Abstract
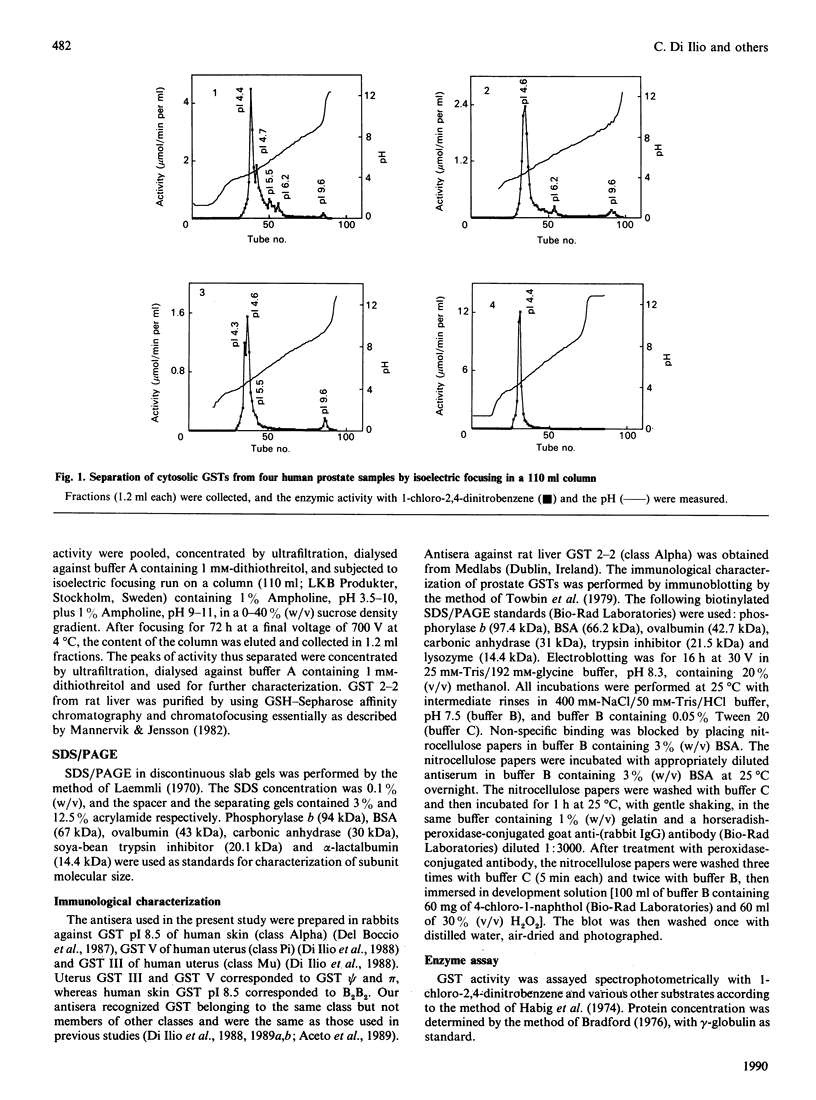
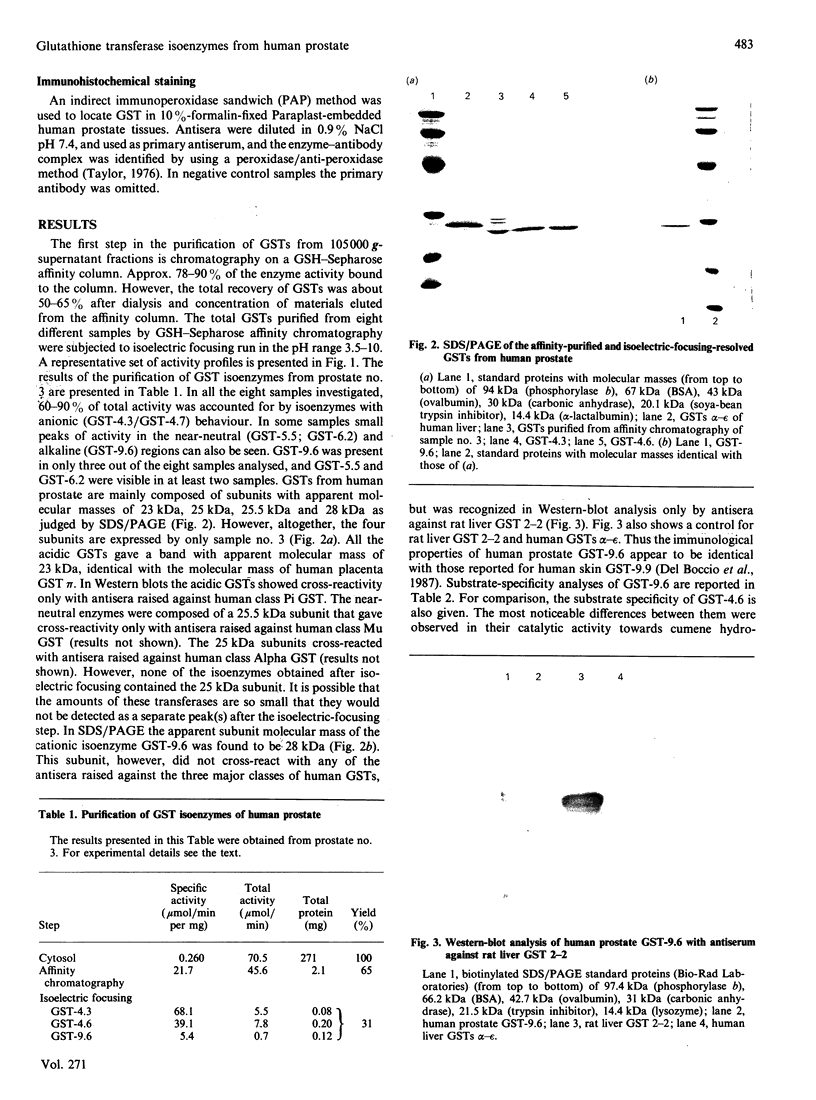
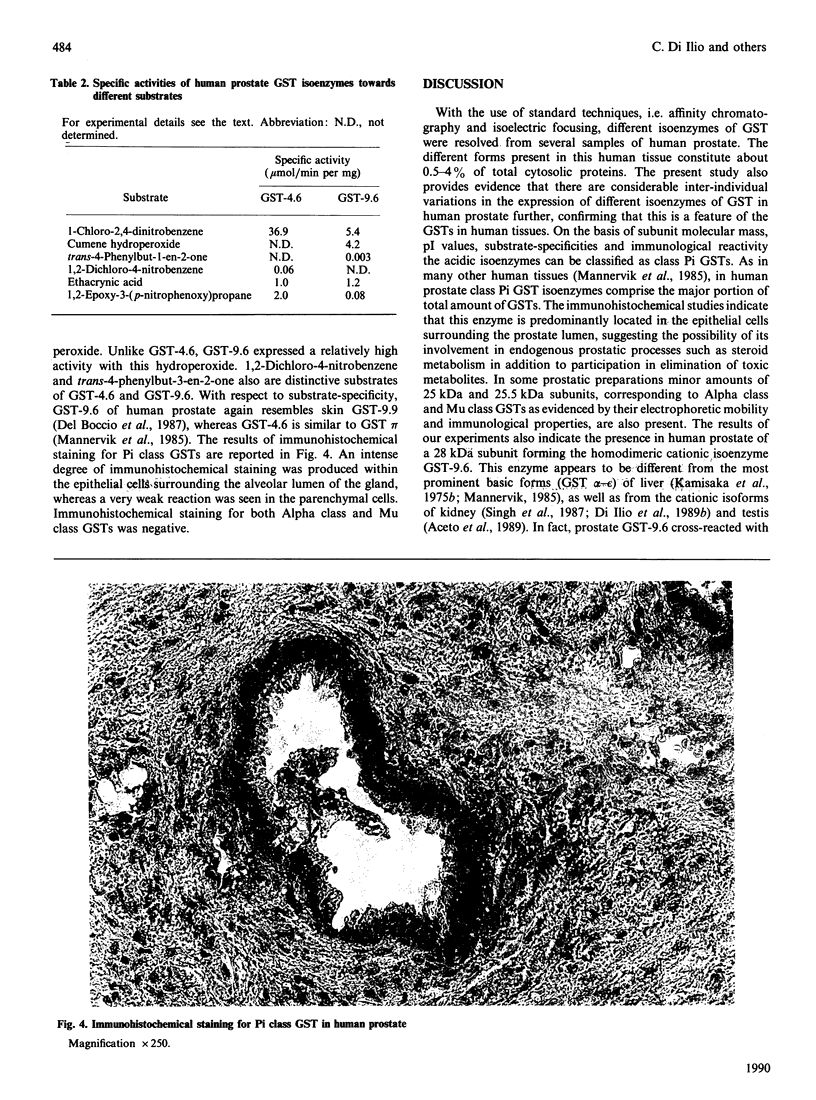
By using affinity-chromatography and isoelectric-focusing techniques, several forms of glutathione transferase (GSTs) were resolved from human prostate cytosol. All the three major classes of GST, i.e. Alpha, Mu and Pi, are present in human prostate. However, large inter-individual variation in the qualitative and quantitative expression of different isoenzymes resulted in the samples investigated. The most abundant group of prostate isoenzymes showed acid (pI 4.3-4.7) behaviour and were classified as Pi class GSTs on the basis of their immunological and structural properties. Immunohistochemical staining of Pi class GSTs was prevalently distributed in the epithelial cells surrounding the alveolar lumen. Class Mu GSTs are also expressed, although in small amounts and in a limited number of samples, by human prostate. The major cationic isoenzyme purified from prostate, GST-9.6; (pI 9.6; apparent subunit molecular mass of 28 kDa), appears to be different from the cationic GST alpha-epsilon forms isolated from human liver and kidney as evidenced by its structural, kinetical and immunological properties. This enzyme, which accounts for about 20-30% (on protein basis) of total amount of GSTs, is expressed by only 40% of samples. GST-9.6 has the ability to cross-react in immunoblotting analysis with antisera raised against rat liver GST 2-2, rather than with antisera raised against members of human Alpha, Mu and Pi class GSTs. Although prostate GST-9.6 shows close relationship with the human skin GST pI 9.9, it does not correspond to any other known human GST.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceto A., Di Ilio C., Angelucci S., Felaco M., Federici G. Glutathione transferase isoenzymes from human testis. Biochem Pharmacol. 1989 Nov 1;38(21):3653–3660. doi: 10.1016/0006-2952(89)90568-6. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Singh S. V. Subunit structure of human and rat glutathione S-transferases. Comp Biochem Physiol B. 1985;82(1):17–23. doi: 10.1016/0305-0491(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Del Boccio G., Di Ilio C., Alin P., Jörnvall H., Mannervik B. Identification of a novel glutathione transferase in human skin homologous with class alpha glutathione transferase 2-2 in the rat. Biochem J. 1987 May 15;244(1):21–25. doi: 10.1042/bj2440021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Del Boccio G., Casalone E., Pennelli A., Federici G. Purification and characterization of five forms of glutathione transferase from human uterus. Eur J Biochem. 1988 Feb 1;171(3):491–496. doi: 10.1111/j.1432-1033.1988.tb13816.x. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Piccolomini R., Allocati N., Faraone A., Cellini L., Ravagnan G., Federici G. Purification and characterization of three forms of glutathione transferase from Proteus mirabilis. Biochem J. 1988 Nov 1;255(3):971–975. doi: 10.1042/bj2550971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Aceto A., Zezza A., Ricci G., Federici G. Electrophoretic and immunological analysis of glutathione transferase isoenzymes of human kidney carcinoma. Biochem Pharmacol. 1989 Apr 1;38(7):1045–1051. doi: 10.1016/0006-2952(89)90247-5. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Del Boccio G., Massoud R., Federici G. Glutathione transferase of human breast is closely related to transferase of human placenta and erythrocytes. Biochem Int. 1986 Aug;13(2):263–269. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Gatmaitan Z., Arias I. M. Interactions of bilirubin and other ligands with ligandin. Biochemistry. 1975 May 20;14(10):2175–2180. doi: 10.1021/bi00681a021. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laisney V., Nguyen Van Cong, Gross M. S., Frezal J. Human genes for glutathione S-transferases. Hum Genet. 1984;68(3):221–227. doi: 10.1007/BF00418392. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Miller D. G., Beattie E. J. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986 May;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Singh S. V., Leal T., Ansari G. A., Awasthi Y. C. Purification and characterization of glutathione S-transferases of human kidney. Biochem J. 1987 Aug 15;246(1):179–186. doi: 10.1042/bj2460179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Faulder C. G., Davis B. A., Hume R., Brown J. A., Cotton W., Hopkinson D. A. The human glutathione S-transferases: studies on the tissue distribution and genetic variation of the GST1, GST2 and GST3 isozymes. Ann Hum Genet. 1984 Jan;48(Pt 1):11–20. doi: 10.1111/j.1469-1809.1984.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Coggan M., Shaw D. C., Board P. G. Electrophoretic and immunological analysis of human glutathione S-transferase isozymes. Ann Hum Genet. 1987 May;51(Pt 2):95–106. doi: 10.1111/j.1469-1809.1987.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Taylor C. R. An immunohistological study of follicular lymphoma, reticulum cell sarcoma and Hodgkin's disease. Eur J Cancer. 1976 Jan;12(1):61–75. doi: 10.1016/0014-2964(76)90125-0. [DOI] [PubMed] [Google Scholar]
- Tew K. D., Clapper M. L., Greenberg R. E., Weese J. L., Hoffman S. J., Smith T. M. Glutathione S-transferases in human prostate. Biochim Biophys Acta. 1987 Oct 8;926(1):8–15. doi: 10.1016/0304-4165(87)90177-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]