Abstract
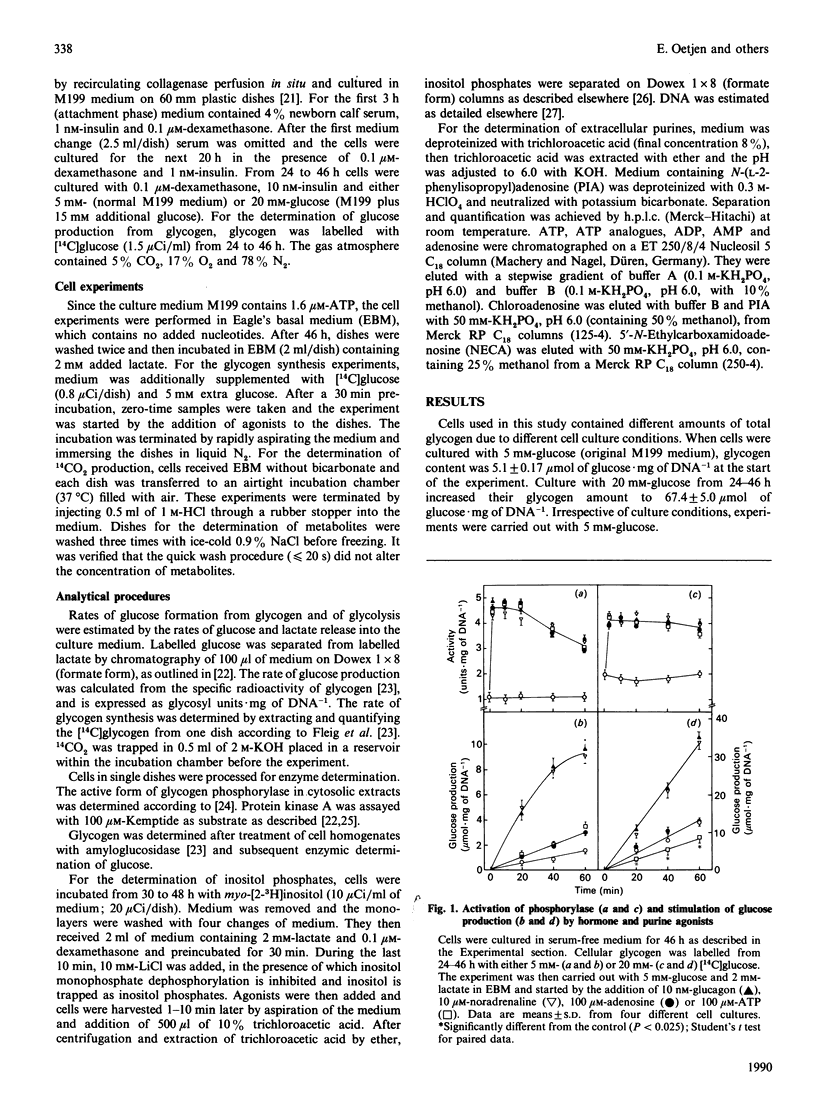
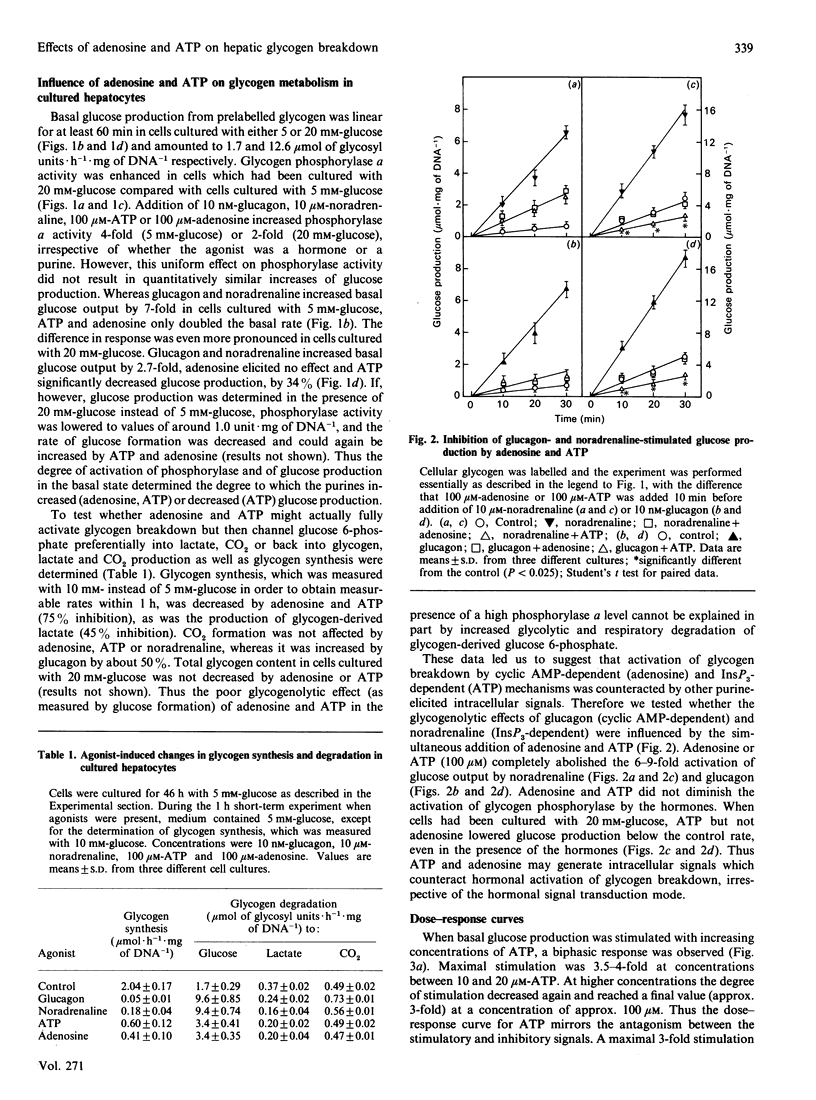
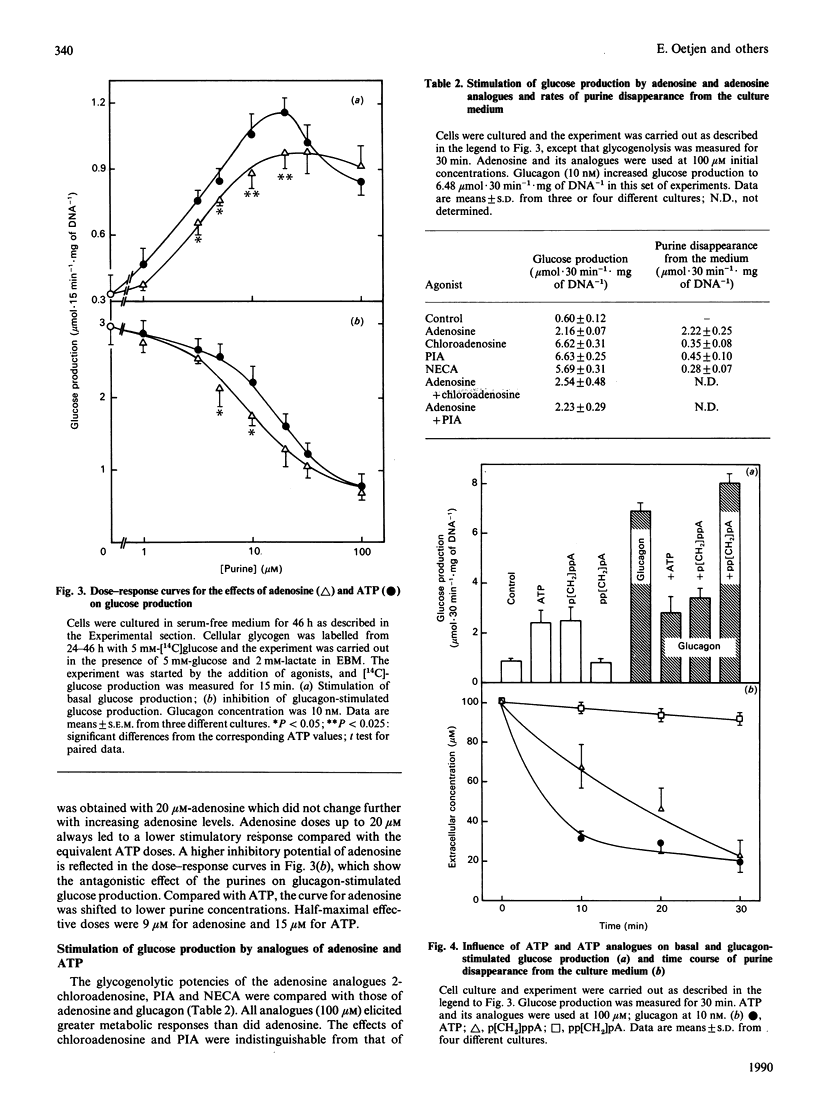
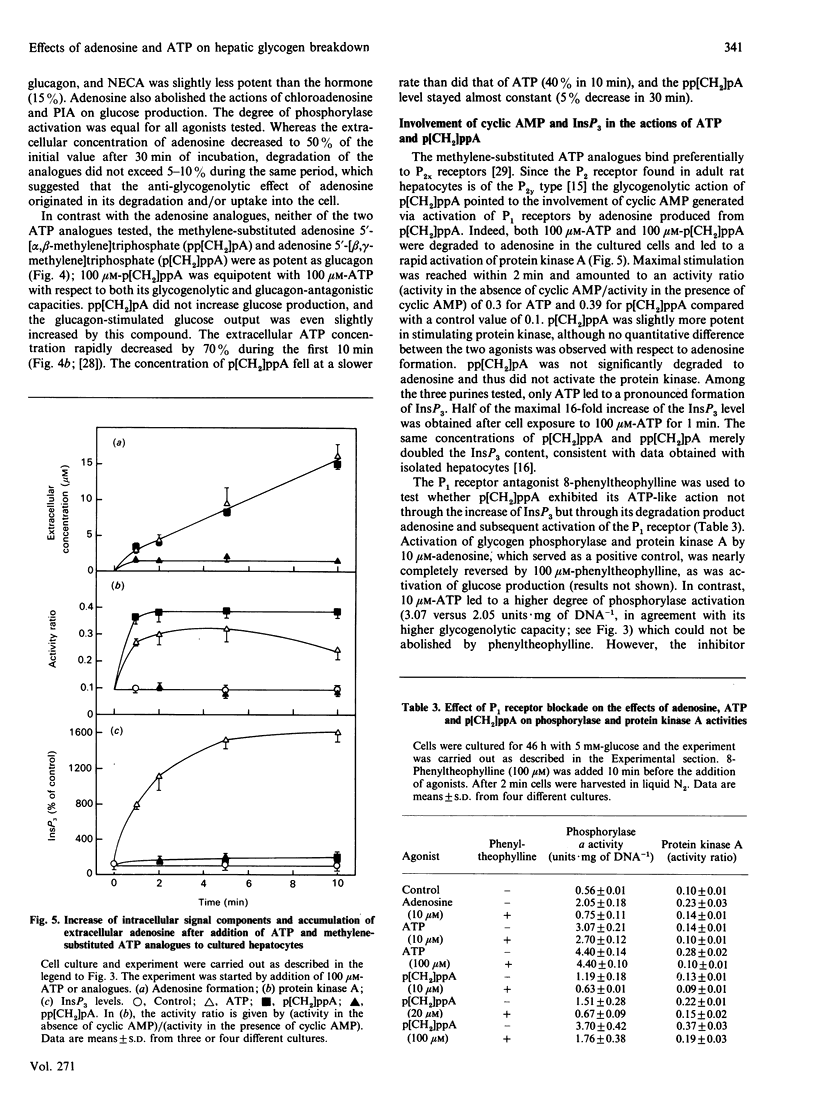
The glycogenolytic potency of adenosine and ATP was studied in adult rat hepatocytes and compared with the action of glucagon and noradrenaline. In cells cultured for 48 h, adenosine and ATP as well as their analogues 2-chloroadenosine, phenylisopropyladenosine, N-ethylcarboxamidoadenosine and beta-gamma-methylene-substituted ATP (p[CH2]ppA) increased glycogen phosphorylase alpha to levels indistinguishable from those obtained by the addition of glucagon or noradrenaline. The P1 receptor antagonist 8-phenyltheophylline abolished the activation of phosphorylase by adenosine and by p[CH2]ppA, but not that by ATP. Protein kinase A was activated by p[CH2]ppA and ATP via their breakdown to adenosine. [14C]Glucose production from glycogen was stimulated only 3-fold by ATP and adenosine, compared with a 7-fold increase produced by the hormones. Stimulation of glucose production by glucagon or noradrenaline was almost completely abolished by ATP or adenosine, with half-maximal effects at around 10 microM. The non-degradable adenosine analogues were equipotent with glucagon with respect to stimulation of glucose production, and their action was also inhibited by adenosine. ATP and p[CH2]ppA, which were both degraded to adenosine, showed comparable metabolic effects, whereas the alpha, beta-methylene analogue was without biological action and also was not degraded to adenosine. In the presence of the adenosine transport inhibitor nitrobenzyl thioinosine (NBTI), adenosine exerted an increased glycogenolytic potency, reaching 80% of the maximal stimulation obtained by glucagon. The glucagon-antagonistic effect of adenosine could be completely abolished by NBTI, but was not affected by phenyltheophylline. It is concluded that, in the hepatocyte culture system, adenosine and ATP decrease the catalytic efficiency of phosphorylase alpha through signals arising from their uptake into the cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athari A., Jungermann K. Direct activation by prostaglandin F2 alpha but not thromboxane A2 of glycogenolysis via an increase in inositol 1,4,5-trisphosphate in rat hepatocytes. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1235–1242. doi: 10.1016/0006-291x(89)91110-8. [DOI] [PubMed] [Google Scholar]
- Bartrons R., Van Schaftingen E., Hers H. G. The ability of adenosine to decrease the concentration of fructose 2,6-bisphosphate in isolated hepatocytes. A cyclic AMP-mediated effect. Biochem J. 1984 Feb 15;218(1):157–163. doi: 10.1042/bj2180157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G., Nicotera P., Orrenius S. Alterations in intracellular calcium compartmentation following inhibition of calcium efflux from isolated hepatocytes. Eur J Biochem. 1984 Oct 1;144(1):19–23. doi: 10.1111/j.1432-1033.1984.tb08425.x. [DOI] [PubMed] [Google Scholar]
- Bollen M., Vandebroeck A., Stalmans W. 1-Deoxynojirimycin and related compounds inhibit glycogenolysis in the liver without affecting the concentration of phosphorylase a. Biochem Pharmacol. 1988 Mar 1;37(5):905–909. doi: 10.1016/0006-2952(88)90179-7. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Robertson S. M., Olson M. S. Stimulation of glycogenolysis and vasoconstriction by adenosine and adenosine analogues in the perfused rat liver. Biochem J. 1987 Nov 15;248(1):35–41. doi: 10.1042/bj2480035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. B., Robertson S. M., Olson M. S. Stimulation of glycogenolysis by adenine nucleotides in the perfused rat liver. Biochem J. 1986 Aug 1;237(3):773–780. doi: 10.1042/bj2370773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteleijn E., Kuiper J., van Rooij H. C., Kamps J. A., Koster J. F., van Berkel T. J. Hormonal control of glycogenolysis in parenchymal liver cells by Kupffer and endothelial liver cells. J Biol Chem. 1988 Feb 25;263(6):2699–2703. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H., Baue A. E. Further evidence for ATP uptake by rat tissues. Biochim Biophys Acta. 1980 Mar 20;628(3):336–342. doi: 10.1016/0304-4165(80)90383-9. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Assimacopoulos F. D., Harper S. C., Corbin J. D., Park C. R., Exton J. H. Studies on the alpha-andrenergic activation of hepatic glucose output. II. Investigation of the roles of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in the actions of phenylephrine in isolated hepatocytes. J Biol Chem. 1976 Sep 10;251(17):5209–5218. [PubMed] [Google Scholar]
- Christ B., Löhne R., Schmidt H., Jungermann K. Modulation of the glucagon-dependent induction of phosphoenolpyruvate carboxykinase by adenosine, but not ketone bodies or ammonia in rat hepatocyte cultures. Possible significance for the zonal heterogeneity of liver parenchyma. Biol Chem Hoppe Seyler. 1987 Dec;368(12):1579–1587. doi: 10.1515/bchm3.1987.368.2.1579. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt L. M., Putney J. W., Jr Stimulation of glycogenolysis in hepatocytes by angiotensin II may involve both calcium release and calcium influx. FEBS Lett. 1983 Aug 22;160(1-2):259–263. doi: 10.1016/0014-5793(83)80978-8. [DOI] [PubMed] [Google Scholar]
- Espinet C., Bartrons R., Carreras J. Effect of adenosine on fructose 2,6-bisphosphate levels and glucose metabolization by chicken erythrocytes. FEBS Lett. 1989 Nov 20;258(1):143–146. doi: 10.1016/0014-5793(89)81635-7. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Shepherd R. E. Adenosine, cyclic AMP metabolism, and glycogenolysis in rat liver cells. J Biol Chem. 1977 Nov 25;252(22):8066–8070. [PubMed] [Google Scholar]
- Fleig W. E., Nöther-Fleig G., Steudter S., Enderle D., Ditschuneit H. Regulation of insulin binding and glycogenesis by insulin and dexamethasone in cultured rat hepatocytes. Biochim Biophys Acta. 1985 Dec 12;847(3):352–361. doi: 10.1016/0167-4889(85)90041-2. [DOI] [PubMed] [Google Scholar]
- Gómez-Foix A. M., Rodriguez-Gil J. E., Guinovart J. J., Bosch F. Prostaglandins E2 and F2 alpha affect glycogen synthase and phosphorylase in isolated hepatocytes. Biochem J. 1989 Jul 1;261(1):93–97. doi: 10.1042/bj2610093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W. Actions of extracellular UTP and ATP in perfused rat liver. A comparative study. Eur J Biochem. 1987 Aug 17;167(1):65–71. doi: 10.1111/j.1432-1033.1987.tb13304.x. [DOI] [PubMed] [Google Scholar]
- Iwai M., Jungermann K. Possible involvement of eicosanoids in the actions of sympathetic hepatic nerves on carbohydrate metabolism and hemodynamics in perfused rat liver. FEBS Lett. 1987 Aug 31;221(1):155–160. doi: 10.1016/0014-5793(87)80371-x. [DOI] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986 Dec 1;240(2):367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. P2-purinergic control of liver glycogenolysis. Biochem J. 1985 Nov 1;231(3):797–799. doi: 10.1042/bj2310797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoinne A., Buc H. A., Claeyssens S., Pinosa M., Matray F. The mechanism by which adenosine decreases gluconeogenesis from lactate in isolated rat hepatocytes. Biochem J. 1987 Sep 1;246(2):449–454. doi: 10.1042/bj2460449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Filkins J. P. Exogenous ATP and carbohydrate metabolism in the rat liver. Circ Shock. 1987;22(3):205–219. [PubMed] [Google Scholar]
- Leser H. G., Holstege A., Gerok W. The role of nonparenchymal and parenchymal liver cells in the catabolism of extracellular purines. Hepatology. 1989 Jul;10(1):66–71. doi: 10.1002/hep.1840100114. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J. C., Lavoinne A., Giroz M., Matray F. The influence of adenosine on intermediary metabolism of isolated hepatocytes. Biochimie. 1979;61(11-12):1273–1282. doi: 10.1016/s0300-9084(80)80286-0. [DOI] [PubMed] [Google Scholar]
- Müller A., Unthan-Fechner K., Probst I. Activation of phosphofructokinase 2 by insulin in cultured hepatocytes without accompanying changes of effector levels or cAMP-stimulated protein kinase activity ratios. Eur J Biochem. 1988 Sep 15;176(2):415–420. doi: 10.1111/j.1432-1033.1988.tb14298.x. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Oliver I. T., Edwards A. M., Pitot H. C. Hormonal regulation of phosphoenolpyruvate carboxykinase in primary cultures of adult-rat liver parenchymal cells. Eur J Biochem. 1978 Jun 15;87(2):221–227. doi: 10.1111/j.1432-1033.1978.tb12369.x. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Oliver J. M. Nucleoside transport. II. Inhibition by p-nitrobenzylthioguanosine and related compounds. Can J Biochem. 1971 Feb;49(2):271–274. doi: 10.1139/o71-039. [DOI] [PubMed] [Google Scholar]
- Peachell P. T., Lichtenstein L. M., Schleimer R. P. Inhibition by adenosine of histamine and leukotriene release from human basophils. Biochem Pharmacol. 1989 Jun 1;38(11):1717–1725. doi: 10.1016/0006-2952(89)90404-8. [DOI] [PubMed] [Google Scholar]
- Probst I., Quentmeier A., Schweickhardt C., Unthan-Fechner K. Stimulation by insulin of glycolysis in cultured hepatocytes is attenuated by extracellular ATP and puromycin through purine-dependent inhibition of phosphofructokinase 2 activation. Eur J Biochem. 1989 Jun 15;182(2):387–393. doi: 10.1111/j.1432-1033.1989.tb14843.x. [DOI] [PubMed] [Google Scholar]
- Probst I., Schwartz P., Jungermann K. Induction in primary culture of 'gluconeogenic' and 'glycolytic' hepatocytes resembling periportal and perivenous cells. Eur J Biochem. 1982 Aug;126(2):271–278. doi: 10.1111/j.1432-1033.1982.tb06775.x. [DOI] [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. Phosphatidylinositol breakdown induced by vasopressin and epinephrine in hepatocytes is calcium-dependent. J Biol Chem. 1982 Oct 10;257(19):11323–11331. [PubMed] [Google Scholar]
- Püschel G. P., Jungermann K. Activation of inositol phosphate formation by circulating noradrenaline but not by sympathetic nerve stimulation with a similar increase of glucose release in perfused rat liver. Eur J Biochem. 1988 Jul 15;175(1):187–191. doi: 10.1111/j.1432-1033.1988.tb14182.x. [DOI] [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Stalmans W., Hers H. G. The stimulation of liver phosphorylase b by AMP, fluoride and sulfate. A technical note on the specific determination of the a and b forms of liver glycogen phosphorylase. Eur J Biochem. 1975 Jun;54(2):341–350. doi: 10.1111/j.1432-1033.1975.tb04144.x. [DOI] [PubMed] [Google Scholar]
- Stojanov I., Proctor K. G. Pharmacological evidence for A1 and A2 adenosine receptors in the skin microcirculation. Circ Res. 1989 Jul;65(1):176–184. doi: 10.1161/01.res.65.1.176. [DOI] [PubMed] [Google Scholar]
- Taylor W. M., Storlien L. H., Jenkins A. B. Effect of fructose on phenylephrine-induced glucose output in perfused rat liver. Horm Metab Res. 1988 Aug;20(8):528–530. doi: 10.1055/s-2007-1010876. [DOI] [PubMed] [Google Scholar]
- Thor H., Hartzell P., Orrenius S. Potentiation of oxidative cell injury in hepatocytes which have accumulated Ca2+. J Biol Chem. 1984 May 25;259(10):6612–6615. [PubMed] [Google Scholar]
- Tran-Thi T. A., Häussinger D., Gyufko K., Decker K. Stimulation of prostaglandin release by Ca2+-mobilizing agents from the perfused rat liver. A comparative study on the action of ATP, UTP, phenylephrine, vasopressin and nerve stimulation. Biol Chem Hoppe Seyler. 1988 Jan;369(1):65–68. doi: 10.1515/bchm3.1988.369.1.65. [DOI] [PubMed] [Google Scholar]
- Vandebroeck A., Uyttenhove K., Bollen M., Stalmans W. The hepatic glycogenolysis induced by reversible ischaemia or KCN is exclusively catalysed by phosphorylase a. Biochem J. 1988 Dec 1;256(2):685–688. doi: 10.1042/bj2560685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstapel F., Waebens M., Van Hecke P., Decanniere C., Stalmans W. The cytosolic concentration of phosphate determines the maximal rate of glycogenolysis in perfused rat liver. Biochem J. 1990 Feb 15;266(1):207–212. doi: 10.1042/bj2660207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. H., Kaslow H. R., Bergman R. N. Fructose effect to suppress hepatic glycogen degradation. J Biol Chem. 1987 Aug 25;262(24):11470–11477. [PubMed] [Google Scholar]


