Abstract
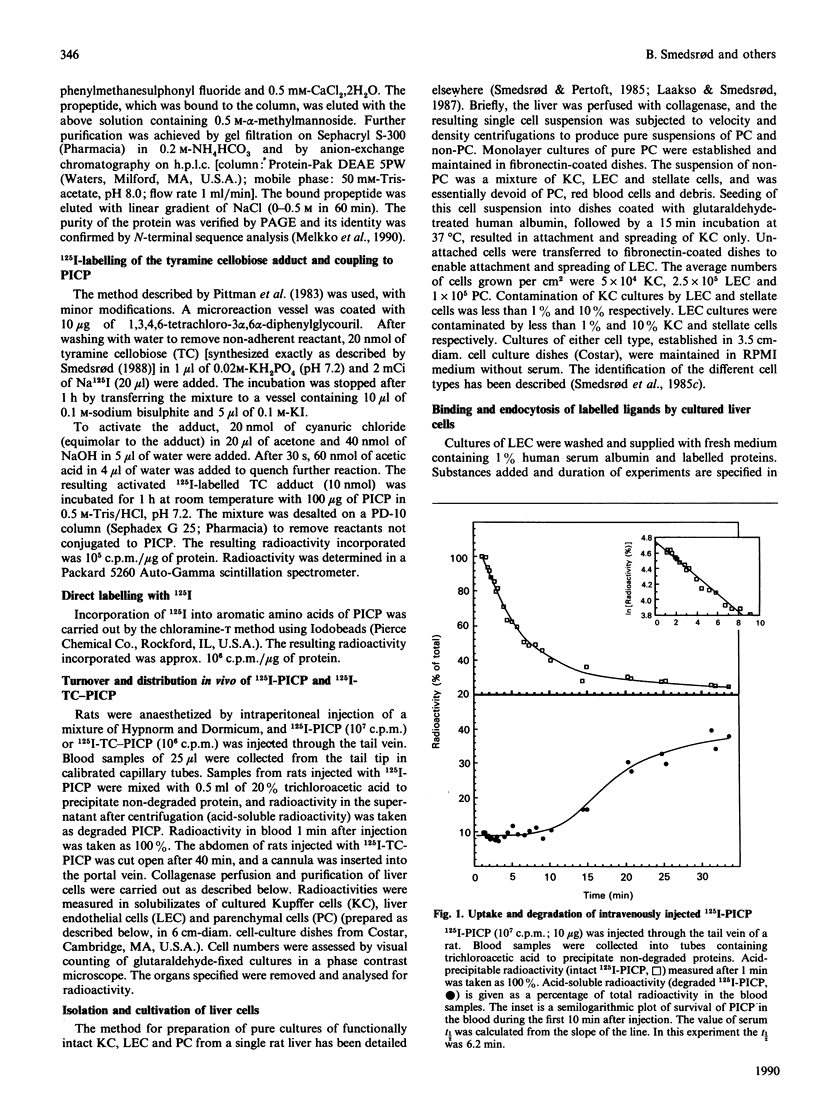
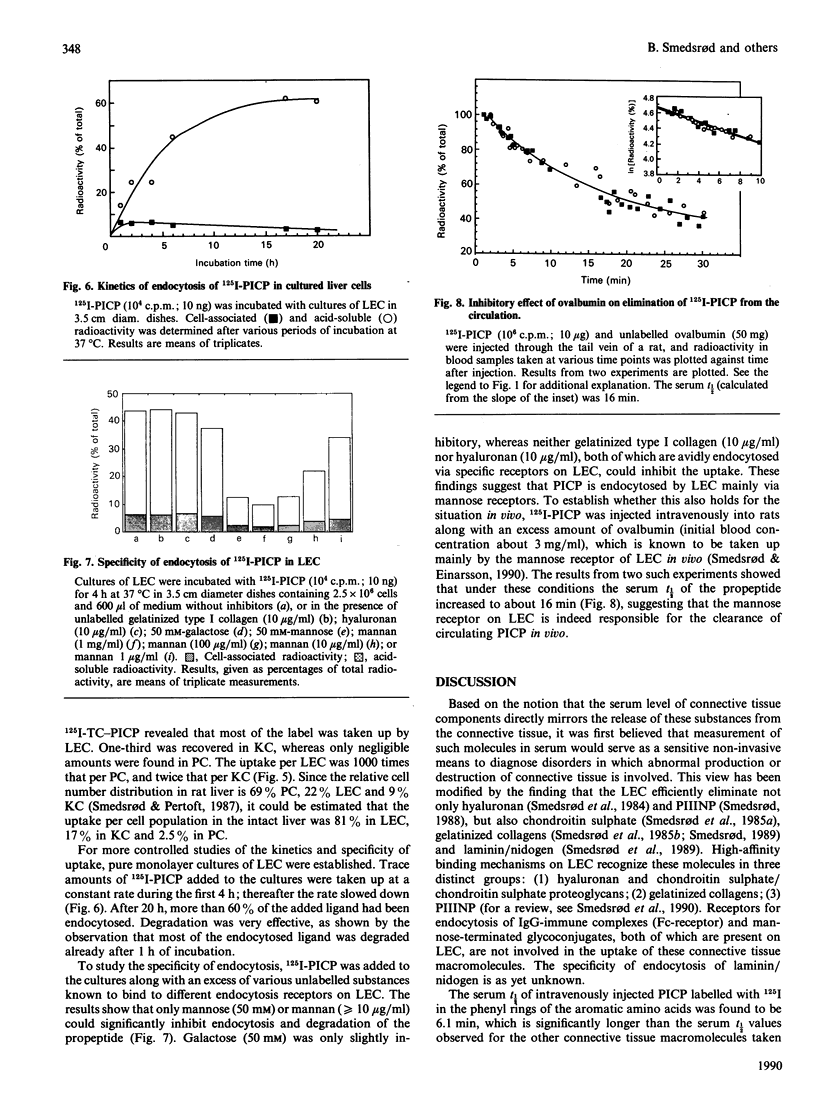
The fate of the circulating C-terminal propeptide of type I procollagen (PICP) was studied. Trace amounts of 125I-PICP administered intravenously to rats disappeared from the blood with an initial t1/2 of 6.1 min. After 45 min the radioactivity was distributed as follows: liver, 36%; blood, 23%; kidneys, 18%; urine, 20%; spleen, 1%; lungs, 2%; heart, 0.4%. To prevent escape of label from the site of uptake, PICP was labelled with 125I-tyramine cellobiose (125I-TC), which is trapped intralysosomally. With this ligand a serum t1/2 of 8.7 min was recorded, and 70% and 20% was traced in the liver and kidneys respectively. The uptake per liver endothelial cell (LEC) was 1000 times that per parenchymal cell and twice that per Kupffer cell. At 1 h and 6 h after addition of 125I-PICP to cultured LEC, 15% and 45% respectively, had been endocytosed. Only ligands for the mannose receptor could compete with PICP for endocytosis. To study whether the same specificity was operative in vivo, 125I-PICP was injected along with an excess of ovalbumin, which is known to be endocytosed by the mannose receptor of LEC. The serum t1/2 was prolonged from 6 to 16 min, signifying that terminal mannose residues are an important signal for clearance of PICP. In conclusion, these studies show that LEC constitute the main site of uptake of circulating PICP. The uptake is mediated by endocytic receptors which recognize terminal mannose residues.
Full text
PDF
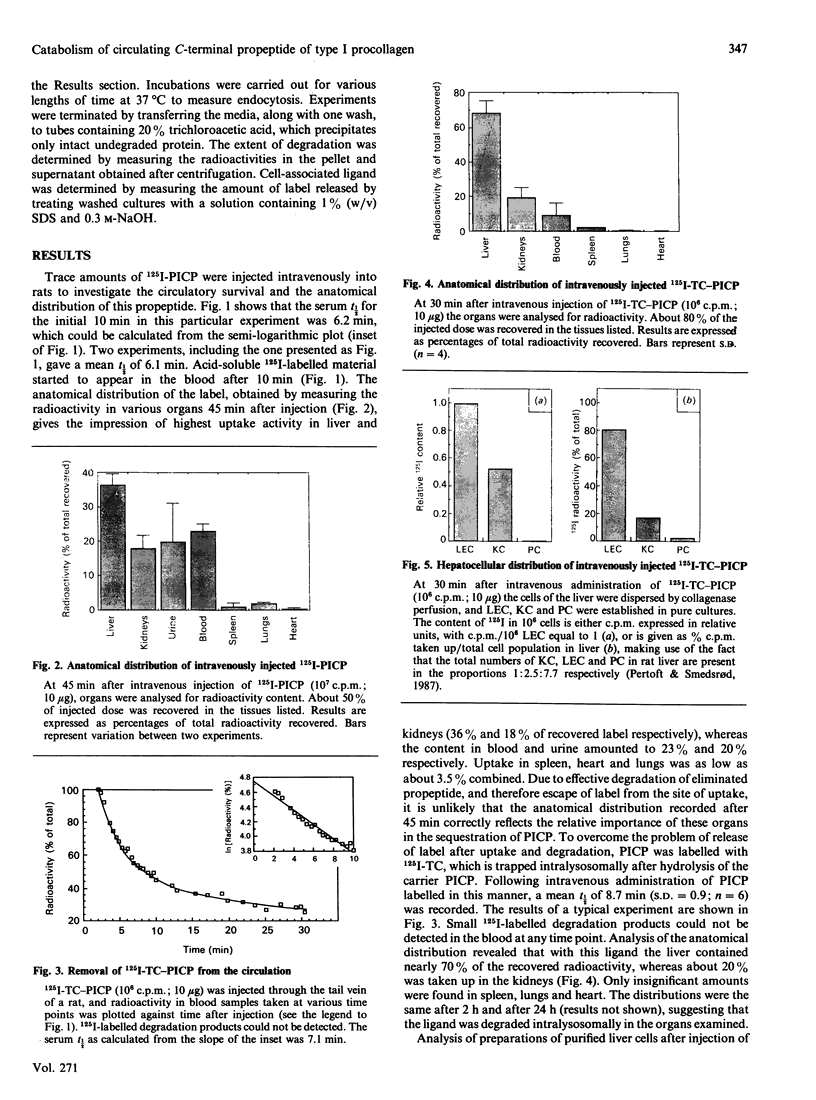


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark C. C., Kefalides N. A. Carbohydrate moieties of procollagen: incorporation of isotopically labeled mannose and glucosamine into propeptides of procollagen secreted by matrix-free chick embryo tendon cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):34–38. doi: 10.1073/pnas.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. C. The distribution and initial characterization of oligosaccharide units on the COOH-terminal propeptide extensions of the pro-alpha 1 and pro-alpha 2 chains of type I procollagen. J Biol Chem. 1979 Nov 10;254(21):10798–10802. [PubMed] [Google Scholar]
- Dahl L. B., Laurent T. C., Smedsrød B. Preparation of biologically intact radioiodinated hyaluronan of high specific radioactivity: coupling of 125I-tyramine-cellobiose to amino groups after partial N-deacetylation. Anal Biochem. 1988 Dec;175(2):397–407. doi: 10.1016/0003-2697(88)90563-5. [DOI] [PubMed] [Google Scholar]
- Melkko J., Niemi S., Risteli L., Risteli J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clin Chem. 1990 Jul;36(7):1328–1332. [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Njieha F. K., Prockop D. J. Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J Biol Chem. 1982 Jul 25;257(14):8442–8448. [PubMed] [Google Scholar]
- Parfitt A. M., Simon L. S., Villanueva A. R., Krane S. M. Procollagen type I carboxy-terminal extension peptide in serum as a marker of collagen biosynthesis in bone. Correlation with Iliac bone formation rates and comparison with total alkaline phosphatase. J Bone Miner Res. 1987 Oct;2(5):427–436. doi: 10.1002/jbmr.5650020510. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Risteli L., Risteli J. Analysis of extracellular matrix proteins in biological fluids. Methods Enzymol. 1987;145:391–411. doi: 10.1016/0076-6879(87)45022-2. [DOI] [PubMed] [Google Scholar]
- Smedsrød B., Einarsson M. Clearance of tissue plasminogen activator by mannose and galactose receptors in the liver. Thromb Haemost. 1990 Feb 19;63(1):60–66. [PubMed] [Google Scholar]
- Smedsrød B., Johansson S., Pertoft H. Studies in vivo and in vitro on the uptake and degradation of soluble collagen alpha 1(I) chains in rat liver endothelial and Kupffer cells. Biochem J. 1985 Jun 1;228(2):415–424. doi: 10.1042/bj2280415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Kjellén L., Pertoft H. Endocytosis and degradation of chondroitin sulphate by liver endothelial cells. Biochem J. 1985 Jul 1;229(1):63–71. doi: 10.1042/bj2290063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Paulsson M., Johansson S. Uptake and degradation in vivo and in vitro of laminin and nidogen by rat liver cells. Biochem J. 1989 Jul 1;261(1):37–42. doi: 10.1042/bj2610037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H., Eggertsen G., Sundström C. Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res. 1985;241(3):639–649. doi: 10.1007/BF00214586. [DOI] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H., Eriksson S., Fraser J. R., Laurent T. C. Studies in vitro on the uptake and degradation of sodium hyaluronate in rat liver endothelial cells. Biochem J. 1984 Nov 1;223(3):617–626. doi: 10.1042/bj2230617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H., Gustafson S., Laurent T. C. Scavenger functions of the liver endothelial cell. Biochem J. 1990 Mar 1;266(2):313–327. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsrød B., Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985 Aug;38(2):213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]


