Abstract
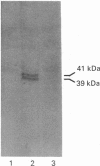
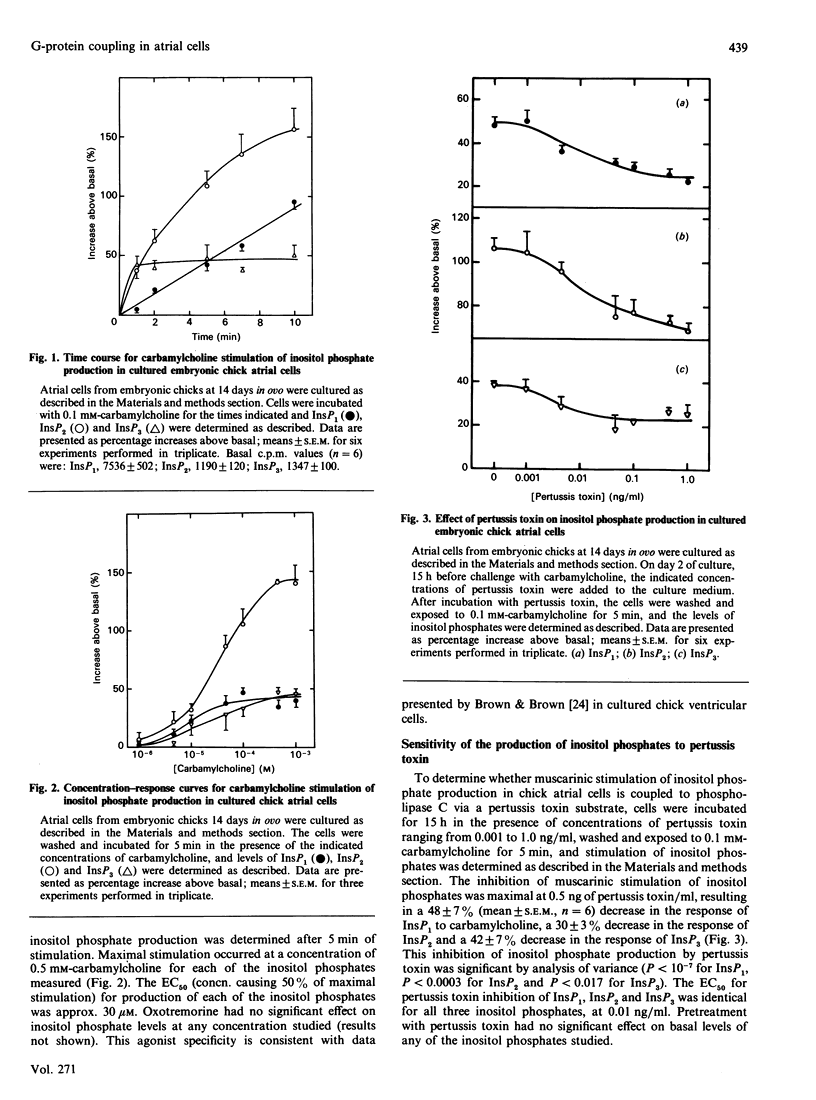
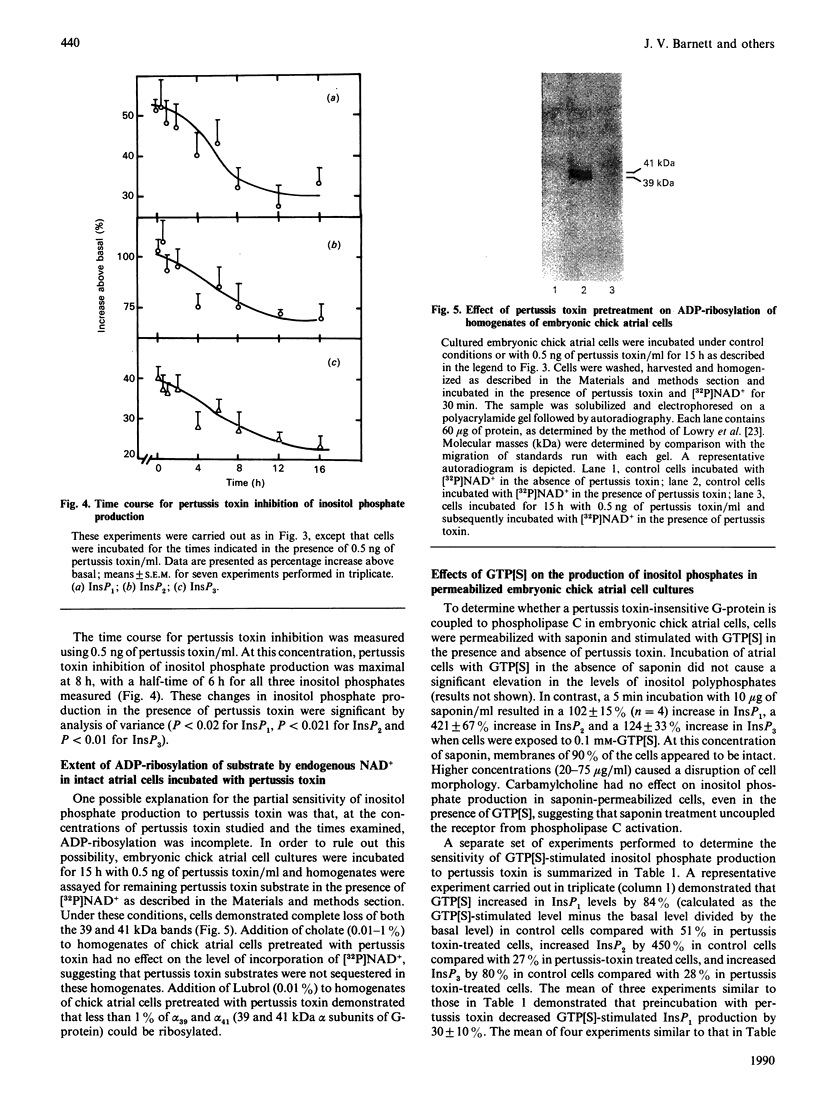
These studies demonstrate a novel mechanism for the coupling of the muscarinic receptor to phospholipase C activity in embryonic chick atrial cells. In monolayer cultures of atrial cells from hearts of embryonic chicks at 14 days in ovo, carbamylcholine stimulated the sequential appearance of InsP3, InsP2 and InsP1 with an EC50 (concn. causing 50% of maximal stimulation) of 30 microM. In the presence of 15 mM-Li, a 5 min exposure to carbamylcholine (0.1 mM) increased InsP3 levels to a maximum of 47 +/- 12% over basal, InsP2 to 108 +/- 13% over basal and InsP1 to 42 +/- 5% over basal. This effect was blocked by 5 microM-atropine. Incubation of these cells with pertussis toxin (15 h; 0.5 ng/ml) inhibited carbamylcholine-stimulated InsP3, InsP2 and InsP1 formation by 42 +/- 7%, 30 +/- 3% and 48 +/- 7% respectively. The IC50 (concn. causing 50% inhibition) for pertussis toxin inhibition of all three inositol phosphates was 0.01 ng/ml, with a half-time of 6 h at 0.5 ng/ml. This partial sensitivity to pertussis toxin was not due to incomplete ADP-ribosylation of the guanine-nucleotide-binding protein (G-protein), since autoradiography of polyacrylamide gels of cell homogenates incubated with [32P]NAD+ in the presence of pertussis toxin demonstrated that incubation of cells with 0.5 ng of pertussis toxin/ml for 15 h resulted in complete ADP-ribosylation of pertussis toxin substrates by endogenous NAD+. In cells permeabilized with saponin (10 micrograms/ml), 0.1 mM-GTP[S] (guanosine 5'-[gamma-thio]triphosphate) stimulated InsP1 by 102 +/- 15% (mean +/- S.E.M., n = 4), InsP2 by 421 +/- 67% and InsP3 by 124 +/- 33% above basal. Incubation of cells for 15 h with 0.5 ng of pertussis toxin/ml decreased GTP[S]-stimulated InsP1 production in saponin-treated cells by 30 +/- 10% (n = 3), InsP2 production by 45 +/- 7% (n = 4) and InsP3 production by 49 +/- 6% (n = 4). These data demonstrate that in embryonic chick atrial cells at least two independent G-proteins, a pertussis toxin-sensitive G-protein and a pertussis toxin-insensitive G-protein, play a role in coupling muscarinic agonist binding to phospholipase C activation and to inositol phosphate production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Brown S. L. Agonists differentiate muscarinic receptors that inhibit cyclic AMP formation from those that stimulate phosphoinositide metabolism. J Biol Chem. 1984 Mar 25;259(6):3777–3781. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- DeHann R. L. Regulation of spontaneous activity and growth of embryonic chick heart cells in tissue culture. Dev Biol. 1967 Sep;16(3):216–249. doi: 10.1016/0012-1606(67)90025-5. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Fukui T., Lutz R. J., Lowenstein J. M. Purification of a phospholipase C from rat liver cytosol that acts on phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate. J Biol Chem. 1988 Nov 25;263(33):17730–17737. [PubMed] [Google Scholar]
- Galper J. B., Smith T. W. Agonist and guanine nucleotide modulation of muscarinic cholinergic receptors in cultured heart cells. J Biol Chem. 1980 Oct 25;255(20):9571–9579. [PubMed] [Google Scholar]
- Hepler J. R., Harden T. K. Guanine nucleotide-dependent pertussis-toxin-insensitive stimulation of inositol phosphate formation by carbachol in a membrane preparation from human astrocytoma cells. Biochem J. 1986 Oct 1;239(1):141–146. doi: 10.1042/bj2390141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Connelly P. A., Sisk R. B., Pobiner B. F., Hewlett E. L., Garrison J. C. Pertussis toxin or phorbol 12-myristate 13-acetate can distinguish between epidermal growth factor- and angiotensin-stimulated signals in hepatocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2032–2036. doi: 10.1073/pnas.83.7.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Garrison J. C. Epidermal growth factor and angiotensin II stimulate formation of inositol 1,4,5- and inositol 1,3,4-trisphosphate in hepatocytes. Differential inhibition by pertussis toxin and phorbol 12-myristate 13-acetate. J Biol Chem. 1987 Dec 25;262(36):17285–17293. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Jones L. G., Goldstein D., Brown J. H. Guanine nucleotide-dependent inositol trisphosphate formation in chick heart cells. Circ Res. 1988 Feb;62(2):299–305. doi: 10.1161/01.res.62.2.299. [DOI] [PubMed] [Google Scholar]
- Josephson I., Sperelakis N. On the ionic mechanism underlying adrenergic-cholinergic antagonism in ventricular muscle. J Gen Physiol. 1982 Jan;79(1):69–86. doi: 10.1085/jgp.79.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liang B. T., Galper J. B. Differential sensitivity of alpha o and alpha i to ADP-ribosylation by pertussis toxin in the intact cultured embryonic chick ventricular myocyte. Relationship to the role of G proteins in the coupling of muscarinic cholinergic receptors to inhibition of adenylate cyclase activity. Biochem Pharmacol. 1988 Dec 1;37(23):4549–4555. doi: 10.1016/0006-2952(88)90671-5. [DOI] [PubMed] [Google Scholar]
- Liang B. T., Hellmich M. R., Neer E. J., Galper J. B. Development of muscarinic cholinergic inhibition of adenylate cyclase in embryonic chick heart. Its relationship to changes in the inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1986 Jul 5;261(19):9011–9021. [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Ramachandran J., Capon D. J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988 Aug 4;334(6181):434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Ryu S. H., Suh P. G., Cho K. S., Lee K. Y., Rhee S. G. Bovine brain cytosol contains three immunologically distinct forms of inositolphospholipid-specific phospholipase C. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6649–6653. doi: 10.1073/pnas.84.19.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Steinberg S. F., Chow Y. K., Robinson R. B., Bilezikian J. P. A pertussis toxin substrate regulates alpha 1-adrenergic dependent phosphatidylinositol hydrolysis in cultured rat myocytes. Endocrinology. 1987 May;120(5):1889–1895. doi: 10.1210/endo-120-5-1889. [DOI] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Moon K. H., Suh H. W., Rhee S. G. Cloning and sequence of multiple forms of phospholipase C. Cell. 1988 Jul 15;54(2):161–169. doi: 10.1016/0092-8674(88)90548-x. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuli I., Snyderman R. Extensive hydrolysis of N-formyl-L-methionyl-L-leucyl-L-[3H] phenylalanine by human polymorphonuclear leukocytes. A potential mechanism for modulation of the chemoattractant signal. J Biol Chem. 1986 Apr 15;261(11):4902–4908. [PubMed] [Google Scholar]