Abstract
Background:
Various oral anticoagulants have been used for stroke prevention in older patients with atrial fibrillation (AF). However, the optimal anticoagulants for stroke prevention has not yet been developed. We performed a systematic review and network meta-analysis to determine the optimal instructions.
Methods:
We searched for randomized controlled trials (RCTs) from PubMed, Embase, and the Cochrane Library without restriction for publication date or language at January 2024. Any RCTs that compared the effectiveness of a direct oral anticoagulant and a vitamin K antagonist (VKA) for stroke prevention in older patients with AF were included in this network meta-analysis. The Bayesian network meta-analysis used a random effects model and surface under the cumulative ranking curve analysis to rank results. All analyses were done using R software with gemtc package, with statistical significance set at P < .05.
Results:
We included 7 RCTs (79,003 patients) comparing 8 different instructions including Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, Edoxaban 60 mg, Rivaroxaban 15 mg, Rivaroxaban 20 mg, and VKA. Apixaban 5 mg, Dabigatran 110 mg, and Dabigatran 150 mg was more effective than the VKA for reducing stroke or systemic embolism risks, and the difference was statistically significant (P < .05). Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, and Edoxaban 60 mg was associated with a reduction of the intracranial hemorrhage rate than the VKA (P < .05). The surface under the cumulative ranking curve shows that Dabigatran 110 mg ranked first for reducing stroke or systemic embolism risks. Edoxaban 60 mg ranked first for major bleeding. Dabigatran 110 mg ranked first for intracranial hemorrhage. Apixaban 5 mg ranked first for all bleeding events.
Conclusions:
Direct oral anticoagulants were found to have lower rates of thromboembolic events compared to VKAs in older patients with AF. Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, and Edoxaban 60 mg were also associated with a reduction of intracranial hemorrhage than VKA.
Keywords: atrial fibrillation, direct oral anticoagulant, network meta-analysis
1. Introduction
Atrial fibrillation (AF) is the most common long-lasting irregular heartbeat in adults globally, with a lifetime risk of up to 1 in 3 people.[1,2] The risk of AF escalates notably with advancing age, particularly in those surpassing 65 years.[3] Over the past 5 decades, the global population aging trend has propelled a substantial surge in individuals living with AF, reaching nearly 60 million globally by 2019.[4] The rise in AF has caused 0.315 million deaths and 8.39 million years of lost life.[5] Projections show that AF could be responsible for 2.5 million deaths by 2034, highlighting its growing importance as a global health issue.[6] A recent Global Burden Disease report indicates that the age-standardized mortality rate for cardiovascular diseases globally in 2022 ranged from 73.6 per 100,000 in high-income Asia-Pacific to 432.3 per 100,000 in Eastern Europe. From 1990 to 2022, the global mortality rate for cardiovascular diseases decreased by 34.9%. Ischemic heart disease has the highest global age-standardized DALYs (Disability-Adjusted Life Years) among all diseases, at 2275.9 per 100,000.[7]
One pivotal aspect of AF management revolves around averting thromboembolic complications.[8] Nonetheless, the benefits of anticoagulation are frequently tempered by an augmented risk of bleeding.[9,10] Various validated scoring systems for risk stratification underscore specific clinical factors that contribute to heightened risks of thromboembolic events and bleeding.[11] For instance, advanced age constitutes a component of scoring systems such as the congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes, stroke (doubled)–vascular disease, age 65 to 74 years, sex category (female) stroke score and the hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly (age ≥ 65 years), drug/alcohol concomitant score, where age ≥ 75 years carries particular weight. Older age is linked to higher risks of stroke and systemic embolism in AF patients, with the likelihood of strokes from AF increasing with age.[9] In older individuals, particularly those over 85, the risk of intracranial hemorrhages during warfarin therapy for stroke prevention increases, complicating stroke prevention in older AF patients.[10]
Direct oral anticoagulant (DOACs) have transformed stroke prevention in AF since the RE-LY trial, showing comparable efficacy and safety to vitamin K antagonists (VKAs).[12] However, older patients, who are at higher risk of events, are underrepresented in trials, making the best treatment choice for them uncertain.[13,14]
This study aimed to compare the effectiveness and safety of different DOACs in patients aged 75 and older, particularly focusing on bleeding outcomes and very old patients, as there is limited direct comparison data available.
2. Methods
2.1. Search strategy
Two authors (H.Z. and F.L.) conducted independent searches of electronic literature databases including PubMed, Embase, and the Cochrane Library, with no restrictions on publication date or language up to January 2024. The search strategy encompassed terms such as “direct oral anticoagulants,” “DOACs,” “novel oral anticoagulants,” “NOACs,” “dabigatran,” “rivaroxaban,” “apixaban,” and “edoxaban,” along with terms related to warfarin, atrial fibrillation, and study types such as randomized controlled trials or clinical trials, and outcomes such as stroke or systemic embolism risks, major bleeding, intracranial hemorrhage, and all bleeding events. Related articles and reference lists were scrutinized to ensure no pertinent studies were overlooked, including a manual search of reference studies from previous systematic reviews, meta-analyses, and randomized controlled trials. In case of any discrepancies between the 2 authors’ assessments of potentially eligible studies, a discussion ensued, and disagreements were resolved with input from a third independent author. The search was limited to studies involving human participants. As this systematic review and meta-analysis did not involve direct patient contact, ethics approval was not deemed necessary. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for systematic reviews that incorporate network meta-analyses in healthcare.
2.2. Inclusion criteria
The inclusion criteria were as follows: (1) patients were diagnosed with AF; (2) studies comparing DOAC with a VKA; (3) randomized controlled trial (RCTs); (4) studies reporting stroke or systemic embolism risks, major bleeding, intracranial hemorrhage, and all bleeding events in patients.
The following studies were excluded: secondary analyses, study protocols, incomplete texts, and animal or basic science experiments.
2.3. Data extraction
Two researchers (H.Z. and F.L.) collected data from trials using a standardized form, including author name, publication year, intervention and control arms, number of participants, inclusion criteria, follow-up duration, patient age and gender, and outcomes. Clinical outcomes included stroke or systemic embolism risks, major bleeding, intracranial hemorrhage, and all bleeding events. Any discrepancies were resolved through discussion.
2.4. Quality assessment and publication bias assessment
Two evaluators (H.Z. and F.L.) independently assessed the quality of individual trials using guidelines from the Cochrane Handbook, evaluating parameters such as random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias. Trials were classified as “low risk,” “high risk,” or “unclear” based on these assessments.
2.5. Statistical analysis
A network meta-analysis was conducted using a random-effect model in a Bayesian framework with the “gemtc” and “rjags” packages in R software. Four iteration chains with 20,000 iterations each were used in the Markov chain Monte Carlo analysis, resulting in a total of 150,000 sample iterations with 10 thinning intervals and 100,000 burn-ins to ensure convergence. Results were calculated using posterior distribution medians and 95% credible intervals. Statistical significance was determined when the intervals did not include 1 for odds ratios and 0 for mean differences, with a significance threshold of P < .05. Top interventions were identified using surface under the cumulative ranking curve (SUCRA) values, with higher values indicating better efficacy on a scale of 0 to 1. A cluster-ranking plot was used to determine the best outcome indicator from multiple outcomes. Heterogeneity was measured with the I2 test, with thresholds of 25%, 50%, and 75% indicating levels of heterogeneity. Global inconsistency was assessed using inconsistency models and a dDIC > 10 indicated significant global inconsistency. Local inconsistency was evaluated using node-splitting analysis, with a P-value > .05 indicating no significant inconsistency between direct and indirect results. Funnel plots were used to detect publication bias in each network.
3. Results
3.1. Included studies
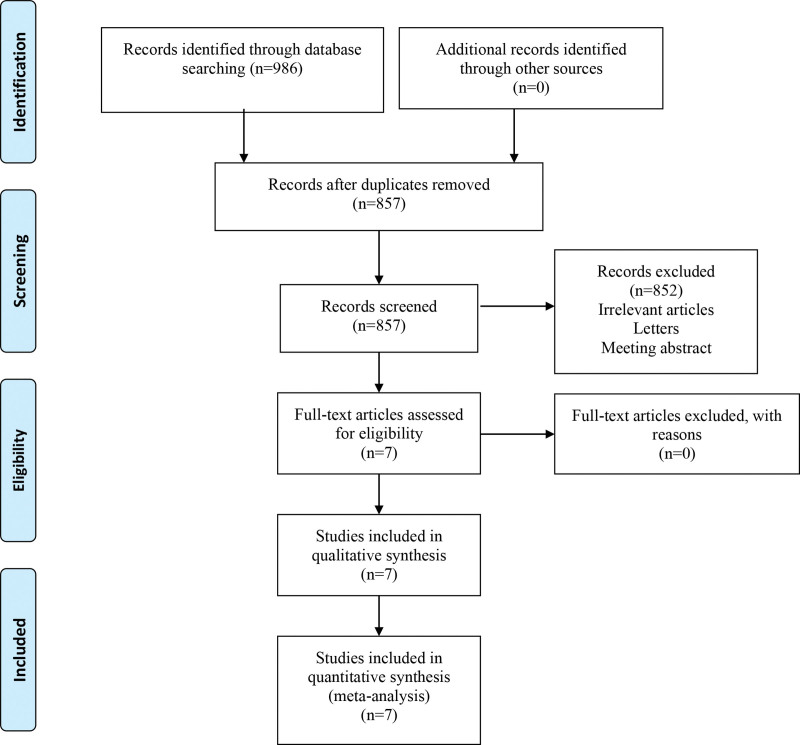
The search retrieved a total of 986 articles which were identified from PubMed (356), Embase (330), and Cochrane Library (300). Of these, 129 were removed as duplicates. Based on our review of the title and abstract, 859 full-text papers were reviewed and 852 were excluded. Then, full-text articles were assessed for eligibility and 0 study were excluded for reasons. Finally, a total of 7 studies[12,15–20] met the inclusion criteria and included for analysis (Fig. 1). General characteristics of the included studies can be seen in Table 1.
Figure 1.
Flow diagram of the literature selection process.
Table 1.
General characteristics of the included studies.
| Study | Intervention arms | Control arm | No. of participants | Inclusion criteria | Follow-up (year) | Age (year) | Female (%) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Connolly 2009[12] | Dabigatran 110 mg twice daily Dabigatran 150 mg twice daily | Warfarin | 18,113 | Patients with AF with at least 1 additional risk factor for stroke | 2 | 71 | 36.3 | Stroke or systemic embolism, Major bleeding, Intracranial hemorrhage |
| Granger 2011[16] | Apixaban 5 mg twice daily | Warfarin | 18,201 | Patients with AF with at least 1 additional risk factor for stroke | 1.8 | 70 | 35.5 | Stroke or systemic embolism, Major bleeding, All bleeding events, Intracranial hemorrhage |
| Patel 2011[19] | Rivaroxaban 20 mg once daily | Warfarin | 14,264 | Patients with AF with moderate-to-high risk for stroke | 1.9 | 73 | 39.7 | Stroke or systemic embolism, Major bleeding, Major and nonmajor clinically relevant bleeding events, Intracranial hemorrhage |
| Hori 2012[17] | Rivaroxaban 15 mg only daily | Warfarin | 1280 | Patients with AF with at least 1 additional risk factor for stroke | 2.5 | 71.1 | 17.1 | Stroke or systemic embolism, Major bleeding, Major and nonmajor clinically relevant bleeding events |
| Giugliano 2013[15] | Edoxaban 60 mg only daily Edoxaban 30 mg only daily | Warfarin | 21,105 | Patients with AF with a CHADS2 score of > 2 | 2.8 | 72 | 38.4 | Stroke or systemic embolism, Major bleeding, Intracranial hemorrhage |
| Lopes 2019[18] | Apixaban 5 mg twice daily | Any VKA | 4614 | Patients with AF with acute coronary syndrome or those who underwent percutaneous coronary intervention within 14 d of randomization | 0.5 | 70.7 | 29.1 | Stroke or systemic embolism, Major and nonmajor clinically relevant bleeding events |
| Van Mieghem 2021[20] | Edoxaban 60 mg only daily Edoxaban 30 mg only daily | Any VKA | 1426 | Patients with AF status after transcatheter aortic valve replacement | 1.5 | 82.1 | 48.7 | Major bleeding |
AF = atrial fibrillation, VKA = vitamin K antagonist.
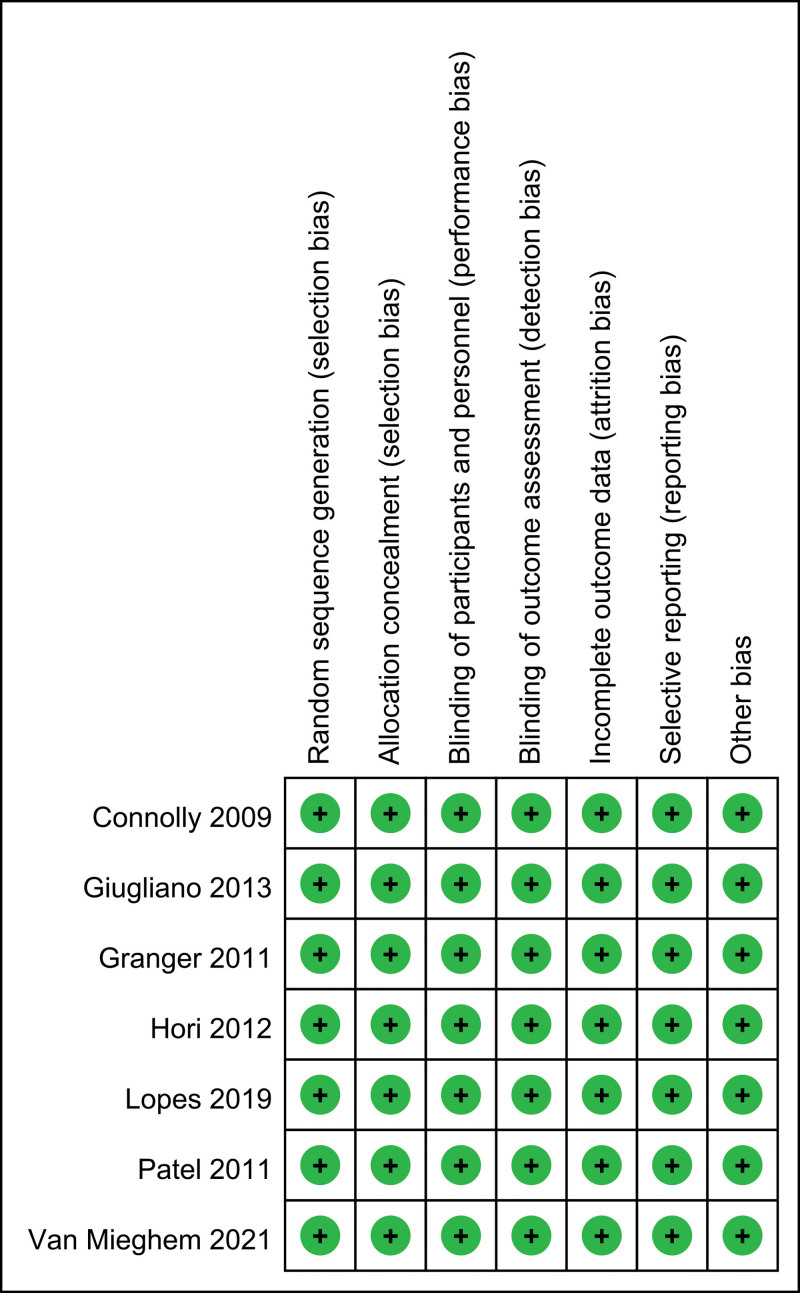
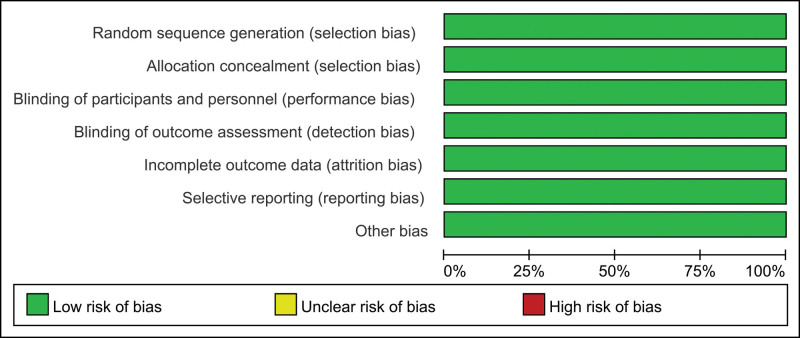
3.2. Risk of bias
Risk of bias summary and risk of bias graph can be seen in Figures 2 and 3, respectively. Of the 7 RCTs, all studies report the random sequence generation and thus should be listed as low risk of bias. All studies report the allocation concealment and should be listed as low risk of bias. All studies report the blinding of participants and personnel and should be listed as low risk of bias.
Figure 2.
Risk of bias graph of the included studies.
Figure 3.
Risk of bias summary of the included studies. stroke or systemic embolism risks, major bleeding, intracranial hemorrhage and all bleeding events.
3.2.1. Stroke or systemic embolism risks
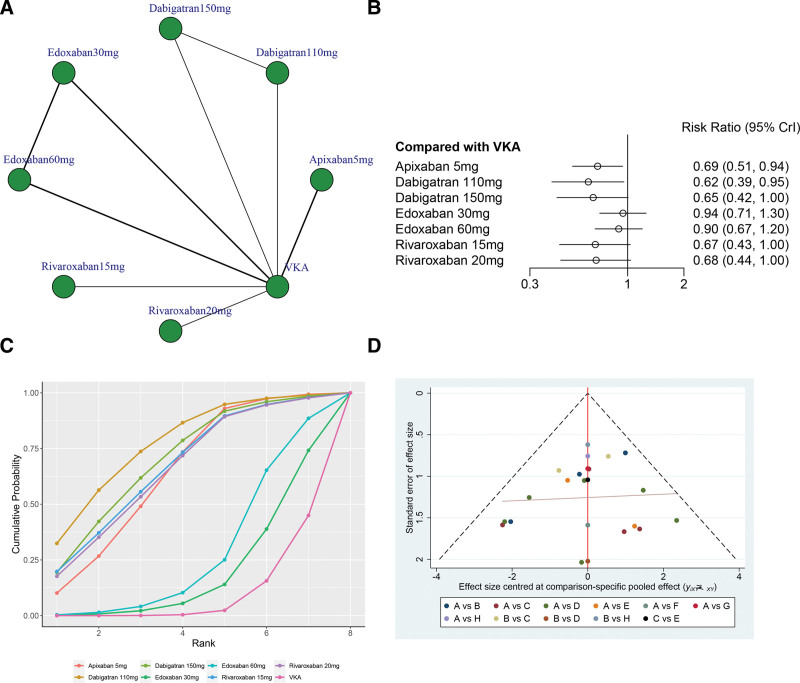
A total of 6 studies (77,577 patients), including 8 treatments (Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, Edoxaban 60 mg, Rivaroxaban 15 mg, Rivaroxaban 20 mg, and VKA) contributed to the clinical outcome of stroke or systemic embolism risks.
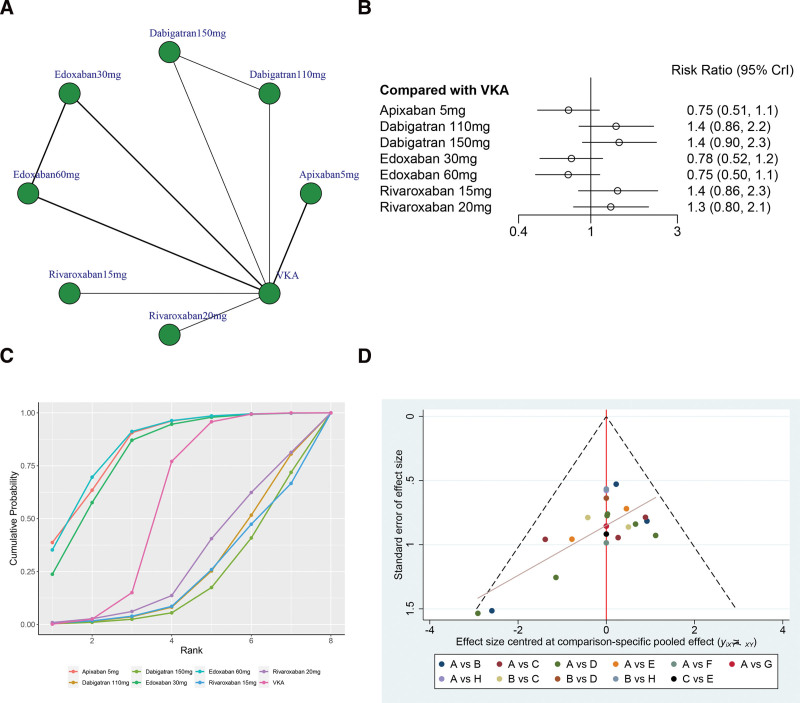
Figure 4A illustrates the network structure diagrams comparing treatments for stroke or systemic embolism risks. The network meta-analysis revealed significant heterogeneity with a global I2 = 0%. In head-to-head comparison, Apixaban 5 mg (OR 0.69, 95% CrI 0.51–0.94), Dabigatran 110 mg (OR 0.62, 95% CrI 0.39–0.95), and Dabigatran 150 mg (OR 0.65, 95% CrI 0.42–1.00) was more effective than the VKA, and the difference was statistically significant. However, there was no statistically significant between other treatments versus VKA in terms of the stroke or systemic embolism risks (P > .05, Fig. 4B).
Figure 4.
(A) Network structure diagrams of stroke or systemic embolism risks. (B) Forest plot of the stroke or systemic embolism risks as compared with VKA. (C) Surface under the cumulative ranking curve (SUCRA) probabilities of different treatments for stroke or systemic embolism risks. (D) Funnel plot of the different treatments for stroke or systemic embolism risks.
The SUCRA shows that Dabigatran 110 mg ranked first (SUCRA, 77.2%), Dabigatran 150 mg ranked second (SUCRA, 69.8%), Rivaroxaban15 mg ranked third (SUCRA, 66.9%), and VKA ranked the last (SUCRA, 9.1%, Fig. 4C). Figure 4D shows a symmetrical inverted funnel plot, indicating a possible small sample effect or publication bias in the study.
3.2.2. Major bleeding
A total of 7 studies (79,003 patients), including 8 treatments (Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, Edoxaban 60 mg, Rivaroxaban 15 mg, Rivaroxaban 20 mg, and VKA) contributed to the clinical outcome of major bleeding.
Figure 5A shows network structure diagrams comparing different treatments for major bleeding. The network meta-analysis revealed significant heterogeneity with an I2 of 5%. In head-to-head comparison, there was no statistically significant between any other treatments versus VKA in terms of the major bleeding (P > .05, Fig. 5B).
Figure 5.
(A) Network structure diagrams of major bleeding. (B) Forest plot of the major bleeding as compared with VKA. (C) Surface under the cumulative ranking curve (SUCRA) probabilities of different treatments for major bleeding. (D) Funnel plot of the different treatments for major bleeding.
The SUCRA analysis indicates that Edoxaban 60 mg achieved the highest ranking (SUCRA, 84.3%), followed by Apixaban 5 mg in second place (SUCRA, 83.9%), Edoxaban 30 mg in third place (SUCRA, 80.0%), and Dabigatran 150 mg in last place (SUCRA, 19.9%, Fig. 5C). Figure 5D shows a symmetrical inverted funnel plot, indicating a possible small sample effect or publication bias in the study.
3.2.3. Intracranial hemorrhage
A total of 7 studies (79,003 patients), including 8 treatments (Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, Edoxaban 60 mg, Rivaroxaban 15 mg, Rivaroxaban 20 mg, and VKA) contributed to the clinical outcome of intracranial hemorrhage.
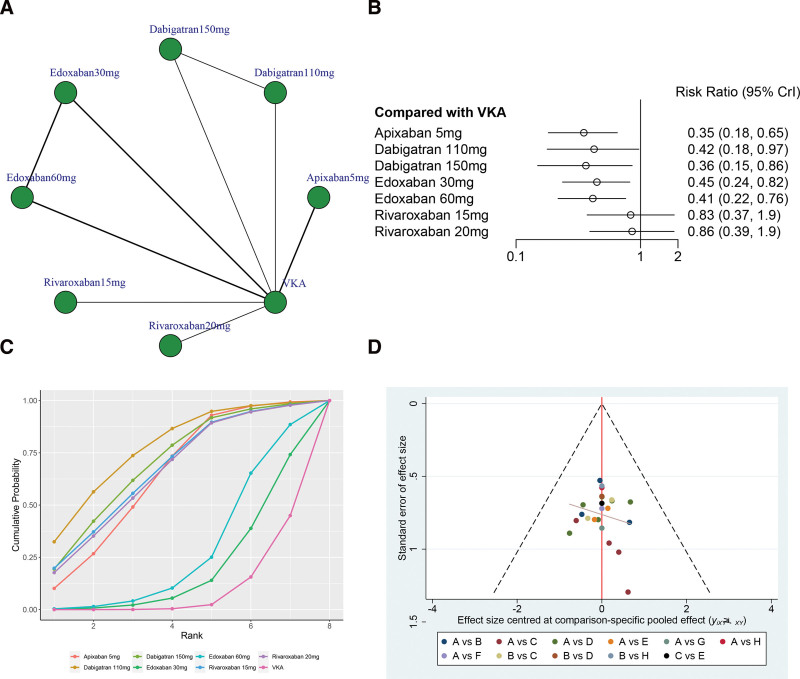
The network structure diagrams in Figure 6A compared different treatments for intracranial hemorrhage, revealing significant heterogeneity with a global I2 = 0%. In head-to-head comparison, Apixaban 5 mg (OR 0.35, 95% CrI 0.18–0.65), Dabigatran 110 mg (OR 0.42, 95% CrI 0.18–0.97), Dabigatran 150 mg (OR 0.36, 95% CrI 0.15–0.86), Edoxaban 30 mg (OR 0.45, 95% CrI 0.24–0.82), and Edoxaban 60 mg (OR 0.41, 95% CrI 0.22–0.76) was associated with a reduction of the intracranial hemorrhage rate than the VKA, and the difference was statistically significant. However, there was no statistically significant between Rivaroxaban 15 mg and Rivaroxaban 20 mg versus VKA in terms of the intracranial hemorrhage (P > .05, Fig. 6B).
Figure 6.
(A) Network structure diagrams of intracranial hemorrhage. (B) Forest plot of the intracranial hemorrhage as compared with VKA. (C) Surface under the cumulative ranking curve (SUCRA) probabilities of different treatments for intracranial hemorrhage. (D) Funnel plot of the different treatments for intracranial hemorrhage.
The SUCRA shows that Dabigatran 110 mg ranked first (SUCRA, 77.2%), Dabigatran 150 mg ranked second (SUCRA, 69.8%), Rivaroxaban 15 mg ranked third (SUCRA, 66.9%), and VKA ranked the last (SUCRA, 9.1%, Fig. 6C). Figure 6D shows a symmetrical inverted funnel plot, indicating a possible small sample effect or publication bias in the study.
3.2.4. All bleeding events
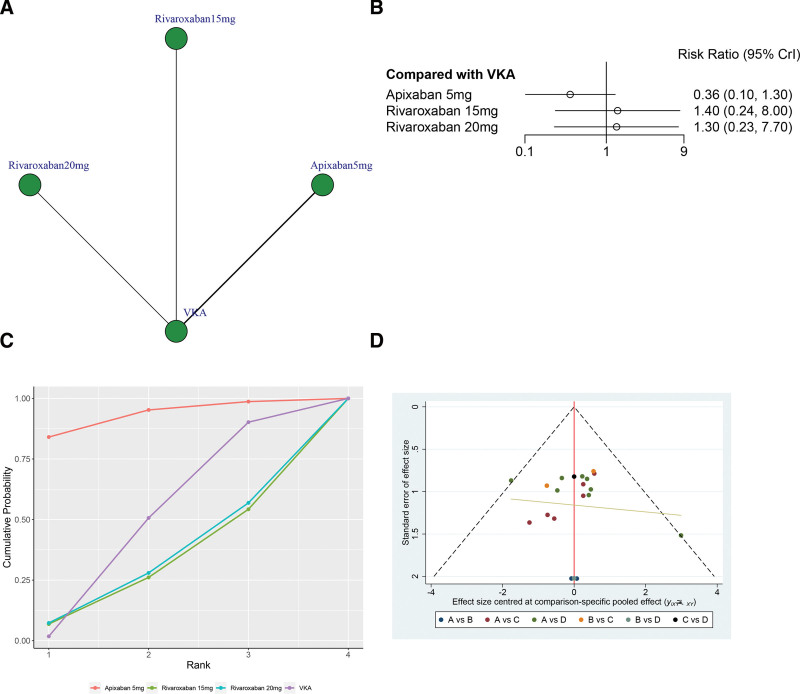
A total of 4 studies (51,858 patients), including 4 treatments (Apixaban 5 mg, Rivaroxaban 15 mg, Rivaroxaban 20 mg, and VKA) contributed to the clinical outcome of all bleeding events.
As illustrated in Figure 7A, the network structure diagrams provide a comprehensive overview of the direct comparisons among various treatments for all bleeding events. The network meta-analysis revealed significant heterogeneity, with a global I2 statistic of 0%. In head-to-head comparison, there was no statistically significant between any other treatments versus VKA in terms of the major bleeding (P > .05, Fig. 7B).
Figure 7.
(A) Network structure diagrams of all bleeding events. (B) Forest plot of the all bleeding events as compared with VKA. (C) Surface under the cumulative ranking curve (SUCRA) probabilities of different treatments for all bleeding events. (D) Funnel plot of the different treatments for all bleeding events.
According to the SUCRA analysis, Apixaban 5 mg demonstrated the highest ranking with a SUCRA value of 92.7%, followed by VKA in second place with a SUCRA value of 47.5%. Rivaroxaban 20 mg ranked third with a SUCRA value of 30.7%, and Rivaroxaban 15 mg ranked last with a SUCRA value of 29.1% (Fig. 7C). Figure 7D shows a symmetrical inverted funnel plot, indicating a possible small sample effect or publication bias in the study.
4. Discussion
A thorough analysis compared the effectiveness and safety of DOACs in preventing stroke in elderly AF patients. All DOACs were equally effective in patients aged 75 and older, except for Apixaban 5 mg, Dabigatran 110 mg, and Dabigatran 150 mg which showed slightly higher efficacy. All DOACs have similar risks of major bleeding compared to VKA. Rivaroxaban has a higher risk of hemorrhagic stroke compared to other DOACs and VKA. Apixaban and Edoxaban were found to lower the risk of major bleeding in elderly patients aged 80 and older compared to Factor IIa inhibitors and VKA.
AF and stroke become more important as people get older.[21] AF is most common in those aged 75 to 80 and continues to increase in prevalence after 90.[22] Older AF patients are at higher risk for ischemic stroke and intracerebral hemorrhage, making it challenging to manage AF in this age group.[23] A meta-analysis found that DOACs are more effective and safer than warfarin in patients aged 75 and older, especially in preventing thromboembolic and bleeding events.[21] Edoxaban showed similar efficacy to VKAs. This observation needs more validation through subgroup analyses from RCTs. Recent research found that reduced-dose Edoxaban was effective in older AF patients at high bleeding risk, reducing the risk of stroke or embolism without significantly increasing major bleeding.[14] These results were consistent across all age groups. The AVERROES trial showed that Apixaban was more effective and had a similar safety profile compared to aspirin in AF patients who could not take VKA.[13] More research is needed to compare DOACs in elderly patients and vulnerable populations with consistent control measures.
Bleeding risk is a key factor in evaluating the effectiveness of anticoagulants.[15] In trials, DOACs showed lower risks of intracerebral hemorrhage compared to VKA, but in older patients, all anticoagulants had similar major bleeding risks. Apixaban has reduced bleeding risks compared to VKA, while rivaroxaban has a slightly higher risk.[24] This suggests older patients may have a higher risk of bleeding with Rivaroxaban compared to the general population.[25] It is unclear if the increased risk of bleeding worsens with age, and the difference in bleeding risk between DOACs and VKA may not be as significant in older patients. The level of time spent in the therapeutic range during VKA therapy is also important, with older patients typically having higher time in therapeutic range (TTR). Meta-analyses show that DOACs have a lower risk of bleeding compared to warfarin, especially in patients from centers with lower TTR.[26] However, in trials like RE-LY and ROCKET AF, there was a trend of increased major bleeding risk with DOACs in patients with higher TTR.[24,25] Older subgroups, which often have higher TTR, likely contributed to the reduced safety benefits of DOACs in our study. Although VKA is still a valid treatment option when DOACs are not suitable, caution should be taken when interpreting the results of this study, as with all post hoc analyses. Our findings provide insights for future research and potential associations to explore further. In the context of treating AF and preventing stroke, the introduction of left atrial appendage closure devices, such as the Watchman device, represents a significant advancement in therapeutic options.[27] Left atrial appendage closure devices offer a non-pharmacological approach to stroke prevention in patients with non-valvular AF who are at increased risk for thromboembolic events.[28] The primary advantage of these devices is their ability to obviate the need for long-term anticoagulation therapy, which is particularly beneficial for patients who are contraindicated for or have a high bleeding risk with oral anticoagulants. The Watchman device, for instance, has been shown to be effective in reducing the risk of stroke in these patients, comparable to warfarin in certain studies.
The RE-LY and ENGAGE AF-TIMI 48 trials found that lower doses of Dabigatran and Edoxaban were more effective with similar safety profiles compared to higher doses.[12,15] High doses were associated with lower risks of stroke or embolism, but both high and low doses had similar safety outcomes, especially in older patients. Worries about bleeding risk are a major issue in anticoagulation therapy for older patients. Some physicians are hesitant to prescribe anticoagulants for stroke prevention in AF due to concerns about advanced age.[29] Lower dosages in older patients may seem like a safe choice, but studies show that underdosing could increase the risk of blood clots without reducing bleeding risk.[30] Our study highlights the need for higher doses of Dabigatran and Edoxaban to prevent AF-related strokes in older individuals. Dose adjustments should follow drug label guidelines, rather than just considering age. In this study, we considered that the dosage of DOACs might need to be adjusted based on patients’ renal function, body weight, age, and other clinical factors. According to the drug product labels and the latest clinical guidelines, we did implement a strategy of dose reduction for patients meeting specific criteria. Specifically, for patients with an estimated glomerular filtration rate of 15 to 30 mL/min/1.73 m², and/or a body weight <60 kg, we reduced the dosage of DOACs as recommended by the guidelines.
This study has limitations due to subgroup analyses within RCTs, potentially leading to underpowered comparisons among individual agents. We conducted a thorough literature review to strengthen statistical robustness, but caution is needed in interpreting the results. Differences in how bleeding events were defined and age groups were categorized in the trials led to varying clinical implications in the results. Our goal was to provide clinical guidance for treating older patients, not to definitively determine causes. We did not conduct further subgroup or meta-regression analyses due to the lack of individual participant data in this study-level meta-analysis. Further research focusing on older patients is necessary to confirm and build upon our results.
5. Conclusions
DOACs were found to have lower rates of thromboembolic events compared to VKAs in older patients with AF. Apixaban 5 mg, Dabigatran 110 mg, Dabigatran 150 mg, Edoxaban 30 mg, and Edoxaban 60 mg were also associated with a reduction of intracranial hemorrhage than VKA. Further analysis and validation from additional high-quality RCTs is needed to confirm the above conclusions.
Author contributions
Methodology: Xueli Lu.
Validation: Feng Liu, Xueli Lu.
Visualization: Feng Liu, Han Zhang.
Writing – original draft: Han Zhang.
Abbreviations:
- AF
- atrial fibrillation
- DOAC
- direct oral anticoagulant
- RCT
- randomized controlled trial
- SUCRA
- surface under the cumulative ranking curve
- TTR
- time in therapeutic range
- VKA
- vitamin K antagonist
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Zhang H, Liu F, Lu X. Efficacy and safety of different oral anticoagulants for stroke prevention in older patients with atrial fibrillation: A network meta-analysis. Medicine 2024;103:42(e39937).
Contributor Information
Feng Liu, Email: liufeng909@qq.com.
Xueli Lu, Email: luxueli909@qq.com.
References
- [1].Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- [2].Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li H, Song X, Liang Y, et al. Global, regional, and national burden of disease study of atrial fibrillation/flutter, 1990-2019: results from a global burden of disease study, 2019. BMC Public Health. 2022;22:2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- [5].Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. [DOI] [PubMed] [Google Scholar]
- [6].Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- [7].Mensah GA, Fuster V, Murray CJL, Roth GA. Global Burden of Cardiovascular Diseases and Risks, 1990-2022. J Am Coll Cardiol. 2023;82:2350–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- [9].Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–10. [DOI] [PubMed] [Google Scholar]
- [10].Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- [11].Singer DE, Chang Y, Fang MC, et al. Should patient characteristics influence target anticoagulation intensity for stroke prevention in nonvalvular atrial fibrillation?: the ATRIA study. Circ Cardiovasc Qual Outcomes. 2009;2:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- [13].Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- [14].Okumura K, Akao M, Yoshida T, et al. Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med. 2020;383:1735–45. [DOI] [PubMed] [Google Scholar]
- [15].Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- [16].Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- [17].Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation–the J-ROCKET AF study. Circ J. 2012;76:2104–11. [DOI] [PubMed] [Google Scholar]
- [18].Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–24. [DOI] [PubMed] [Google Scholar]
- [19].Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- [20].Van Mieghem NM, Unverdorben M, Hengstenberg C, et al. Edoxaban versus vitamin K antagonist for atrial fibrillation after TAVR. N Engl J Med. 2021;385:2150–60. [DOI] [PubMed] [Google Scholar]
- [21].Malik AH, Yandrapalli S, Aronow WS, Panza JA, Cooper HA. Meta-analysis of direct-acting oral anticoagulants compared with warfarin in patients >75 years of age. Am J Cardiol. 2019;123:2051–7. [DOI] [PubMed] [Google Scholar]
- [22].Kuroda M, Tamiya E, Nose T, et al. Effect of 15-mg edoxaban on clinical outcomes in 3 age strata in older patients with atrial fibrillation: a prespecified subanalysis of the ELDERCARE-AF randomized clinical trial. JAMA Cardiol. 2022;7:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ng KH, Shestakovska O, Connolly SJ, et al. Efficacy and safety of apixaban compared with aspirin in the elderly: a subgroup analysis from the AVERROES trial. Age Ageing. 2016;45:77–83. [DOI] [PubMed] [Google Scholar]
- [24].Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–83. [DOI] [PubMed] [Google Scholar]
- [25].Piccini JP, Hellkamp AS, Lokhnygina Y, et al. Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial. J Am Heart Assoc. 2014;3:e000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- [27].Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:3122–35. [DOI] [PubMed] [Google Scholar]
- [28].Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–75. [DOI] [PubMed] [Google Scholar]
- [29].Fumagalli S, Potpara TS, Bjerregaard Larsen T, et al. Frailty syndrome: an emerging clinical problem in the everyday management of clinical arrhythmias. The results of the European Heart Rhythm Association survey. Europace. 2017;19:1896–902. [DOI] [PubMed] [Google Scholar]
- [30].Chan YH, Chao TF, Chen SW, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm. 2020;17:2102–10. [DOI] [PubMed] [Google Scholar]