Abstract
Introduction:
A large epiphrenic esophageal diverticulum can cause troublesome symptoms for patients, including dysphagia and reflux, ultimately, leading to debilitating weight loss.
Case Description/Technique Description:
We present a case of a 68-year-old female with a history of systemic lupus erythematosus presented with a large epiphrenic esophageal diverticulum with dysphagia, gastroesophageal reflux disease, and associated weight loss. The patient underwent a robotic-assisted laparoscopic epiphrenic diverticulectomy with esophageal myotomy. Intraoperative findings were consistent with epiphrenic esophageal diverticulum 7.5 × 6.0 × 4.0 cm with severe adhesions to the pericardium and pleura bilaterally. The diverticulum was transected using a stapler, and a myotomy was performed on the opposite side of the diverticulectomy. The patient tolerated the surgery without complication and was discharged home on postoperative day 5. Pathology was consistent with moderate chronic inflammation.
Discussion:
The robotic trans hiatal approach offers a safe alternative to the transthoracic approach for the surgical management of epiphrenic diverticula.
Keywords: Epiphrenic diverticulum, Minimally invasive surgery, Robotic epiphrenic diverticulectomy
INTRODUCTION
Esophageal diverticula are a rare pathology with a prevalence of around 0.06–4% in the general population.1 It is categorized into pharyngoesophageal (Zenker), para bronchial (mid esophageal) and epiphrenic (supra diaphragmatic). True diverticulum has all three layers of the esophagus, namely, mucosa, submucosa and muscularis, however, false diverticulum will contain mucosa and submucosa only. Zenker and epiphrenic diverticulum are pulsion diverticula caused by increased intra luminal pressures secondary to abnormal esophageal motility. Epiphrenic diverticula are most often found within 10 cm of the gastroesophageal junction (GEJ).2 Patients can present with symptoms of heartburn, regurgitation of food, chest pain, indigestion, and acid reflux. Some patients might also have subsequent weight loss due to decreased oral intake secondary to the above-mentioned symptoms.3 For patients with debilitating symptoms, definitive surgical management is recommended. Surgical treatment includes resection of the diverticulum with a generous distal esophageal myotomy. Partial fundoplication can be performed in patients with lower esophageal sphincter (LES) dysfunction. Conventionally, the surgical approach was trans thoracic through a left posterolateral thoracotomy with good functional outcomes.4 With the advancement in minimally invasive surgery, laparoscopic trans hiatal approach is also feasible option with good outcome.5 It is only recently, that robotic surgery is being implemented in the treatment of epiphrenic diverticulum via trans hiatal approach. We present a successful outcome after robotic trans hiatal approach in a patient with symptomatic giant epiphrenic diverticulum.
CASE DETAILS
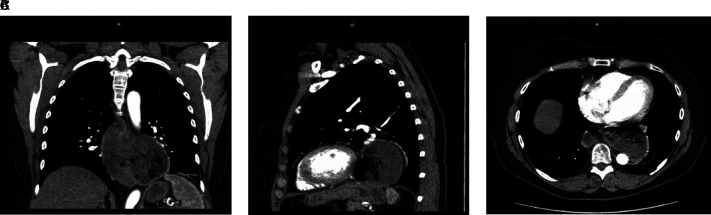
A 68-year-old female with history of systemic lupus erythematosus (SLE) presented with long standing symptoms of dysphagia, reflux of undigested food, and resultant weight loss due to poor oral intake. Her past medical history was significant for SLE, hypothyroidism, and cervical cancer. She has had laparoscopic cholecystectomy and total abdominal hysterectomy and bilateral salpingo-oophrectomy in the past. She was a nonsmoker. Her CT revealed a giant epiphrenic diverticulum measuring around 8 cm in size (Figures 1A, 1B, and 1C). Her esophagram revealed a large distal esophageal diverticulum (Figure 2). After discussion with the patient, a decision was made to proceed with robotic trans hiatal esophageal diverticulectomy with myotomy.
Figure 1.
(A) Coronal view of CT chest revealing large epiphrenic diverticulum. (B) Sagittal view of CT chest revealing large epiphrenic diverticulum. (C) Axial of CT chest revealing large epiphrenic diverticulum.
Figure 2.
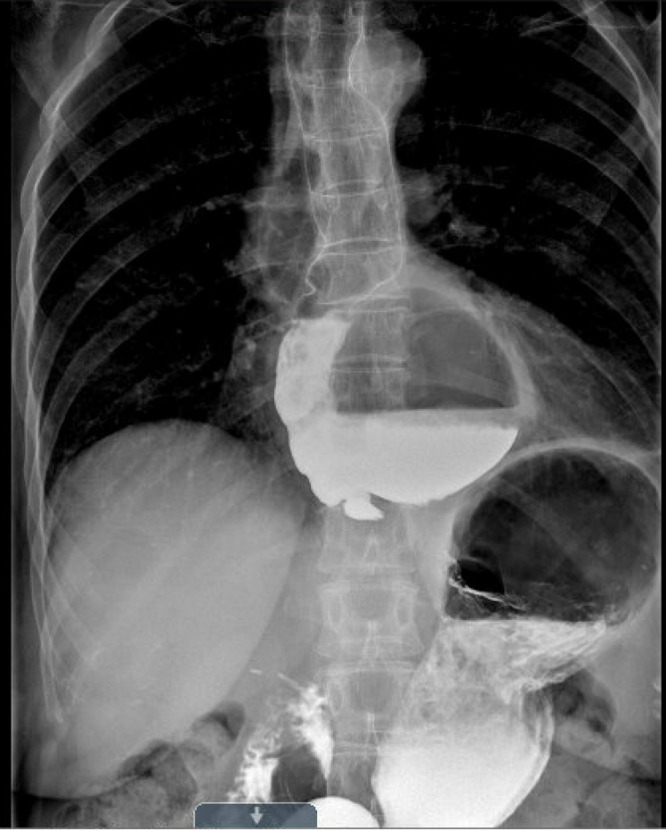
Preoperative esophagram study revealing a giant distal epiphrenic diverticulum.
The patient was placed in supine split leg position. 8 mm robotic trocars were placed in the midclavicular line in the right upper quadrant, right anterior axillary line, and supra umbilical region, a 12-mm port was placed in the left lower quadrant, and an assistant port was placed in the left upper quadrant in the anterior axillary line. Xi robot was docked, and the surgery was initiated with entry into the lesser sac after the greater omentum was taken down. Trans hiatal dissection was performed to gain access to the diverticulum. The diverticulum was found to measure 7.5 × 6.0 × 4.0 cm. Due to the severe desmoplastic reaction, there were dense adhesions surrounding the diverticulum; hence, a small tear was made in the left pleura during the dissection. Due to the extensive dissection and prolonged duration of the surgery, a residual postoperative pneumothorax was anticipated. Hence, a pigtail was placed for decompression. This was removed on postoperative day (POD)2 and did not contribute to an increase in the length of stay.
A 44 Fr bougie was advanced into the stomach, and the diverticulum was transected longitudinally with a tan load of 60-mm Endo GIA stapler. The bougie was replaced with an NG tube. The staple line was reinforced in a continuous fashion using an absorbable suture. A 10-cm Heller myotomy was performed on the opposite side of the diverticulum. Intraoperative esophagogastroduodenoscopy (EGD) was performed, and a hydropneumatic test for staple line leak was negative. A cruroplasty was performed with confirmation of adequate closure of the hiatus without narrowing of the esophagus. The intra-abdominal portion of the staple line was covered with omentum. No hiatal hernia could be appreciated at the end of the procedure.
On POD2, the patient’s upper gastrointestinal series (UGI) did not reveal contrast extravasation (Figure 3). Her NG tube was removed on POD4 and she was started on a liquid diet. Her thoracostomy tube was also removed on POD4. She was discharged on POD6.
Figure 3.
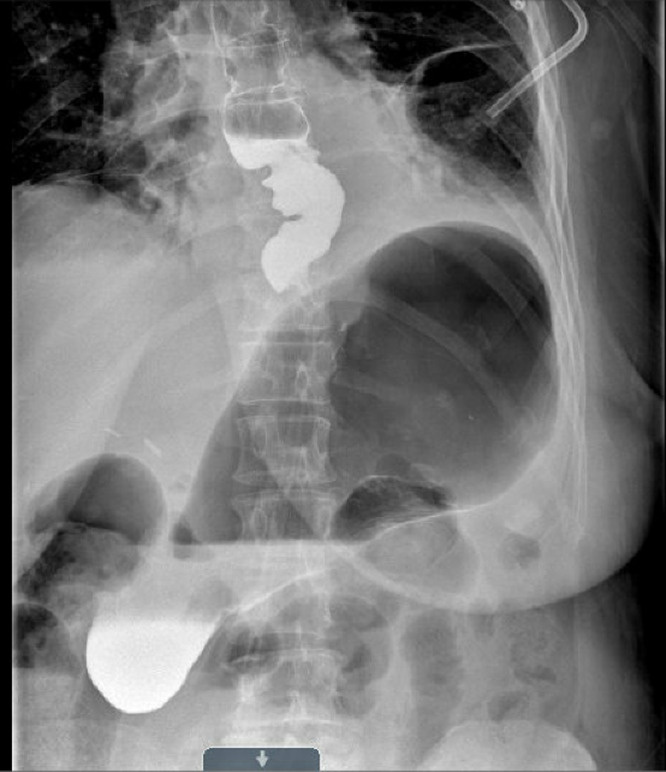
Postoperative esophagram.
She had complete symptom resolution on follow up clinic visit 2 weeks from surgery. Her diet was advanced to regular diet. On 3 months follow up, she remained symptom free. Her histopathology revealed esophageal tissue with moderate chronic inflammation.
DISCUSSION
Esophageal diverticula are categorized according to their location. Zenker is pharyngoesophageal, mid esophageal diverticula are para bronchial and supra diaphragmatic are epiphrenic. True or traction diverticula are a result of a mechanical external pull by a mediastinal mass (e.g., lymph nodes). The esophagus that is adherent to the mass gradually develops an outpouching due to this force resulting in a true diverticulum (with all 3 layers of the esophageal wall). A false pulsion diverticulum is due to increased intra luminal pressure secondary to the esophageal dysmotility. This results in mucosa and submucosa herniating through the muscular wall.2
Epiphrenic diverticula are classified as false pulsion diverticula that occur in the distal esophagus within 10 cm of the gastroesophageal junction. Diagnosis is established with barium esophagram however, manometric studies can be utilized to evaluate for underlying motility disorder.6 Achalasia is the most documented disorder associated with these diverticula, however, the diffuse esophageal spasm, hypercontractile peristalsis, and hypertensive LES are also found to be associated with their formation7 Our patient had systemic sclerosis, which contributed to the impaired motility of the esophagus and, hence, predisposed her to developing a pulsion diverticulum.8
Diagnosis can be made with an esophagram and CT scan. EGD can help with direct visualization of the diverticular ostium and rule out possible neoplastic lesions. Manometry assists with assessment of overall motility of the esophagus and peristalsis derangements. Patients with debilitating symptoms are offered surgical management as definitive treatment.9
Effective surgical management requires a stapled diverticulectomy along with an esophageal myotomy, with or without anti reflux procedure, for wide neck or large diverticula.10
There has always been a debate between trans hiatal vs trans thoracic approach for esophageal procedures. Trans thoracic approach has a higher risk of pulmonary complications, chylous leaks, and wound infections. Trans hiatal approach has increased risk of recurrent laryngeal nerve injury. In patients with esophageal cancer, trans hiatal approach has an increased incidence of anastomotic leak, however, due to its location, it is not as morbid as a trans thoracic leak. Trans thoracic approach is also associated with longer stay in ICU and an increased mortality.11
In recent years, the advancement in minimally invasive surgery has shifted the paradigm from open to minimally invasive surgery. Among its benefits are faster recovery time, decreased post operative pain and shorter hospital stays.12
Robotic surgery has further made superior 3-dimensional visualization and better depth perception possible.13
Our patient had long standing symptoms with a giant epiphrenic diverticulum. There was a dense desmoplastic reaction from years of inflammation. Hence, robotic approach greatly facilitated with the dissection.
Sommer et al. recently reported a similar case of robotic trans hiatal approach for epiphrenic diverticulectomy for a giant diverticulum measuring 7 × 7 cm with esophageal myotomy. Their patient progressed well and was discharged on POD4.14
Pernazza et al. also describe a similar trans hiatal approach for a patient with an epiphrenic diverticulum with Dor fundoplication with the patient resuming oral intake on POD6 with no return of symptoms.15
CONCLUSION
Epiphrenic diverticulum, even though rare, can be a very debilitating disease. Its definitive treatment is surgical, with options of transthoracic or hiatal approach. Performing a contralateral myotomy is essential for the long-term success of the procedure. Although in its infancy, the robotic trans hiatal approach offers a promising alternative to the conventional transthoracic approach. However, multicenter trials are needed before it can be rendered the standard of care.
Footnotes
Disclosure: none.
Conflict of interests: none.
Funding sources: none.
Contributor Information
Najiha Farooqi, Department of Surgery, Central Michigan University, Saginaw, MI. (Drs. Farooqi, Lossia, Pacheco, Shaheen, and Ghanem).
Olivia Lossia, Department of Surgery, Central Michigan University, Saginaw, MI. (Drs. Farooqi, Lossia, Pacheco, Shaheen, and Ghanem).
Felipe Pacheco, Department of Surgery, Central Michigan University, Saginaw, MI. (Drs. Farooqi, Lossia, Pacheco, Shaheen, and Ghanem).
Samuel Shaheen, Department of Surgery, Central Michigan University, Saginaw, MI. (Drs. Farooqi, Lossia, Pacheco, Shaheen, and Ghanem).
Maher Ghanem, Department of Surgery, Central Michigan University, Saginaw, MI. (Drs. Farooqi, Lossia, Pacheco, Shaheen, and Ghanem).
References:
- 1.Herbella FAM, Patti MG. Modern pathophysiology and treatment of esophageal diverticula. Langenbecks Arch Surg. 2012;397(1):29–35. [DOI] [PubMed] [Google Scholar]
- 2.Malangoni MA, Rosen MJ. Sabiston Textbook of Surgery, 20th ed. Sabiston Textbook of Surgery: the Biological Basis of Modern Surgical Practice. 2017. [Google Scholar]
- 3.Yuan M-C, Chou C-K, Chen C-C, Wang H-P, Wu J-F, Tseng P-H. Characteristics of esophageal motility and associated symptom profiles in patients with esophageal diverticulum: a study based on high-resolution impedance manometry. Dig Dis Sci. 2024;69(2):510–520. [DOI] [PubMed] [Google Scholar]
- 4.Varghese TK, Marshall B, Chang AC, Pickens A, Lau CL, Orringer MB. Surgical treatment of epiphrenic diverticula: a 30-year experience. Ann Thorac Surg. 2007;84(6):1801–1809. [DOI] [PubMed] [Google Scholar]
- 5.Soares RV, Montenovo M, Pellegrini CA, Oelschlager BK. Laparoscopy as the initial approach for epiphrenic diverticula. Surg Endosc. 2011;25(12):3740–3746. [DOI] [PubMed] [Google Scholar]
- 6.Smith CD. Esophageal strictures and diverticula. Surg Clin North Am. 2015;95(3):669–681. [DOI] [PubMed] [Google Scholar]
- 7.Vicentine FPP, Herbella FAM, Silva LC, Patti MG. High resolution manometry findings in patients with esophageal epiphrenic diverticula. Am Surg. 2011;77(12):1661–1664. [PubMed] [Google Scholar]
- 8.Li B, Yan J, Pu J, Tang J, Xu S, Wang X. Esophageal dysfunction in systemic sclerosis: an update. Rheumatol Ther. 2021;8(4):1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbella FAM, Patti MG. Achalasia and epiphrenic diverticulum. World J Surg. 2015;39(7):1620–1624. [DOI] [PubMed] [Google Scholar]
- 10.Andolfi C, Wiesel O, Fisichella PM. Surgical treatment of epiphrenic diverticulum: technique and controversies. J Laparoendosc Adv Surg Tech A. 2016;26(11):905–910. [DOI] [PubMed] [Google Scholar]
- 11.Barreto JC, Posner MC. Transhiatal versus transthoracic esophagectomy for esophageal cancer. World J Gastroenterol. 2010;16(30):3804–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackroyd R, Watson DI, Majeed AW, Troy G, Treacy PJ, Stoddard CJ. Randomized clinical trial of laparoscopic versus open fundoplication for gastro-oesophageal reflux disease. Br J Surg. 2004;91(8):975–982. [DOI] [PubMed] [Google Scholar]
- 13.Wong SW, Crowe P. Visualisation ergonomics and robotic surgery. J Robot Surg. 2023;17(5):1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommer R, Machado Grossi JV, Grossi Harada GR, Seabra MK, Cavazzola LT, Seabra AP. Treatment of giant esophageal epiphrenic diverticulum using robotic-assisted surgery. CRSLS. 2022;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pernazza G, Monsellato I, Pende V, Alfano G, Mazzocchi P, D’Annibale A. Fully robotic treatment of an epiphrenic diverticulum: report of a case. Minim Invasive Ther Allied Technol. 2012;21(2):96–100. [DOI] [PubMed] [Google Scholar]