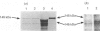Abstract
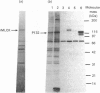
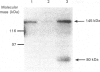
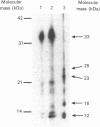
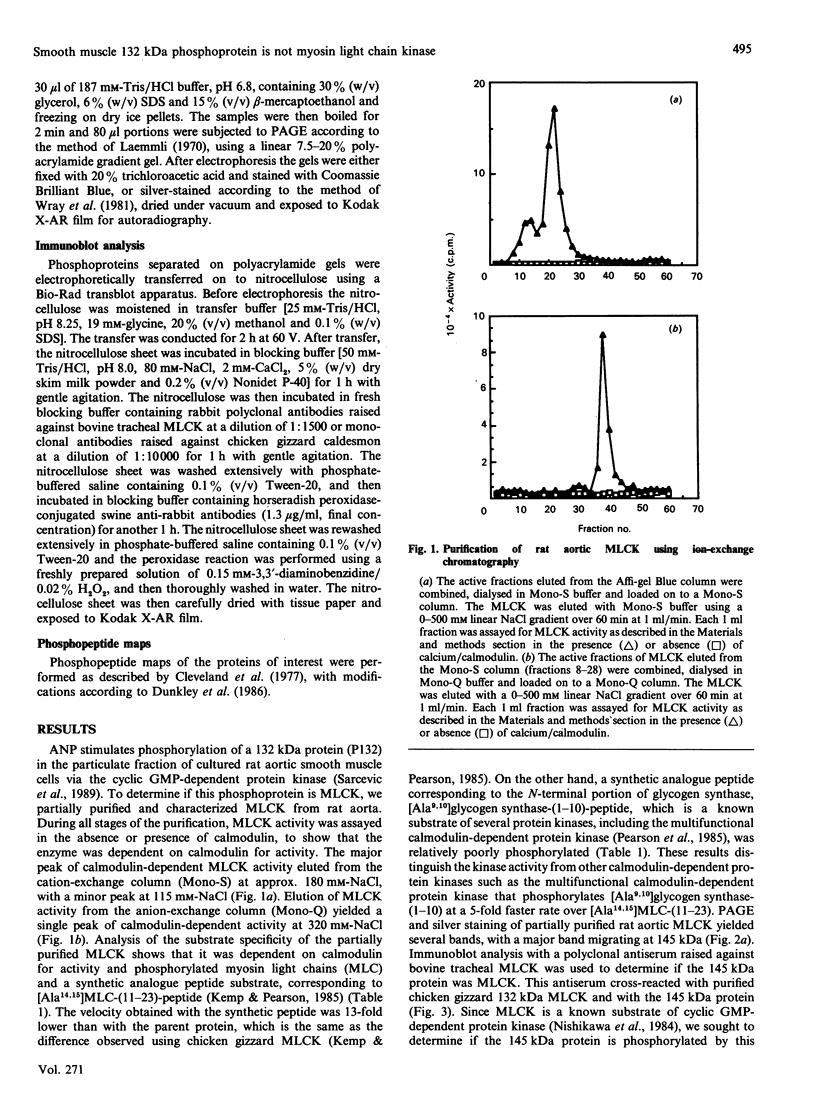
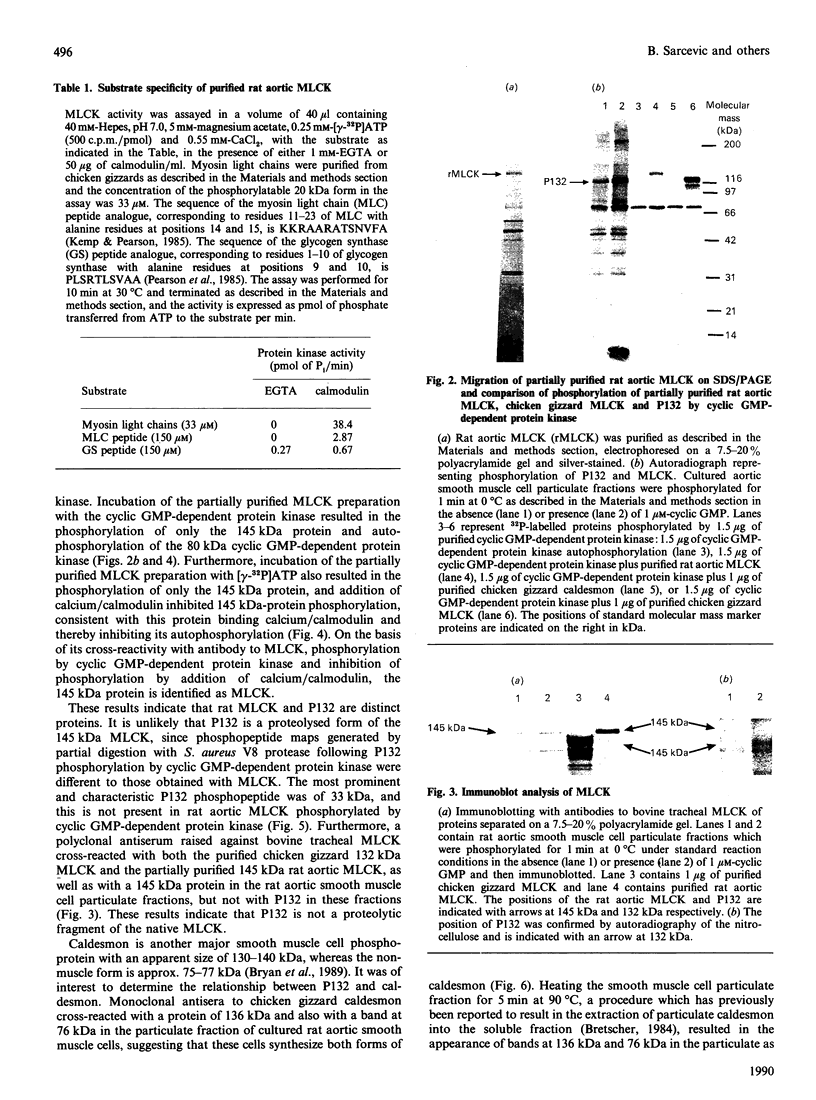
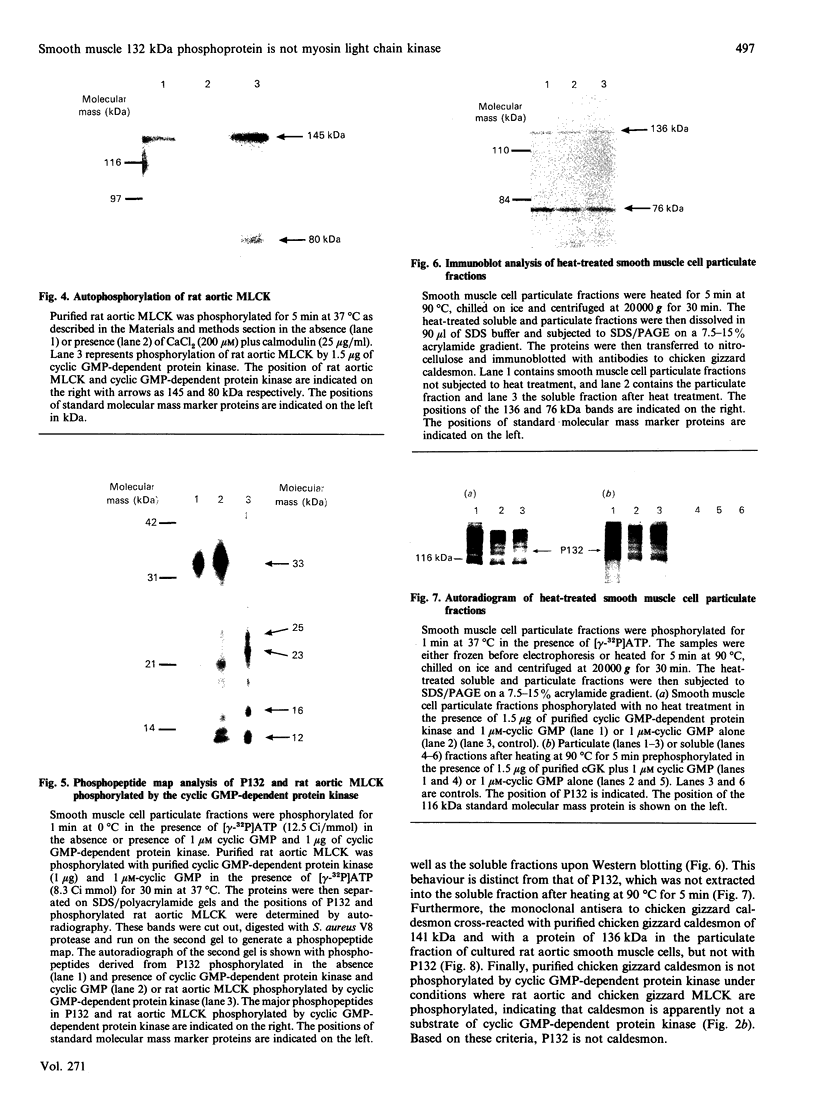
Atrial natriuretic peptide (ANP) stimulates the phosphorylation of three cyclic GMP-dependent protein kinase substrate proteins of 225, 132, and 11 kDa (P225, P132 and P11 respectively) in the particulate fraction of cultured rat aortic smooth muscle cells [Sarcevic, Brookes, Martin, Kemp & Robinson (1989) J. Biol. Chem. 264, 20648-20654]. Vrolix, Raeymaekers, Wuytack, Hofmann & Casteels [(1988) Biochem. J. 255, 855-863] have reported the presence of a 130 kDa cyclic GMP-dependent protein kinase substrate protein in the membrane fraction of pig aorta or stomach, and suggested that it may be myosin light chain kinase (MLCK). The aim of the present study was to determine whether P132 from rat aorta was MLCK or caldesmon. Although P132 co-migrates with purified chicken gizzard MLCK on SDS/polyacrylamide gels, it is distinct from rat aortic MLCK. Partially purified MLCK from rat aorta migrated as a 145 kDa protein on SDS/polyacrylamide gels. Immunoblotting the partially purified rat aortic MLCK with antibody to bovine tracheal MLCK identified rat aortic MLCK (145 kDa) and a corresponding 145 kDa protein in the particulate fraction of cultured rat aortic smooth muscle cells, but did not detect the 132 kDa protein. Phosphopeptide maps of purified rat aortic MLCK prepared by digestion with Staphylococcus aureus V8 protease were distinct from those of P132. P132 was not caldesmon, since antibodies to caldesmon cross-reacted with 136 and 76 kDa proteins in the particulate fraction of rat aortic cells, but not with P132. Furthermore, caldesmon was partially extracted from the particulate into the soluble fraction by heating at 90 degrees C, whereas P132 was not. These results demonstrate that the ANP-responsive cyclic GMP-dependent protein kinase substrate of 132 kDa from rat aortic smooth muscle cells is not MLCK or caldesmon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam L. P., Haeberle J. R., Hathaway D. R. Phosphorylation of caldesmon in arterial smooth muscle. J Biol Chem. 1989 May 5;264(13):7698–7703. [PubMed] [Google Scholar]
- Adelstein R. S., Conti M. A., Hathaway D. R., Klee C. B. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3': 5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Dec 10;253(23):8347–8350. [PubMed] [Google Scholar]
- Baltensperger K., Carafoli E., Chiesi M. The Ca2+-pumping ATPase and the major substrates of the cGMP-dependent protein kinase in smooth muscle sarcolemma are distinct entities. Eur J Biochem. 1988 Feb 15;172(1):7–16. doi: 10.1111/j.1432-1033.1988.tb13848.x. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Casnellie J. E., Greengard P. Guanosine 3':5'-cyclic monophosphate-dependent phosphorylation of endogenous substrate proteins in membranes of mammalian smooth muscle. Proc Natl Acad Sci U S A. 1974 May;71(5):1891–1895. doi: 10.1073/pnas.71.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989 Jan 15;264(2):1146–1155. [PubMed] [Google Scholar]
- Dedman J. R., Kaetzel M. A. Calmodulin purification and fluorescent labeling. Methods Enzymol. 1983;102:1–8. doi: 10.1016/s0076-6879(83)02003-0. [DOI] [PubMed] [Google Scholar]
- Dunkley P. R., Baker C. M., Robinson P. J. Depolarization-dependent protein phosphorylation in rat cortical synaptosomes: characterization of active protein kinases by phosphopeptide analysis of substrates. J Neurochem. 1986 Jun;46(6):1692–1703. doi: 10.1111/j.1471-4159.1986.tb08486.x. [DOI] [PubMed] [Google Scholar]
- Fiscus R. R., Rapoport R. M., Waldman S. A., Murad F. Atriopeptin II elevates cyclic GMP, activates cyclic GMP-dependent protein kinase and causes relaxation in rat thoracic aorta. Biochim Biophys Acta. 1985 Jul 30;846(1):179–184. doi: 10.1016/0167-4889(85)90124-7. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Nakamura H. Cyclic GMP regulation of the plasma membrane (Ca2+-Mg2+)ATPase in vascular smooth muscle. J Biochem. 1987 Jan;101(1):287–290. doi: 10.1093/oxfordjournals.jbchem.a121904. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Merrifield R. B. Monitoring of solid phase peptide synthesis by an automated spectrophotometric picrate method. Anal Biochem. 1975 May 12;65(1-2):241–272. doi: 10.1016/0003-2697(75)90509-6. [DOI] [PubMed] [Google Scholar]
- Hogaboom G. K., Emler C. A., Butcher F. R., Fedan J. S. Concerted phosphorylation of endogenous tracheal smooth muscle membrane proteins by Ca2+ . calmodulin-, cyclic GMP- and cyclic AMP-dependent protein kinases. FEBS Lett. 1982 Mar 22;139(2):309–312. doi: 10.1016/0014-5793(82)80877-6. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., House C. Phosphorylation of a synthetic heptadecapeptide by smooth muscle myosin light chain kinase. J Biol Chem. 1982 Nov 25;257(22):13349–13353. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., House C. Role of basic residues in the phosphorylation of synthetic peptides by myosin light chain kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7471–7475. doi: 10.1073/pnas.80.24.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Spatial requirements for location of basic residues in peptide substrates for smooth muscle myosin light chain kinase. J Biol Chem. 1985 Mar 25;260(6):3355–3359. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M. Effects of nitroprusside and 8-bromo-cyclic GMP on the contractile activity of the rat aorta. J Pharmacol Exp Ther. 1983 Jan;224(1):100–107. [PubMed] [Google Scholar]
- Lincoln T. M. cGMP-dependent protein kinase. Methods Enzymol. 1983;99:62–71. doi: 10.1016/0076-6879(83)99041-9. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Properties of caldesmon isolated from chicken gizzard. Biochem J. 1985 Sep 15;230(3):695–707. doi: 10.1042/bj2300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. The effects of phosphorylation of smooth-muscle caldesmon. Biochem J. 1987 Jun 1;244(2):417–425. doi: 10.1042/bj2440417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T. M., Adelstein R. S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem. 1984 Jul 10;259(13):8429–8436. [PubMed] [Google Scholar]
- Parks T. P., Nairn A. C., Greengard P., Jamieson J. D. The cyclic nucleotide-dependent phosphorylation of aortic smooth muscle membrane proteins. Arch Biochem Biophys. 1987 Jun;255(2):361–371. doi: 10.1016/0003-9861(87)90404-8. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Woodgett J. R., Cohen P., Kemp B. E. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985 Nov 25;260(27):14471–14476. [PubMed] [Google Scholar]
- Raeymaekers L., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J. 1988 May 15;252(1):269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Sarcevic B., Brookes V., Martin T. J., Kemp B. E., Robinson P. J. Atrial natriuretic peptide-dependent phosphorylation of smooth muscle cell particulate fraction proteins is mediated by cGMP-dependent protein kinase. J Biol Chem. 1989 Dec 5;264(34):20648–20654. [PubMed] [Google Scholar]
- Vorotnikov A. V., Shirinsky V. P., Gusev N. B. Phosphorylation of smooth muscle caldesmon by three protein kinases: implication for domain mapping. FEBS Lett. 1988 Aug 29;236(2):321–324. doi: 10.1016/0014-5793(88)80047-4. [DOI] [PubMed] [Google Scholar]
- Vrolix M., Raeymaekers L., Wuytack F., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988 Nov 1;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Davies P. J., Pastan I. Cyclic AMP-dependent phosphorylation of filamin in mammalian smooth muscle. J Biol Chem. 1978 Jul 10;253(13):4739–4745. [PubMed] [Google Scholar]
- Walsh M. P., Hinkins S., Dabrowska R., Hartshorne D. J. Smooth muscle myosin light chain kinase. Methods Enzymol. 1983;99:279–288. doi: 10.1016/0076-6879(83)99063-8. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Itoh K., Sobue K., Mori T., Shibata N. Purification of caldesmon and myosin light chain (MLC) kinase from arterial smooth muscle: comparisons with gizzard caldesmon and MLC kinase. J Biochem. 1987 Jan;101(1):1–9. doi: 10.1093/oxfordjournals.jbchem.a121879. [DOI] [PubMed] [Google Scholar]