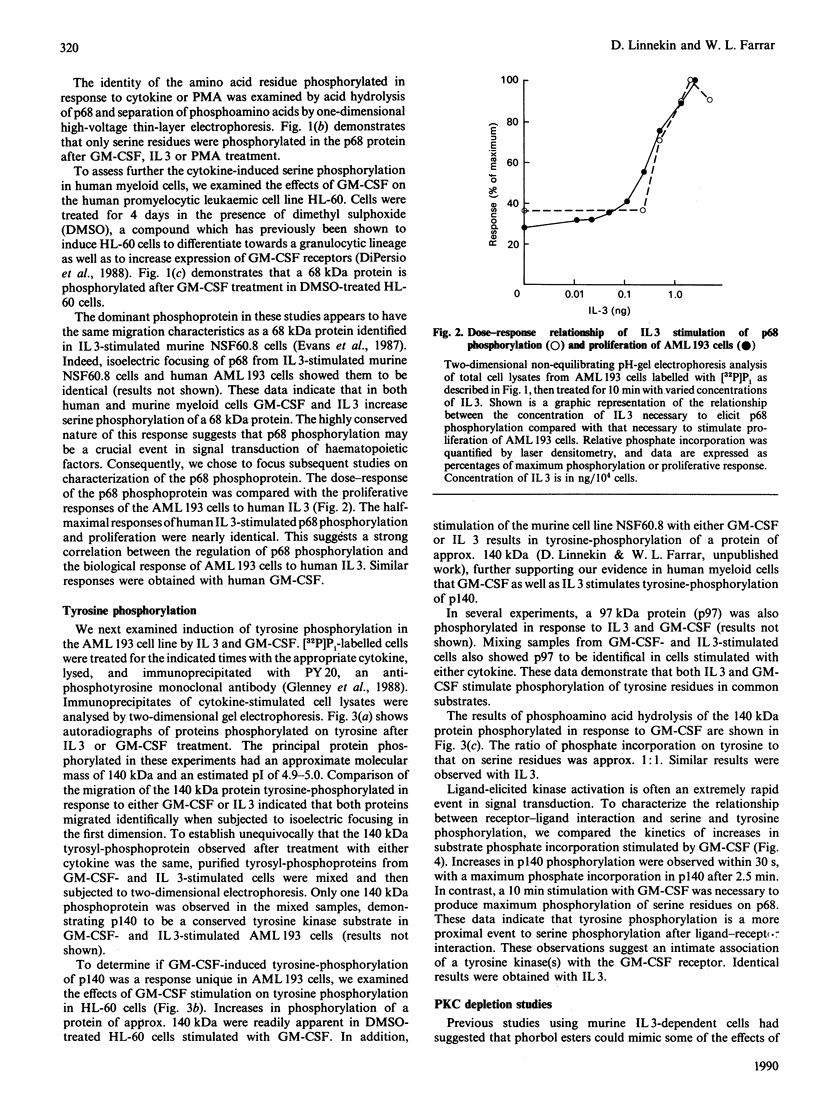
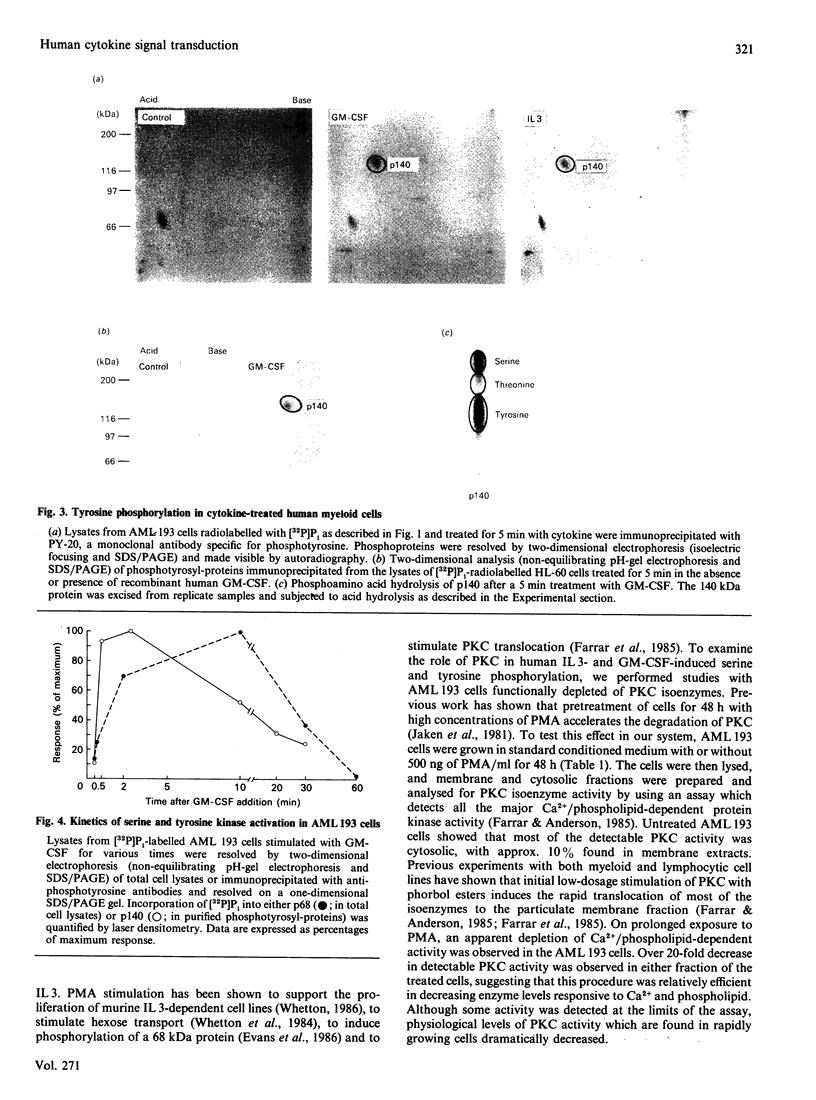
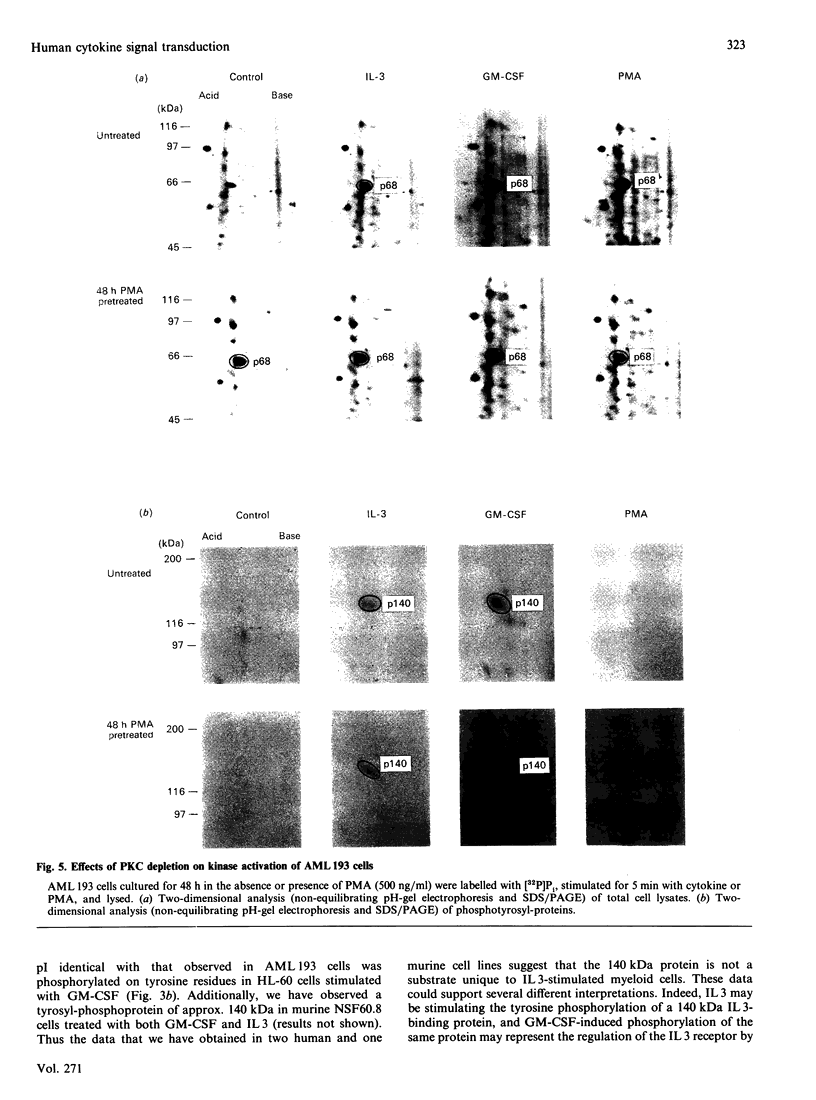
To elucidate the rapid events in signal transduction of human granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 3 (IL 3), we examined phosphorylation of proteins on both serine and tyrosine residues in a cytokine-stimulated human myeloid cell line. We found increases in tyrosine phosphorylation within 30 s of stimulation with GM-CSF or IL 3, with peak responses occurring within 2 min. IL 3 and GM-CSF also induced serine phosphorylation, though 10 min of stimulation was required for maximum phosphate incorporation. Interestingly, both IL 3 and GM-CSF stimulated phosphate incorporation in identical substrates, a 68 kDa seryl-phosphoprotein (p68) and a 140 kDa tyrosyl-phosphoprotein (p140). Treatment of AML 193 cells with phorbol myristate acetate resulted in serine phosphorylation of p68; however, p140 was not phosphorylated on tyrosine. Depletion of protein kinase C isoenzymes with high concentrations of phorbol myristate acetate resulted in p68 phosphorylation, which was not further increased by IL 3 or GM-CSF. In contrast, cytokine-induced phosphorylation on tyrosine of p140 was observed after protein kinase C depletion. These data demonstrate the co-ordinate yet independent serine and tyrosine phosphorylation in IL 3- and GM-CSF-treated human myeloid cells, and thus suggest a common set of protein kinases stimulated by each separate ligand.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cook N., Dexter T. M., Lord B. I., Cragoe E. J., Jr, Whetton A. D. Identification of a common signal associated with cellular proliferation stimulated by four haemopoietic growth factors in a highly enriched population of granulocyte/macrophage colony-forming cells. EMBO J. 1989 Oct;8(10):2967–2974. doi: 10.1002/j.1460-2075.1989.tb08446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J., Billing P., Kaufman S., Eghtesady P., Williams R. E., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1988 Feb 5;263(4):1834–1841. [PubMed] [Google Scholar]
- Evans S. W., Rennick D., Farrar W. L. Identification of a signal-transduction pathway shared by haematopoietic growth factors with diverse biological specificity. Biochem J. 1987 Jun 15;244(3):683–691. doi: 10.1042/bj2440683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. W., Rennick D., Farrar W. L. Multilineage hematopoietic growth factor interleukin 3 and direct activators of protein kinase C stimulate phosphorylation of common substrates. Blood. 1986 Oct;68(4):906–913. [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Thomas T. P., Anderson W. B. Altered cytosol/membrane enzyme redistribution on interleukin-3 activation of protein kinase C. Nature. 1985 May 16;315(6016):235–237. doi: 10.1038/315235a0. [DOI] [PubMed] [Google Scholar]
- Ferris D. K., Willet-Brown J., Martensen T., Farrar W. L. Interleukin 3 stimulation of tyrosine kinase activity in FDC-P1 cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):991–996. doi: 10.1016/0006-291x(88)90237-9. [DOI] [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Linnekin D., Farrar W. L. Comparative analysis of IL-2 and IL-3 induced tyrosine phosphorylation. Lymphokine Res. 1989 Fall;8(3):215–224. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Yamazaki M., Metwally F., Molski T. F., Bonak V. A., Huang C. K., Becker E. L., Sha'afi R. I. Granulocyte-macrophage colony-stimulating factor and human neutrophils: role of guanine nucleotide regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3569–3573. doi: 10.1073/pnas.86.10.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P., Huang K. P., Paul W. E. Lamin B is rapidly phosphorylated in lymphocytes after activation of protein kinase C. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2279–2283. doi: 10.1073/pnas.85.7.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Stevens D., May W. S., Ihle J. N. Interleukin 3 binds to a 140-kDa phosphotyrosine-containing cell surface protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7982–7986. doi: 10.1073/pnas.85.21.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Jaken S., Tashjian A. H., Jr, Blumberg P. M. Characterization of phorbol ester receptors and their down-modulation in GH4C1 rat pituitary cells. Cancer Res. 1981 Jun;41(6):2175–2181. [PubMed] [Google Scholar]
- Koyasu S., Tojo A., Miyajima A., Akiyama T., Kasuga M., Urabe A., Schreurs J., Arai K., Takaku F., Yahara I. Interleukin 3-specific tyrosine phosphorylation of a membrane glycoprotein of Mr 150,000 in multi-factor-dependent myeloid cell lines. EMBO J. 1987 Dec 20;6(13):3979–3984. doi: 10.1002/j.1460-2075.1987.tb02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. S., Moore P. B. Expression of 67,000 MW calcimedin and its binding protein in resident and thioglycolate-elicited macrophages. Immunology. 1988 Dec;65(4):537–541. [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Takai Y., Kawahara Y., Kimura S., Nishizuka Y. A new possible regulatory system for protein phosphorylation in human peripheral lymphocytes. I. Characterization of a calcium-activated, phospholipid-dependent protein kinase. J Immunol. 1981 Oct;127(4):1369–1374. [PubMed] [Google Scholar]
- Owens R. J., Crumpton M. J. Isolation and characterization of a novel 68,000-Mr Ca2+-binding protein of lymphocyte plasma membrane. Biochem J. 1984 Apr 1;219(1):309–316. doi: 10.1042/bj2190309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Park L. S., Waldron P. E., Friend D., Sassenfeld H. M., Price V., Anderson D., Cosman D., Andrews R. G., Bernstein I. D., Urdal D. L. Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood. 1989 Jul;74(1):56–65. [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D., Yang Y. C., Clark S. C., Kreider B. L., Caracciolo D., Rovera G. Synergistic and antagonistic effects of recombinant human interleukin (IL) 3, IL-1 alpha, granulocyte and macrophage colony-stimulating factors (G-CSF and M-CSF) on the growth of GM-CSF-dependent leukemic cell lines. J Immunol. 1987 Nov 15;139(10):3348–3354. [PubMed] [Google Scholar]
- Schreurs J., Sugawara M., Arai K., Ohta Y., Miyajima A. A monoclonal antibody with IL-3-like activity blocks IL-3 binding and stimulates tyrosine phosphorylation. J Immunol. 1989 Feb 1;142(3):819–825. [PubMed] [Google Scholar]
- Sorensen P. H., Mui A. L., Murthy S. C., Krystal G. Interleukin-3, GM-CSF, and TPA induce distinct phosphorylation events in an interleukin 3-dependent multipotential cell line. Blood. 1989 Feb;73(2):406–418. [PubMed] [Google Scholar]
- Sorensen P., Mui A. L., Krystal G. Interleukin-3 stimulates the tyrosine phosphorylation of the 140-kilodalton interleukin-3 receptor. J Biol Chem. 1989 Nov 15;264(32):19253–19258. [PubMed] [Google Scholar]
- Tabarini D., Garcia de Herreros A., Heinrich J., Rosen O. M. Purification of a bovine liver S6 kinase. Biochem Biophys Res Commun. 1987 Apr 29;144(2):891–899. doi: 10.1016/s0006-291x(87)80048-7. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977 Nov 10;252(21):7603–7609. [PubMed] [Google Scholar]
- Valent P., Besemer J., Muhm M., Majdic O., Lechner K., Bettelheim P. Interleukin 3 activates human blood basophils via high-affinity binding sites. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5542–5546. doi: 10.1073/pnas.86.14.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Bazill G. W., Dexter T. M. Haemopoietic cell growth factor mediates cell survival via its action on glucose transport. EMBO J. 1984 Feb;3(2):409–413. doi: 10.1002/j.1460-2075.1984.tb01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Heyworth C. M., Dexter T. M. Phorbol esters activate protein kinase C and glucose transport and can replace the requirement for growth factor in interleukin-3-dependent multipotent stem cells. J Cell Sci. 1986 Aug;84:93–104. doi: 10.1242/jcs.84.1.93. [DOI] [PubMed] [Google Scholar]





