Abstract
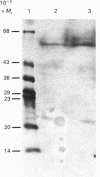
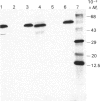
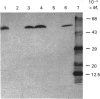
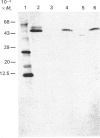
The aim of this work was to discover whether fructose-1,6-bisphosphatase (FBPase) is present in higher-plant cells that synthesize storage starch. The following were examined: suspension cultures of soybean (Glycine max), tubers of potato (Solanum tuberosum), florets of cauliflower (Brassica oleracea), developing endosperm of maize and of sweet corn (Zea mays), roots of pea (Pisum sativum), and the developing embryos of round and wrinkled varieties of pea. Unfractionated extracts of each tissue readily converted fructose 1,6-bisphosphate to fructose 6-phosphate in assays for both plastidic and cytosolic FBPase. These conversions were not inhibited by 20 microM-fructose 2,6-bisphosphate. Except in extracts of pea embryos and sweet-corn endosperm, treatment with affinity-purified antibodies to pyrophosphate: fructose-6-phosphate 1-phosphotransferase reduced the above fructose 6-phosphate production to the rate found with boiled extracts. The antibody-resistant activity from sweet corn was slight. In immunoblot analyses, antibody to plastidic FBPase did not react positively with any protein in extracts of soybean cells, potato tuber, cauliflower florets, maize endosperm and pea roots. Positive reactions were found for extracts of embryos of both round and wrinkled varieties of peas and endosperm of sweet corn. For pea embryos, but not for sweet-corn endosperm, the Mr of the recognized protein corresponded to that of plastidic FBPase. It is argued that soybean cells, potato tuber, cauliflower florets, maize (var. White Horse Tooth) endosperm and pea roots lack significant activity of plastidic FBPase, but that this enzyme is present in developing embryos of pea. The data for sweet corn (var. Golden Bantam) are not decisive. It is also argued that, where FBPase is absent, carbon for starch synthesis does not enter the amyloplast as triose phosphate.
Full text
PDF

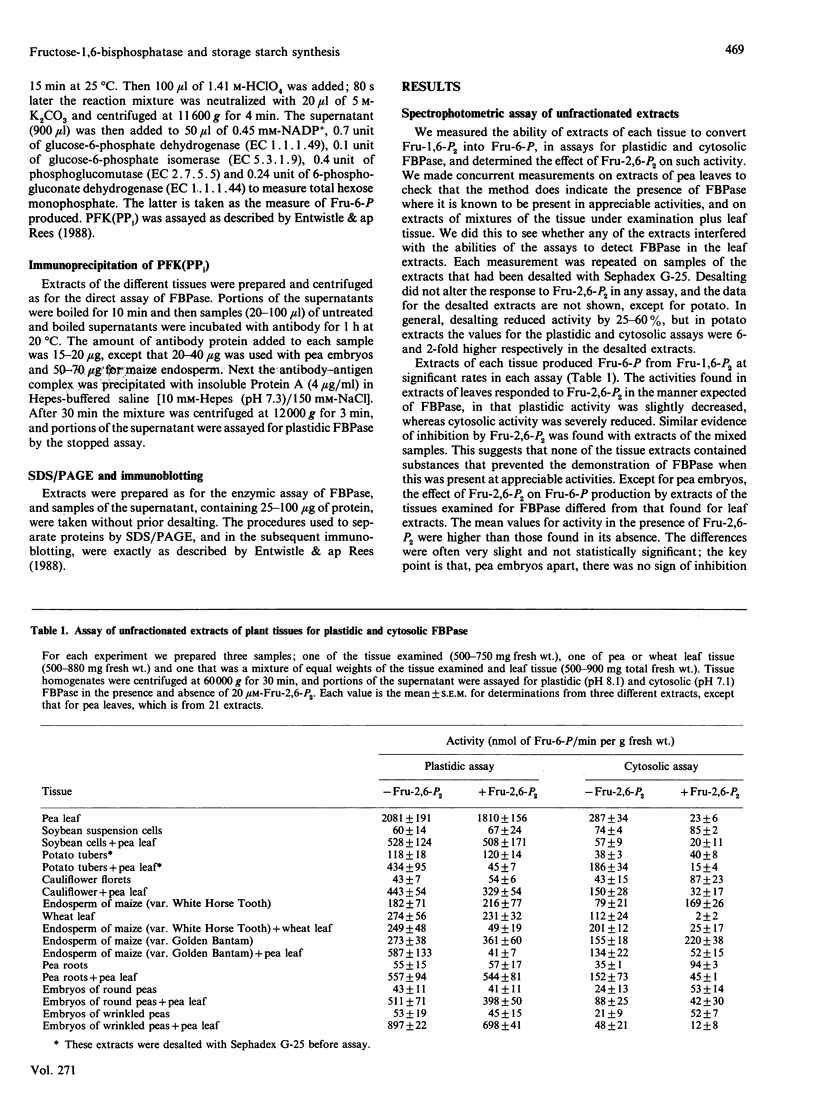

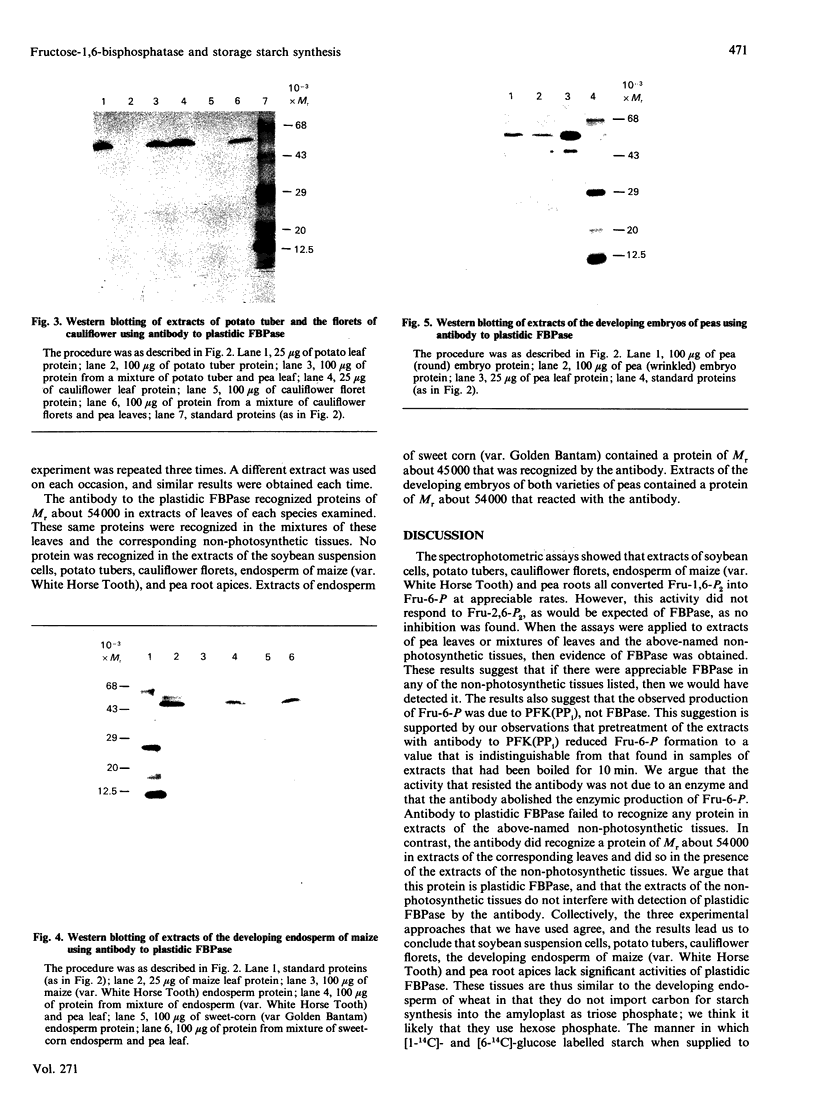

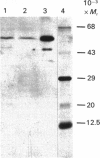
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allington W. B., Cordry A. L., McCullough G. A., Mitchell D. E., Nelson J. W. Electrophoretic concentration of macromolecules. Anal Biochem. 1978 Mar;85(1):188–196. doi: 10.1016/0003-2697(78)90289-0. [DOI] [PubMed] [Google Scholar]
- Cadet F., Meunier J. C., Ferté N. Effects of pH and fructose 2,6-bisphosphate on oxidized and reduced spinach chloroplastic fructose-1,6-bisphosphatase. Eur J Biochem. 1987 Jan 15;162(2):393–398. doi: 10.1111/j.1432-1033.1987.tb10614.x. [DOI] [PubMed] [Google Scholar]
- Echeverria E., Boyer C. D., Thomas P. A., Liu K. C., Shannon J. C. Enzyme activities associated with maize kernel amyloplasts. Plant Physiol. 1988 Mar;86(3):786–792. doi: 10.1104/pp.86.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entwistle G., Rees T. A. Enzymic capacities of amyloplasts from wheat (Triticum aestivum) endosperm. Biochem J. 1988 Oct 15;255(2):391–396. doi: 10.1042/bj2550391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B., Stitt M., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : III. Properties of the Cytosolic Fructose 1,6-Bisphosphatase. Plant Physiol. 1984 Jul;75(3):561–565. doi: 10.1104/pp.75.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Douce R. Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 1985 Oct;79(2):458–467. doi: 10.1104/pp.79.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling P. L., Wood J. R., Tyson R. H., Bridges I. G. Starch Biosynthesis in Developing Wheat Grain : Evidence against the Direct Involvement of Triose Phosphates in the Metabolic Pathway. Plant Physiol. 1988 Jun;87(2):311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Kruger N. J., Beevers H. Kinetic properties of pyrophosphate:fructose-6-phosphate phosphotransferase from germinating castor bean endosperm. Plant Physiol. 1984 Feb;74(2):395–401. doi: 10.1104/pp.74.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger N. J., Dennis D. T. Molecular properties of pyrophosphate:fructose-6-phosphate phosphotransferase from potato tuber. Arch Biochem Biophys. 1987 Jul;256(1):273–279. doi: 10.1016/0003-9861(87)90446-2. [DOI] [PubMed] [Google Scholar]
- Mohabir G., John P. Effect of temperature on starch synthesis in potato tuber tissue and in amyloplasts. Plant Physiol. 1988 Dec;88(4):1222–1228. doi: 10.1104/pp.88.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa A. N., Buchanan B. B. Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked fructose bisphosphatase and sedoheptulose bisphosphatase from corn leaves. J Biol Chem. 1981 Jun 25;256(12):6119–6126. [PubMed] [Google Scholar]