Abstract
Acute kidney injury (AKI) frequently complicates the repair of Stanford type A aortic dissection (TAAD). This systematic review, meta-analysis, and meta-regression analysis aimed to elucidate the prognostic impact of AKI in these patients. A literature search in PubMed, EMBASE, and Google Scholar identified relevant studies on the predictors and outcomes of AKI following TAAD repair. The primary endpoint was 30-day mortality; secondary endpoints included stroke, dialysis/continuous renal replacement therapy (CRRT), and other complications. Random-effects meta-analyses were used, with significance set at P < 0.05. Twenty-one studies (10 396 patients) were analyzed. AKI was associated with higher risks of 30-day mortality (risk ratio = 3.98), stroke (risk ratio = 2.05), dialysis/CRRT (risk ratio = 32.91), cardiovascular (risk ratio = 2.85) and respiratory complications (risk ratio = 2.13), sepsis (risk ratio = 4.92), and re-exploration for bleeding (risk ratio = 2.46). No significant differences were noted in sternal wound infection, tracheostomy, paraplegia, or hepatic failure. AKI significantly increases mortality, morbidity, hospital, and ICU stay duration in TAAD repair patients.
Keywords: acute kidney injury, aortic dissection, cardiology, meta-analysis, mortality, prevention
Introduction
Type A aortic dissection (TAAD) is defined as separation of the aortic wall involving the ascending aorta, irrespective of the site of the intimal tear (Stanford Classification) [1]. Acute TAAD is a common cardiac emergency, and despite advances in diagnostic modalities, intraoperative techniques, and perioperative care, TAAD repair is associated with approximately 12% in-hospital mortality [2,3]. Postoperative acute kidney injury (AKI) after repair of TAAD is an early and common complication with an incidence ranging from 20.2 to 66.7%. It ranges from mild renal dysfunction to renal failure, requiring renal replacement therapy (RRT) [4–6]. It is associated with adverse outcomes such as increased in-hospital morbidity and mortality, longer hospital stay, and reduced long-term survival [7–9]. The causative mechanisms of postoperative AKI are not always evident and are multifactorial. There is no known pharmacological treatment to prevent or treat AKI, and many patients require continuous renal replacement therapy (CRRT), which is associated with increased mortality [10,11]. Although several studies have explored the prognostic significance of postoperative AKI, there remains a lack of sufficient investigation into its impact on the occurrence of 30-day postoperative mortality and morbidities, including cardiovascular, respiratory, and other complications. Some studies have found only severe AKI is associated with increased 30-day postoperative mortality, whereas others have demonstrated that AKI, regardless of severity, increases mortality [4,11–13].
Recently, several systematic reviews and meta-analyses have attempted to establish the relative significance of risk factors and adverse outcomes [12–14]. Kidney disease was defined in several ways, including the Kidney Disease: Improving Global Outcomes (KDIGO) definition and staging system, the Risk, Injury, Failure, Loss of kidney function (RIFLE) criteria, and the Acute Kidney Injury Network (AKIN) classification [15–17]. Owing to the inter-definition variability and evaluation of different outcomes in each study, there are inconsistencies in establishing the relative significance of AKI on prognosis after repair of TAAD. Thus, our systematic review and meta-analysis aimed to understand the prognostic significance of the development of AKI in patients who have undergone repair of TAAD, by understanding its effects on the risk of mortality and several other outcomes.
Materials and methodology
We adhered to the guidelines established by the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) for our systematic review and meta-analysis [18]. We registered our protocol in the International PROSPERO Registry for Systematic Reviews and Meta-Analyses (CRD42023486286). Our study has been reported in line with the AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines [19].
Data sources and search strategy
Two authors independently conducted an extensive literature search to identify relevant studies on the predictors and outcomes of AKI following TAAD repair.
The authors meticulously searched for relevant articles using various databases, including PubMed, Google Scholar, EMBASE, Scopus, and the Cochrane Library. To ensure the comprehensiveness of the review, references from retrieved studies, prior meta-analyses, and review articles were examined. Additionally, they meticulously scanned citations on Google Scholar to identify any pertinent literature. In instances where deemed appropriate, attempts were made to contact the authors via email to request additional data; however, no responses were received.
The search strategy employed a string of keywords and related Medical Subject Headings terms, encompassing ‘Acute Kidney Injury’, ‘AKI’, ‘Type A Aortic Dissection’, ‘Predictors’, and ‘Outcomes’.
Eligibility criteria
Utilizing the Population, Exposure, Control, and Outcomes (PECO) framework for systematic reviews and meta-analyses, we assessed the inclusion criteria. In our study, ‘P’ represented patients who had undergone repair for TAAD, ‘E’ referred to patients who developed AKI, ‘C’ denoted patients who did not develop AKI, and ‘O’ encompassed various outcomes as subsequently discussed. The exclusion criteria included non-adult populations, interventions other than repair, and studies lacking relevant AKI outcome data. Additionally, case reports, review articles, and abstracts without full text were excluded from the analysis.
Endpoints
This study aimed to evaluate the 30-day mortality rate (primary endpoint) and various secondary endpoints, including stroke incidence, need for dialysis/CRRT, cardiovascular and respiratory complications, sepsis, re-exploration for bleeding, sternal wound infection, tracheostomy requirement, paraplegia, hepatic failure, and length of hospital and ICU stay.
Study selection and data extraction
After retrieving all relevant studies through a thorough literature search, they were imported into EndNote X9 (Clarivate Analytics) for removal of duplicates. Two authors independently reviewed the abstracts and those meeting the eligibility criteria were included after a full-text assessment. Disagreements were resolved through discussion and consensus between the two authors. A pre-piloted Microsoft Excel sheet was used to facilitate data extraction.
Quality assessment of included studies
The current meta-analysis included observational studies, and the quality of the included studies was evaluated using the Newcastle–Ottawa Scale. This was accomplished by two researchers whose findings were compared to eliminate inconsistencies. The Newcastle–Ottawa scale focuses on the selection criteria of studies, comparability between groups, exposure, and outcomes [20].
Data synthesis
Data synthesis for this meta-analysis was performed using RevMan version 5.4, developed by the Nordic Cochrane Center in Copenhagen, Denmark. Pooled analysis of studies was represented in the form of forest plots, with statistical significance set at P < 0.05, within a 95% confidence interval (CI). All analyses were performed using the Mantel–Haenszel random-effects model. The effect measure was the risk ratio for dichotomous variables and mean difference for continuous variables.
To evaluate the degree of heterogeneity arising from differences in methodologies, study designs, and populations, the Higgins I2 metric was used [21]. A value of less than 50% indicated low heterogeneity, exceeding 50% indicated moderate heterogeneity, and a value greater than 75% indicated significant heterogeneity. To gauge the robustness of the findings, sensitivity analysis was performed by systematically excluding one study at a time. Meta-regression analysis was performed to explore various covariates (baseline mean age, baseline BMI, baseline kidney function, and gender) that may have led to potential heterogeneity among the studies. To assess publication bias, a visual analysis of the funnel plots was conducted.
Results
Literature search results
Using the predefined search strategy, 1147 references were identified through four electronic database searches. After removing 298 duplicates, 849 articles were screened based on their titles and abstracts, resulting in the further exclusion of 788 articles. The remaining 61 articles underwent full-text review, leading to the exclusion of 40 studies due to irrelevant populations, lack of relevant outcomes, irrelevant study design, and unavailability of full text or full text not in the English language. Consequently, 21 studies that met the eligibility criteria were included in our meta-analysis [4,6,14,20,22–38]. A comprehensive overview of the literature search and study selection process is shown in Fig. 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram (2020) for systematic reviews and meta-analyses.
Study characteristics and risk of bias assessment
Of the included studies, 19 were retrospective [4,6,22,23,25–27,29–33,35,37–42], while two followed a prospective design [24,28]. Fifteen studies originated in China [6,22,23,26–33,35,38–40], two each from Japan [4,37] and the USA [24,25], and one each from Italy [42] and Iceland [41]. The collective cohort comprised 10 396 patients undergoing aortic arch repair, with 3928 in the AKI group and 6468 in the non-AKI group, respectively. The mean ages across the studies ranged from 33 to 68 years, with the proportion of male participants varying from 39.9% to 100%. Additionally, 15 of the included studies used the KDIGO criteria to diagnose AKI. The most common comorbidities reported were diabetes and hypertension. The baseline characteristics of all studies are summarized in Table 1.
Table 1.
Baseline characteristics of patients among the included studies
| Author (year, country) | Study type | Total no. of participants, n | Sample size, n (AKI/Non-AKI) | Diagnosis of patients | Diagnostic criteria of AKI | AKI incidence, n (%) | Mean age, years (AKI/non-AKI) | Mean BMI, kg/m2 (AKI/non-AKI) | Male, % (AKI/non-AKI) | Comorbidities, % (AKI/non-AKI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Arnaoutakis et al. (2023, USA) [25] | Retrospective, Single center | 3307 | 761/2546 | Acute TAAD | RIFLE | 761 (23) | 61.4/60.2 | 28.6/27.6 | 71.2/64.3 | DM (15.7/11.2) HTN (84.1/74.6) Smoking (31.3/28.9) COPD (9.8/7.9) PAD (5.1/2.7) Previous cardiac surgery (4.4/6.1) Previous coronary disease (3.9/3.6) CKD (16.4/5.1) Marfan syndrome (2/2.8) Bicuspid aortic valve (2/3.7) |
| Chen et al. (2023, China) [27] | Retrospective, Single center | 382 | 153/229 | TAAD | KDIGO | 153 (40.1) | 50.6/48.9/ | 25.8/25.9 | 68.6/75.1 | DM (9.2/3.1) HTN (81.7/83) Hyperlipidemia (13.1/7.9) COPD (6.4/2.6) Previous cardiac surgery (11.1/23.4) Previous coronary disease (20.9/13.1) Cerebrovascular disease (9.2/7) Cardiac tamponade (11.8/9.2) |
| Brown et al. (2022, USA) [24] | Prospective, Single center | 601 | 85/516 | Acute TAAD | RIFLE | 85 (14.1) | 62/61.3 | 30.1/30 | 40/39.9 | DM (14.1/9.9) HTN (77.7/75.8) COPD (17.7/13.8) PAD (42.4/33.1) Previous cardiac surgery (8.2/10.5) Previous coronary disease (17.7/13.8) Aortic valve insufficiency (49.4/40.7) Cardiac tamponade (30/37.7) |
| Fang et al. (2023, China) [28] | Prospective, Single center | 621 | 314/307 | TAAD | KDIGO | 314 (50.6) | 54.2/51.4 | NR | 82.8/78.5 | DM (2.5/2.6) HTN (63.4/55.6) Hyperlipidemia (2.2/1.9) Previous coronary disease (3.5/2.9) CKD (1.6/1.6) |
| Ko et al. (2015, Japan) [37] | Retrospective, Single center | 375 | 165/210 | Acute TAAD | KDIGO | 165 (44) | 65.5/67 | 24/22.6 | 60/46 | DM (5/8) HTN (81/81) Smoking (29/24) PAD (0.6/1.4) Previous cardiac surgery (7/5) Previous coronary disease (4/4) Cerebrovascular disease (9/9) Cardiac tamponade (14/13) |
| Li et al. (2021, China) [26] | Retrospective, Single center | 421 | 193/228 | Acute TAAD | KDIGO | 228 (54.2) | 49/46.1 | 25.9/26.6 | 76.6/78.5 | NR |
| Guan et al. (2023, China) [29] | Retrospective, Single center | 172 | 88/84 | Acute TAAD | KDIGO | 88 (51.2) | 48.8/47.9 | 28.1/25 | 70.5/73.8 | DM (4.5/2.4) HTN (76.1/76.2) Smoking (54.5/38.1) Marfan syndrome (8/7.1) Cerebrovascular disease (4.5/7.1) |
| Li et al. (2020, China) [38] | Retrospective, Single center | 335 | 241/94 | TAAD | KDIGO | 241 (71.94) | 47.9/46.6 | 25.3/23.5 | 83.4/73.4 | DM (2.9/3.1) HTN (57.6/44.6) Hyperlipidemia (7.8/1.1) COPD (6.2/2.1) Previous cardiac surgery (3.3/9.5) Previous coronary disease (31.9/25.5) CKD (9.1/1.1) Marfan syndrome (3.3/3.1) |
| Liu T et al. (2021, China) [30] | Retrospective, Single center | 115 | 61/54 | Acute DeBakey Type I AD | KDIGO | 61 (53) | 48.7/46.8 | 27.2/25 | 72.1/77.8 | DM (9.8/1.9) HTN (82/77.8) Smoking (55.7/40.7) Alcohol consumption (19.7/20.4) Previous coronary disease (4.9/5.6) Cerebrovascular disease (3.3/7.4) |
| Wang-1 et al. (2020, China) [22] | Retrospective, Single center | 214 | 114/100 | Acute TAAD | KDIGO | 114 (53.3) | 68/66 | 24.2/24.2 | 56.1/59 | DM (2.6/1) HTN (73.7/74) Previous cardiac surgery (7.9/5) Previous coronary disease (6.1/8) Cerebrovascular disease (6.1/5) Pericardial effusion (4.4/4) |
| Wang-2 et al. (2020, China) [23] | Retrospective, Single center | 712 | 359/353 | Acute TAAD | KDIGO | 359 (40.4) | 53.7/51.3 | 25.9/24.7 | 73.3/73.4 | DM (1.9/1.4) HTN (74.7/63.5) Previous cardiac surgery (5.8/4.2) Previous coronary disease (3.9/3.7) Cerebrovascular disease (3.6/3.1) Pericardial effusion (2.5/5.4) |
| Zhang et al. (2022, China) [35] | Retrospective, Single center | 224 | 53/171 | Acute TAAD | KDIGO | (23.66) | 55.2/51.8 | 26.6/26.7 | 71.7/78.4 | DM (0/2.9) HTN (79.2/67.3) Smoking (54.7/46.2) Alcohol consumption (24.5/22.8) Previous cardiac surgery (1.9/2.3) Cerebrovascular disease (7.5/6.4) |
| Sasabuchi et al. (2016, Japan) [4] | Retrospective, Single center | 403 | 181/222 | Acute TAAD | KDIGO | 181 (44.9) | 63/66 | NR | 59.7/44.6 | DM (7.7/5.9) HTN (75.7/68) Hyperlipidemia (16.6/14) Smoking (40.3/29.7) COPD (1.7/3.2) PAD (1.7/0.5) Previous cardiac surgery (0.6/1.8) Previous coronary disease (6.6/2.7) Marfan syndrome (1.7/3.6) Bicuspid aortic valve (1.1/0.9) Cerebrovascular disease (9.9/7.7) |
| Qiu et al. (2015, China) [32] | Retrospective, Single center | 155 | 56/99 | Acute TAAD | AKIN | 56 (36.13) | 56.1/56.1 | NR | 82.1/71 | DM (8.9/8) HTN (78.5/67) |
| Sansone et al. (2015, Italy) [42] | Prospective, Single center | 37 | 14/23 | Acute TAAD | NR | (37.8) | 65/65 | NR | 92.8/78.2 | Cardiac tamponade (50/60.8) |
| Xu et al. (2023, China) [33] | Retrospective, Single center | 624 | 235/389 | Acute TAAD | KDIGO | 235 (37.7) | 50.9/47 | 26/26.1 | 67.7/77.4 | DM (5.5/2.1) HTN (59.6/49.1) Smoking (33.6/33.4) COPD (0.9/0.3) Previous cardiac surgery (2.1/1.5) Previous coronary disease (7.2/5.9) Cerebrovascular disease (1.7/2.3) |
| Yang et al. (2022, China) [39] | Retrospective, Single center | 398 | 268/130 | TAAD | KDIGO | 268 (67.3) | 49/47.2 | 25.8/24.3 | 82.1/75.4 | DM (1.9/0) HTN (76.1/70) COPD (0.7/2.3) Previous cardiac surgery (3.7/3.1) Previous coronary disease (1.9/1.5) Marfan syndrome (1.1/3.1) |
| Zhao et al. (2015, China) [6] | Retrospective, Single center | 108 | 72/36 | Acute TAAD | AKIN | 72 (66.7) | 44/43 | 29.6/29.7 | 94.4/100 | DM (2.8/5.6) HTN (80.6/83.3) Smoking (81.9/27.8) COPD (1.4/0) Previous cardiac surgery (51.4/33.3) Previous coronary disease (6.9/5.6) Cerebrovascular disease (1.4/0) |
| Zong et al. (2020, China) [40] | Retrospective, Single center | 121 | 51/p70 | Acute TAAD | KDIGO | 51 (42.1) | 35/33 | 26.3/24.7 | 78.4/84.3 | DM (2/0) HTN (70.6/35.7) Previous cardiac surgery (5.9/4.3) Previous coronary disease (5.9/0) Cerebrovascular disease (2/0) Pericardial effusion (0/1.4) |
| Liu Y et al. (2020, China) [31] | Retrospective, Single center | 130 | 82/48 | Acute TAAD | KDIGO | 82 (63.08) | 54.7/53.1 | NR | 80.3/68.7 | DM (7.8/2.1) HTN (66.6/45.8) COPD (5.8/4.1) Previous coronary disease (3.9/2.1) Cerebrovascular disease (5.8/6.2) |
| Helgason et al. 2021 [41] | Retrospective, multicenter | 941 | 382/5559/941 | Acute TAAD | RIFLE | 382(40.6) | 63.1/60.3 | 27.6/ 26.1 | 70.2/64.9 | DM (2.6/1.8) HTN (58.6/47.8) CAD (5.3/2.9) Smoking (35.1/34.5) Cerebrovascular disease (5.3/2.9) Bicuspid valve (6.1/6.3) |
AD, aortic dissection; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; KDIGO, Kidney Disease Improving Global Outcome; PAD, peripheral artery disease; RIFLE, Risk, Injury, Failure, Loss and End stage kidney disease; TAAD, Stanford type A aortic dissection.
All studies underwent high-quality assessment, scoring eight or higher on the Newcastle-Ottawa Scale (Table 2). Funnel plots for nearly all outcomes were symmetrical, demonstrating a minimal publication bias (Supplementary Fig. 1, Supplemental digital content 1, http://links.lww.com/CAEN/A61).
Table 2.
Risk of bias summary of included observational studies using Newcastle-Ottawa Scale
| Study | Representative of exposed cohorts | Selection of nonexposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at the start of study | Comparability of cohort on the basis of design or analysis | Assessment of outcome | Was follow-up long enough for the outcomes to occur | Adequacy of follow-up of cohorts | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Li 2021 et al. [26] | * | * | * | * | * | * | * | * | 8 |
| Arnaoutakis et al. [25] | * | * | * | * | * | * | * | * | 8 |
| Brown et al. [24] | * | * | * | * | ** | * | * | * | 9 |
| Chen et al. [27] | * | * | * | * | ** | * | * | * | 9 |
| Fang et al. [28] | * | * | * | * | * | * | * | * | 8 |
| Guan et al. [29] | * | * | * | * | * | * | * | * | 8 |
| Helgason et al. [41] | * | * | * | * | * | * | * | * | 8 |
| Ko et al. [37] | * | * | * | * | * | * | * | * | 8 |
| Li 2020 et al. [38] | * | * | * | * | * | * | * | * | 8 |
| Liu 2020 et al. [31] | * | * | * | * | ** | * | * | * | 9 |
| Liu 2021 Y et al. [30] | * | * | * | * | ** | * | * | * | 9 |
| Qiu et al. [32] | * | * | * | * | * | * | * | * | 8 |
| Sansone et al. [42] | * | * | * | * | ** | * | * | 8 | |
| Sasabuci et al. [4] | * | * | * | * | * | * | * | * | 8 |
| Wang Aug et al. [22] | * | * | * | * | ** | * | * | * | 9 |
| Wang July et al. [23] | * | * | * | * | * | * | * | * | 8 |
| Xu et al. [33] | * | * | * | * | * | * | * | * | 8 |
| Yang et al. [39] | * | * | * | * | ** | * | * | * | 9 |
| Zhang et al. [35] | * | * | * | * | ** | * | * | * | 9 |
| Zhao et al. [6] | * | * | * | * | ** | * | * | * | 9 |
| Zong et al. [40] | * | * | * | * | ** | * | * | * | 9 |
*Score>7 was considered as a good quality study with low risk of bias.
Endpoints
This study showed that 37.8% of patients undergoing TAAD repair develop AKI, highlighting its potential impact on patient outcomes.
Primary endpoint of 30-day mortality
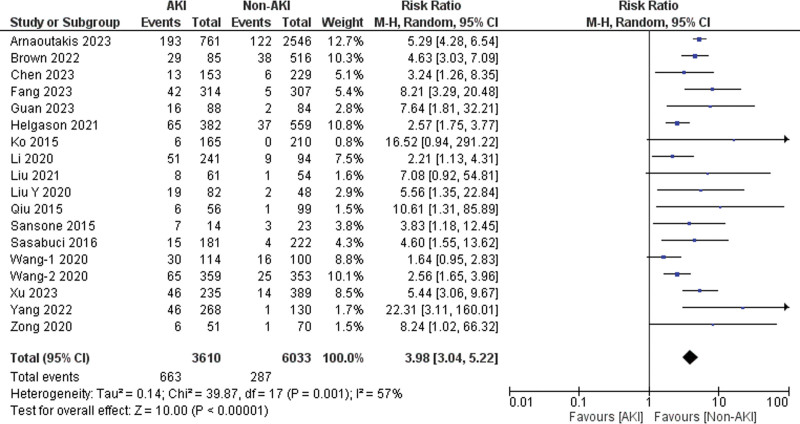
Eighteen of the 21 included studies reported data on 30-day mortality [4,22–25,27–33,37–42]. Patients with AKI exhibited a significantly higher risk of 30-day mortality as compared to those without AKI (risk ratio = 3.98, 95% CI: 3.04–5.22, P < 0.001), with 57% heterogeneity detected across studies (I2 = 57%, P for heterogeneity = 0.001) (Fig. 2). The sensitivity analysis showed that ‘Wang-1 et al. (2020)’ [22] might be the source of heterogeneity. After excluding this study, the heterogeneity between the studies was reduced (I2 = 44%).
Fig. 2.
Forest plot for the outcome of 30-day mortality.
Secondary endpoints
Stroke
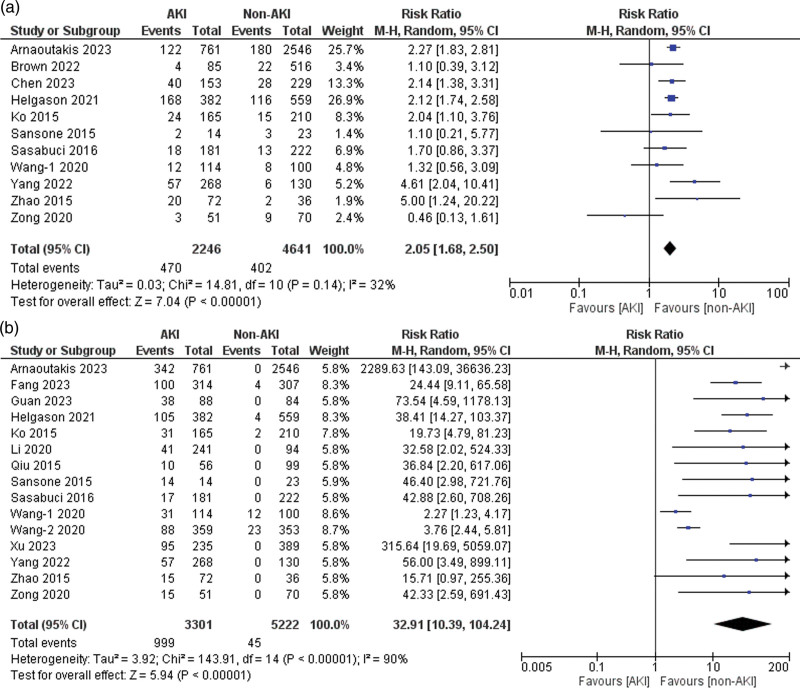
Eleven of the 21 included studies reported data on stroke [4,6,22,24,25,27,37,39–42]. Patients with AKI had a significantly higher risk of experiencing stroke than those without AKI (risk ratio = 2.05, 95% CI: 1.68–2.50, P < 0.001), with 32% heterogeneity detected across studies (I2 = 32%, P for heterogeneity = 0.14) (Fig. 3a).
Fig. 3.
Forest plots for the outcome of (a) stroke and (b) the need for dialysis/CRRT. CRRT, continuous renal replacement therapy.
Need for dialysis/continuous renal replacement therapy
Fifteen of the 21 included studies reported data on patients who required dialysis or CRRT [4,6,22–25,29,32,33,37–42]. A significantly increased risk of dialysis/CRRT was observed in the AKI group compared to that in the non-AKI group (risk ratio = 32.91, 95% CI: 10.39–104.24, P < 0.001), with 90% heterogeneity detected across studies (I2 = 90%, P for heterogeneity < 0.001) (Fig. 3b). Upon performing sensitivity analysis, no significant reduction in heterogeneity was observed.
Cardiovascular complications
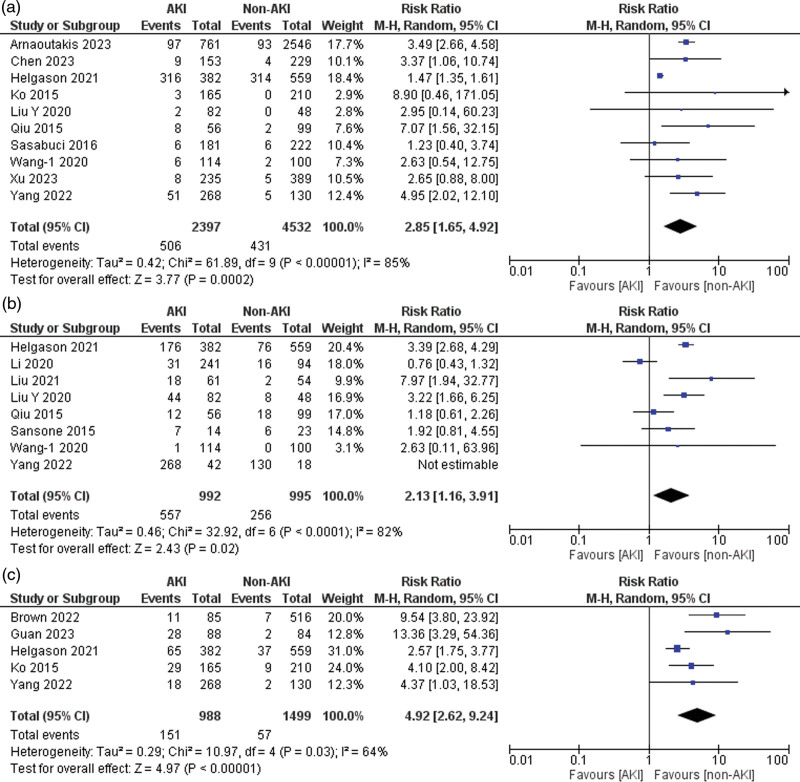
Of the 21 included studies, 10 reported data on postoperative cardiovascular complications [4,22,25,27,31–33,37,39,41]. Patients with AKI had a significantly higher risk of cardiovascular complications than those in the non-AKI group (risk ratio = 2.85, 95% CI: 1.65–4.92, P < 0.001), with 85% heterogeneity detected across studies (I2 = 85%, P for heterogeneity < 0.001) (Fig. 4a). Sensitivity analysis showed ‘Helgason et al. (2021)’ [41] might be the source of heterogeneity. After excluding this study, no heterogeneity was observed (I2 = 0%, P for heterogeneity > 0.1).
Fig. 4.
Forest plots for the outcomes of (a) cardiovascular complications, (b) respiratory complications, and (c) sepsis.
Respiratory complications
Eight of the 21 included studies reported data on postoperative respiratory complications (pneumonia and respiratory failure) [23,30–32,38,39,41,42]. Patients with AKI demonstrated a significantly higher risk of respiratory complications than those without AKI (risk ratio = 2.13, 95% CI: 1.16–3.91, P = 0.02), with 82% heterogeneity detected across studies (I2 = 82%, P for heterogeneity < 0.001) (Fig. 4b). A sensitivity analysis showed that ‘Li et al. (2020)’ [38] might be a source of heterogeneity. After excluding this study, the heterogeneity between the studies was reduced (I2 = 58%, P for heterogeneity > 0.1).
Sepsis
Five of the 21 included studies reported sepsis data [24,29,37,39,41]. A significantly higher risk of sepsis was observed in the AKI group than in the non-AKI group (risk ratio = 4.92, 95% CI: 2.62–9.24, P < 0.001), with 64% heterogeneity detected across studies (I2 = 64%, P for heterogeneity = 0.03) (Fig. 4c). Sensitivity analysis showed ‘Helgason et al. (2021)’ [41] might be the source of heterogeneity. After excluding this study, the heterogeneity between the studies was reduced (I2 = 14%, P for heterogeneity > 0.1).
Reexploration for bleeding
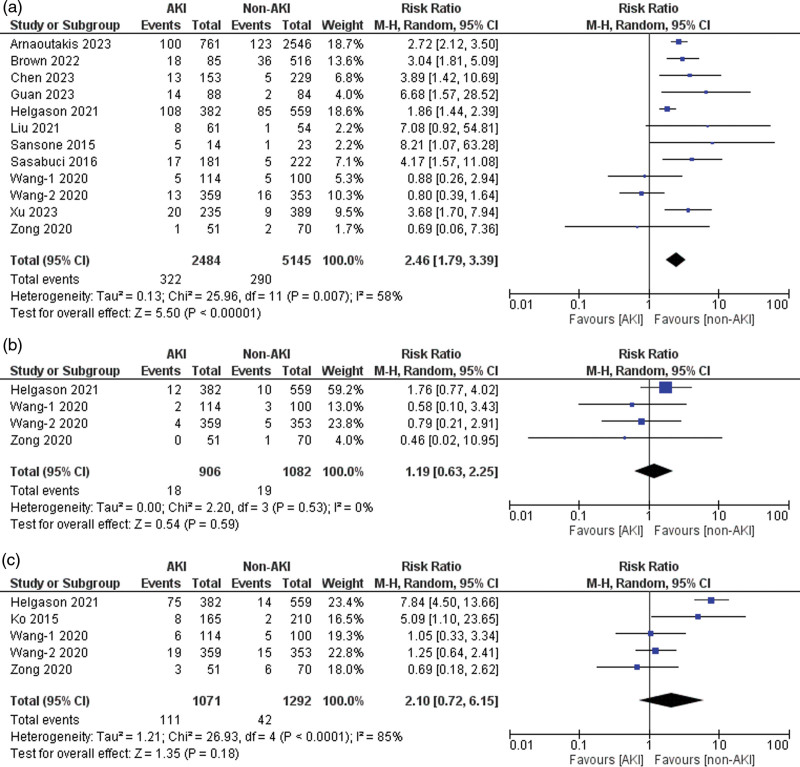
Twelve of the 21 included studies reported data for reexploration for bleeding [4,22–25,27,29,30,33,40–42]. Patients with AKI had a significantly higher risk of undergoing re-exploration for bleeding than those without AKI (risk ratio = 2.46, 95% CI: 1.79–3.39, P < 0.001), with 58% heterogeneity detected across studies (I2 = 58%, P for heterogeneity = 0.007) (Fig. 5a). The sensitivity analysis showed that ‘Wang-2 et al. (2020)’ [23] might be the source of heterogeneity. After excluding this study, the heterogeneity between the studies was reduced (I2 = 42%, P for heterogeneity > 0.1).
Fig. 5.
Forest plots for the outcomes of (a) reexploration for bleeding, (b) sternal wound infection, and (c) the need for tracheostomy.
Sternal wound infection
Four of the 21 included studies reported data on sternal wound infection [22,23,40,41]. A nonsignificant increase in the risk of sternal wound infection was noted in the AKI group compared to the non-AKI group (risk ratio = 1.19, 95% CI: 0.63–2.25, P = 0.59), with no heterogeneity detected across studies (I2 = 0%, P for heterogeneity = 0.53) (Fig. 5b).
Need for tracheostomy
Five of the 21 included studies reported data on the need for a tracheostomy [22,23,37,40,41]. Patients with AKI exhibited a nonsignificantly increased risk of tracheostomy compared to those without AKI (risk ratio = 2.10, 95% CI: 0.72–6.15, P = 0.18), with 85% heterogeneity detected across studies (I2 = 85%, P for heterogeneity < 0.001) (Fig. 5c). Sensitivity analysis showed ‘Helgason et al. (2021)’ [41] might be the source of heterogeneity. After excluding this study, the heterogeneity between the studies was reduced (I2 = 27%, P for heterogeneity > 0.1).
Paraplegia
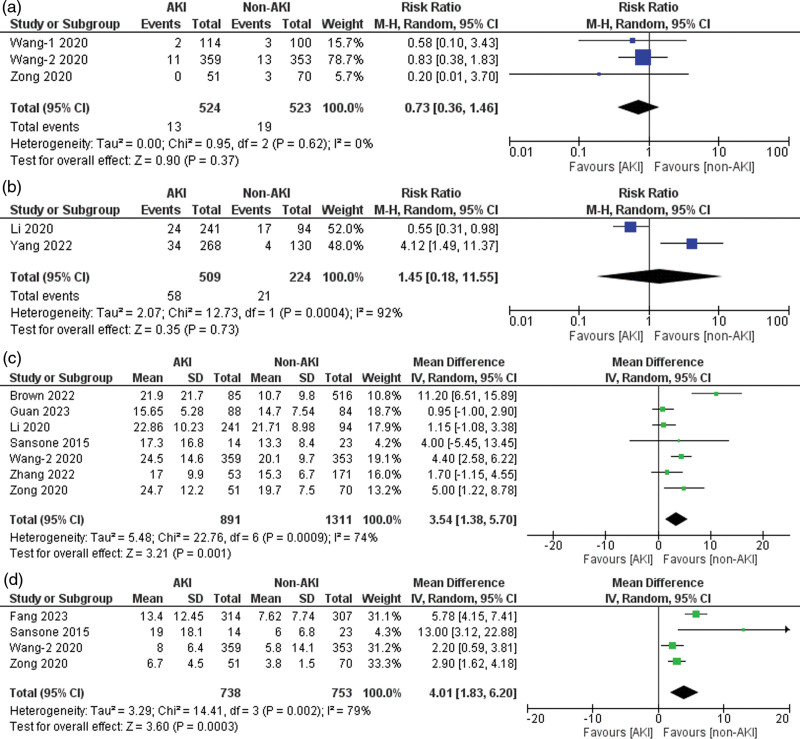
Three of the 21 included studies reported data on paraplegia [22,23,40]. A nonsignificantly increased risk of paraplegia was observed in the AKI group compared to the non-AKI group (risk ratio = 0.73, 95% CI: 0.36–1.46, P = 0.37), with no heterogeneity detected across studies (I2 = 0%, P for heterogeneity = 0.62) (Fig. 6a).
Fig. 6.
Forest plots for the outcomes of (a) paraplegia, (b) hepatic failure, (c) lengths of stay in hospital, and (d) lengths of stay in ICU.
Hepatic failure
Two of the 21 included studies reported data on hepatic failure [38,39]. Patients with AKI had a nonsignificantly increased risk of hepatic failure compared to the non-AKI group (risk ratio = 1.45, 95% CI: 0.18–11.55, P = 0.73), with 92% heterogeneity detected across studies (I2 = 92%, P for heterogeneity < 0.001) (Fig. 6b). Upon performing sensitivity analysis, no significant reduction in heterogeneity was observed.
Length of stay
Seven [23,24,29,35,38,40,42] of the 21 included studies reported data for length of hospital stay, with 74% heterogeneity detected across studies (I2 = 74%, P for heterogeneity < 0.001) (Fig. 6c). The sensitivity analysis showed that ‘Brown et al. (2022)’ [24] might be a source of heterogeneity. After excluding this study, the heterogeneity between the studies was reduced (I2 = 51%, P for heterogeneity > 0.1). Four of the 21 included studies reported data on the length of stay in the ICU [23,28,40,42]. Patients with AKI had a significantly longer duration of ICU stay than those without AKI (mean difference = 4.01, 95% CI: 1.83–6.20, P < 0.001), with 79% heterogeneity detected across studies (I2 = 79%, P for heterogeneity < 0.001) (Fig. 6d). Upon performing sensitivity analysis, no significant reduction in heterogeneity was observed.
Meta regression analysis
We performed a meta-regression analysis for numerous covariates, namely mean age of patients in the study, mean baseline BMI of patients, gender, and baseline kidney function, against the outcomes of 30-day mortality, stroke, and need for dialysis/CRRT. Our analysis found no significant correlation between the outcomes and covariates.
Discussion
This meta-analysis revealed a combined incidence of 37.8% for postoperative AKI after TAAD repair, supporting a previously reported range of 18–87% [34,36,43]. Our findings demonstrate that patients experiencing AKI face a significantly elevated risk of adverse events, including mortality, stroke, the need for dialysis, cardiovascular and respiratory complications, sepsis, re-exploration for bleeding, prolonged hospital stay, and an extended ICU stay. These results underscore the severity of AKI and highlight the importance of promptly identifying and managing it to improve patient outcomes. The risk of sternal wound infection, the need for tracheostomy, hepatic failure, and paraplegia appeared comparable between the two groups. However, this observation may be attributed to the limited number of studies reporting these outcomes, resulting in insufficient power to draw definitive conclusions.
Our study revealed a nearly four-fold increase in the 30-day mortality risk among patients with AKI (18.36% compared to 4.75%). These findings align with those of Ko et al., who reported a higher 30-day mortality rate in their AKI group. Notably, they reported that the severity of AKI was a significant predictor of mortality. Specifically, AKI stage 3, the most severe stage, was identified as an independent risk factor for mortality with a hazard ratio of 6.83, even after adjusting for potential confounding factors. Additionally, extracorporeal circulation time, BMI, elevated perioperative peak serum C-reactive protein concentration, reduced renal perfusion, and perioperative sepsis have been identified as risk factors for AKI development [37]. Furthermore, a study by Li et al. revealed a significantly higher overall postoperative in-hospital mortality rate in the AKI group than that in the non-AKI group (21.2% vs. 9.6%). The mortality rate increased with each stage of AKI, with stage 3 exhibiting the highest mortality rate (70.59%) [38].
AKI has been associated with a complex and challenging postoperative course in numerous studies [4,13,14,23,41,42,44]. Our meta-analysis builds on this existing knowledge by comprehensively examining the intricate relationship between AKI and postoperative complications following TAAD repair. Our findings demonstrate a significant association between AKI and a multitude of adverse events. Patients with AKI exhibited a nearly threefold increased risk of cardiovascular complications (P < 0.001), an over two-fold increased risk of respiratory complications (P = 0.02), a significantly higher risk of sepsis (P < 0.001), and a more than two-fold increased risk of stroke (P < 0.001). Additionally, patients with AKI had a greater than two-fold increased risk of re-exploration for bleeding (P < 0.001), experienced prolonged hospital stay, and required extended ICU stay. However, there was no significant difference between the two groups in terms of sternal wound infection, need for tracheostomy, paraplegia, and hepatic failure.
Early initiation of RRT has been suggested as a means of improving patient outcomes [14]. However, findings from the study by Wang et al. challenged this notion, indicating that even with timely postoperative RRT, patients still experience elevated rates of perioperative mortality and postoperative morbidity [13]. Our findings align with these observations, demonstrating a significantly higher prevalence of dialysis/CRRT in the AKI group than in the non-AKI group (30.2% vs. 0.8%). Moreover, the RRT procedure itself carries potential complications, including circulatory instability, infection, thrombosis, and electrolyte imbalance, which could negatively affect patient recovery and prognosis [45].
Recent advances have shed light on the complex mechanisms that underlie AKI. The pathophysiology of AKI involves a cascade of events, including hemodynamic imbalances, inflammatory responses, immune system dysfunction, dysregulation of iron metabolism, increased oxidative stress, and associated inflammation [7]. Since there is currently no specific treatment for postoperative AKI in TAAD patients, preventive measures such as careful management of blood pressure and anemia during repair, utilizing new technologies to shorten the time organs are deprived of blood flow, and avoiding exposure to nephrotoxic substances may be crucial for protecting kidney function [46]. The early diagnosis of AKI can lead to timely treatment and improved outcomes [47]. Currently, AKI is diagnosed on the basis of serum creatinine levels, glomerular filtration rate, and urine output. However, these tests are not always accurate early in the course of AKI and cannot predict the outcomes. Recently, researchers have looked at biomarkers for the early diagnosis of AKI. Two promising biomarkers are neutrophil gelatinase-associated lipocalin and cystatin C [38,48–50]. While these studies focused on cardiac procedures, they did not investigate the diagnostic effectiveness of these biomarkers in TAAD patients. Further research is warranted to validate the utility of these biomarkers for TAAD diagnosis.
Overall, this meta-analysis provides valuable insights into the association between AKI and adverse outcomes after TAAD repair. Further clinical trials are necessary to determine preventive strategies and reduce the burden of AKI in this patient population.
Limitations
Our study had several limitations. First, the studies included in this review were observational, and while they offer valuable insights, they are inherently susceptible to biases that are not present in randomized controlled trials. Second, although the quality of these studies, as evaluated using the Newcastle-Ottawa Scale, was high, the results varied, which may have affected the outcomes. Third, more than half of the studies included in this analysis focused on the Chinese population, thereby limiting the generalizability of the results to patients from other ethnic backgrounds. Fourth, significant heterogeneity was observed across the studies owing to differences in study design, patient characteristics, repair techniques, and definitions of outcomes. Although the sensitivity analysis attempted to address some of the heterogeneity, it remained high for certain outcomes, indicating that the results should be interpreted with caution. Fifth, in the process of conducting an extensive literature search, there may still be a risk of publication bias as studies with negative findings are often less published. This could potentially skew the findings of this study. Sixth, for some outcomes, the data were limited, leading to wider confidence intervals and less precise estimates. This limitation highlights the need for further research to confirm our findings. Finally, most studies had a follow-up duration of at least 30 days, but long-term outcomes were not considered. This could have provided more information about the effects of AKI following TAAD repair, indicating a gap in the current research.
Despite these limitations, this systematic review and meta-analysis provides valuable insights into the outcomes of AKI following TAAD repair. Future research should address these limitations and provide more definitive conclusions.
Conclusion
In conclusion, this meta-analysis confirms that postoperative AKI is significantly associated with adverse outcomes following repair of TAAD, including increased mortality, stroke, dialysis/CRRT requirement, cardiovascular and respiratory complications, sepsis, re-exploration for bleeding, and prolonged hospital and ICU stays. Future research should aim to standardize patient populations and outcome definitions to enhance understanding of AKI’s prognostic significance in these patients. Additionally, large-scale multicenter studies, including randomized controlled trials, prospective studies, are required to validate these findings and to better understand long-term outcomes in these patients.
Acknowledgements
A.G. contributed to the conceptualization, investigation, data curation, methodology, formal analysis, and writing – original draft. S.M. contributed to the investigation, data curation, and formal analysis. H.Q.A. contributed to the data curation, methodology, and writing – original draft. Y.M. contributed to the investigation, data curation, and writing – original draft. U.S. and M.C. contributed to the methodology and writing – original draft. A.K. contributed to the writing – original draft. D.S. and H.J. contributed to the writing – review and editing and supervision. P.P. contributed to the writing – original draft. H.S. and IU contributed to the writing – review and editing and supervision. All authors read and approved the final manuscript.
All datasets generated and analyzed are available in the article and supplementary materials.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.cardiovascularendocrinology.com.
References
- 1.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg 1970; 10:237–247. [DOI] [PubMed] [Google Scholar]
- 2.Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015; 66:350–358. [DOI] [PubMed] [Google Scholar]
- 3.Parikh N, Trimarchi S, Gleason TG, Kamman AV, di Eusanio M, Myrmel T, et al. Changes in operative strategy for patients enrolled in the International Registry of Acute Aortic Dissection interventional cohort program. J Thorac Cardiovasc Surg 2017; 153:S74–S79. [DOI] [PubMed] [Google Scholar]
- 4.Sasabuchi Y, Kimura N, Shiotsuka J, Komuro T, Mouri H, Ohnuma T, et al. Long-term survival in patients with acute kidney injury after acute type A aortic dissection repair. Ann Thorac Surg 2016; 102:2003–2009. [DOI] [PubMed] [Google Scholar]
- 5.Collins JS, Evangelista A, Nienaber CA, Bossone E, Fang J, Cooper JV, et al. Differences in clinical presentation, management, and outcomes of acute type A aortic dissection in patients with and without previous cardiac surgery. Circulation 2004; 110(11 Suppl 1):II237–II242. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Pan X, Gong Z, Zheng J, Liu Y, Zhu J, Sun L. Risk factors for acute kidney injury in overweight patients with acute type A aortic dissection: a retrospective study. J Thorac Dis. 2015; 7:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J Am Heart Assoc 2018; 7:e008834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006; 1:19–32. [DOI] [PubMed] [Google Scholar]
- 9.Seese L, Sultan I, Gleason TG, Navid F, Wang Y, Thoma F, Kilic A. The impact of major postoperative complications on long-term survival after cardiac surgery. Ann Thorac Surg 2020; 110:128–135. [DOI] [PubMed] [Google Scholar]
- 10.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol 2015; 10:500–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Ge M, Chen T, Chen C, Zong Q, Lu L, Wang D. Independent risk factors and the long-term outcomes for postoperative continuous renal replacement treatment in patients who underwent emergency surgery for type A acute aortic dissection. J Cardiothorac Surg 2020; 15:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zhong G, Lv X, Dong Y, Hou Y, Dai X, Chen L. Risk factors for acute kidney injury after Stanford type A aortic dissection repair surgery: a systematic review and meta-analysis. Ren Fail 2022; 44:1462–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Yu W, Zhai G, Liu N, Sun L, Zhu J. Independent risk factors for postoperative AKI and the impact of the AKI on 30-day postoperative outcomes in patients with type A acute aortic dissection: an updated meta-analysis and meta-regression. J Thorac Dis. 2018; 10:2590–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roh GU, Lee JW, Nam SB, Lee J, Choi JR, Shim YH. Incidence and risk factors of acute kidney injury after thoracic aortic surgery for acute dissection. Ann Thorac Surg 2012; 94:766–771. [DOI] [PubMed] [Google Scholar]
- 15.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International Journal of Surgery (2021):88;105906. Ann Intern Med 2015; 162:777–784. [DOI] [PubMed] [Google Scholar]
- 19.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. 2011;2(1):1-2. [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Ge M, Chen T, Chen C, Zong Q, Lu L, et al. Risk factors and long-term outcomes of elderly patients complicating with acute kidney injury after type A acute aortic dissection surgery: a retrospective study. J Thorac Dis. 2020; 12:5833–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Ge M, Chen T, Chen C, Zong Q, Lu L, Wang D. Acute kidney injury in patients operated on for type A acute aortic dissection: incidence, risk factors and short-term outcomes. Interact Cardiovasc Thorac Surg 2020; 31:697–703. [DOI] [PubMed] [Google Scholar]
- 24.Brown JA, Serna-Gallegos D, Navid F, Thoma FW, Zhu J, Kumar R, et al. The long-term impact of acute renal failure after aortic arch replacement for acute type A aortic dissection. J Card Surg 2022; 37:2378–2385. [DOI] [PubMed] [Google Scholar]
- 25.Arnaoutakis GJ, Ogami T, Patel HJ, Pai CW, Woznicki EM, Brinster DR, et al. Acute kidney injury in patients undergoing surgery for type A acute aortic dissection. Ann Thorac Surg 2023; 115:879–885. [DOI] [PubMed] [Google Scholar]
- 26.Li CN, Ge YP, Liu H, Zhang CH, Zhong YL, Chen SW, et al. Blood transfusion and acute kidney injury after total aortic arch replacement for acute Stanford type A aortic dissection. Heart Lung Circ 2022; 31:136–143. [DOI] [PubMed] [Google Scholar]
- 27.Chen S, Zhang C, Zhong Y, Tang B, Xie Q, Guo R, et al. Association between preoperative serum myoglobin and acute kidney injury after Stanford type A aortic dissection surgery. Clin Chim Acta 2023; 541:117232. [DOI] [PubMed] [Google Scholar]
- 28.Fang M, Li J, Fang H, Wu J, Wu Z, He L, et al. Prediction of acute kidney injury after total aortic arch replacement with serum cystatin C and urine N-acetyl-β-d-glucosaminidase: a prospective observational study. Clin Chim Acta 2023; 539:105–113. [DOI] [PubMed] [Google Scholar]
- 29.Guan XL, Li L, Jiang WJ, Gong M, Li HY, Liu YY, et al. Low preoperative serum fibrinogen level is associated with postoperative acute kidney injury in patients with in acute aortic dissection. J Cardiothorac Surg 2023; 18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Fu Y, Liu J, Liu Y, Zhu J, Sun L, et al. Body mass index is an independent predictor of acute kidney injury after urgent aortic arch surgery for acute DeBakey Type I aortic dissection. J Cardiothorac Surg 2021; 16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Shang Y, Long D, Yu Li. Intraoperative blood transfusion volume is an independent risk factor for postoperative acute kidney injury in type A acute aortic dissection. BMC Cardiovasc Disord 2020; 20:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Z, Chen L, Cao H, Zhang G, Xu F, Chen Q. Analysis of risk factors for acute kidney injury after ascending aortic replacement combined with open placement of triple-branched stent graft in type A aortic dissection: a new technique versus the traditional method in a single Chinese center. Med Sci Monit 2015; 21:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S, Wu Z, Liu Y, Zhu J, Gong M, Sun L, et al. Influence of preoperative serum albumin on acute kidney injury after aortic surgery for acute type A aortic dissection: a retrospective cohort study. J Clin Med 2023; 12:1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K, Shang J, Chen Y, Huo Y, Li B, Hu Z. The prognosis and risk factors for acute kidney injury in high-risk patients after surgery for type A aortic dissection in the ICU. J Thorac Dis. 2021; 13:4427–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Lan Y, Chen T, Chen Q, Guo Z, Jiang N. Prediction of acute kidney injury for acute type A aortic dissection patients who underwent sun’s procedure by a perioperative nomogram. Cardiorenal Med 2022; 12:117–130. [DOI] [PubMed] [Google Scholar]
- 36.Kim WH, Lee JH, Kim E, Kim G, Kim HJ, Lim HW. Can we really predict postoperative acute kidney injury after aortic surgery? Diagnostic accuracy of risk scores using gray zone approach. Thorac Cardiovasc Surg 2016; 64:281–289. [DOI] [PubMed] [Google Scholar]
- 37.Ko T, Higashitani M, Sato A, Uemura Y, Norimatsu T, Mahara K, et al. Impact of acute kidney injury on early to long-term outcomes in patients who underwent surgery for type A acute aortic dissection. Am J Cardiol 2015; 116:463–468. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Zhou J, Hao X, Zhang W, Yu D, Xie Y, et al. The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after surgery for acute type A aortic dissection: a single-center retrospective analysis of 335 patients. Front Med (Lausanne) 2020; 7:557044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C, Hou P, Wang D, Wang Z, Duan W, Liu J, et al. Serum myoglobin is associated with postoperative acute kidney injury in Stanford type A aortic dissection. Front Med (Lausanne) 2022; 9:821418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zong Q, Ge M, Chen T, Chen C, Wang Z, Wang D. Risk factors and long-term outcomes of acute kidney injury complication after type A acute aortic dissection surgery in young patients. J Cardiothorac Surg 2020; 15:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helgason D, Helgadottir S, Ahlsson A, Gunn J, Hjortdal V, Hansson EC, et al. Acute kidney injury after acute repair of type A aortic dissection. Ann Thorac Surg 2021; 111:1292–1298. [DOI] [PubMed] [Google Scholar]
- 42.Sansone F, Morgante A, Ceresa F, Salamone G, Patanè F. Prognostic implications of acute renal failure after surgery for type A acute aortic dissection. Aorta (Stamford) 2015; 3:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori Y, Sato N, Kobayashi Y, Ochiai R. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J Anesth 2011; 25:799–804. [DOI] [PubMed] [Google Scholar]
- 44.Tsai HS, Tsai FC, Chen YC, Wu L-S, Chen S-W, Chu J-J, et al. Impact of acute kidney injury on one‐year survival after surgery for aortic dissection. Ann Thorac Surg 2012; 94:1407–1412. [DOI] [PubMed] [Google Scholar]
- 45.Hanafusa N, Nakai S, Iseki K, Tsubakihara Y. Japanese society for dialysis therapy renal data registry-a window through which we can view the details of Japanese dialysis population. Kidney Int Suppl (2011) 2015; 5:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostermann M, Cennamo A, Meersch M, Kunst G. A narrative review of the impact of surgery and anaesthesia on acute kidney injury. Anaesthesia 2020; 75:121–133. [DOI] [PubMed] [Google Scholar]
- 47.Hoogmoed RC, Patel HJ, Kim KM, Williams DM, Deeb GM, Yang B. Acute kidney injury in acute type B aortic dissection: outcomes over 20 years. Ann Thorac Surg 2019; 107:486–492. [DOI] [PubMed] [Google Scholar]
- 48.KDIGO (Kidney Disease: Improving Global Outcomes) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Inter Suppl 2012; 2:1–138. [Google Scholar]
- 49.Nishigawa K, Fukui T, Uemura K, Takanashi S, Shimokawa T. Preoperative renal malperfusion is an independent predictor for acute kidney injury and operative death but not associated with late mortality after surgery for acute type A aortic dissection. Eur J Cardiothorac Surg 2020; 58:302–308. [DOI] [PubMed] [Google Scholar]
- 50.Qin H, Li Y, Zhang N, Wang T, Fan Z. Prediction efficiency of postoperative acute kidney injury in acute Stanford type A aortic dissection patients with renal resistive index and semiquantitative color doppler. Cardiol Res Pract 2019; 2019:4381052. [DOI] [PMC free article] [PubMed] [Google Scholar]