Abstract
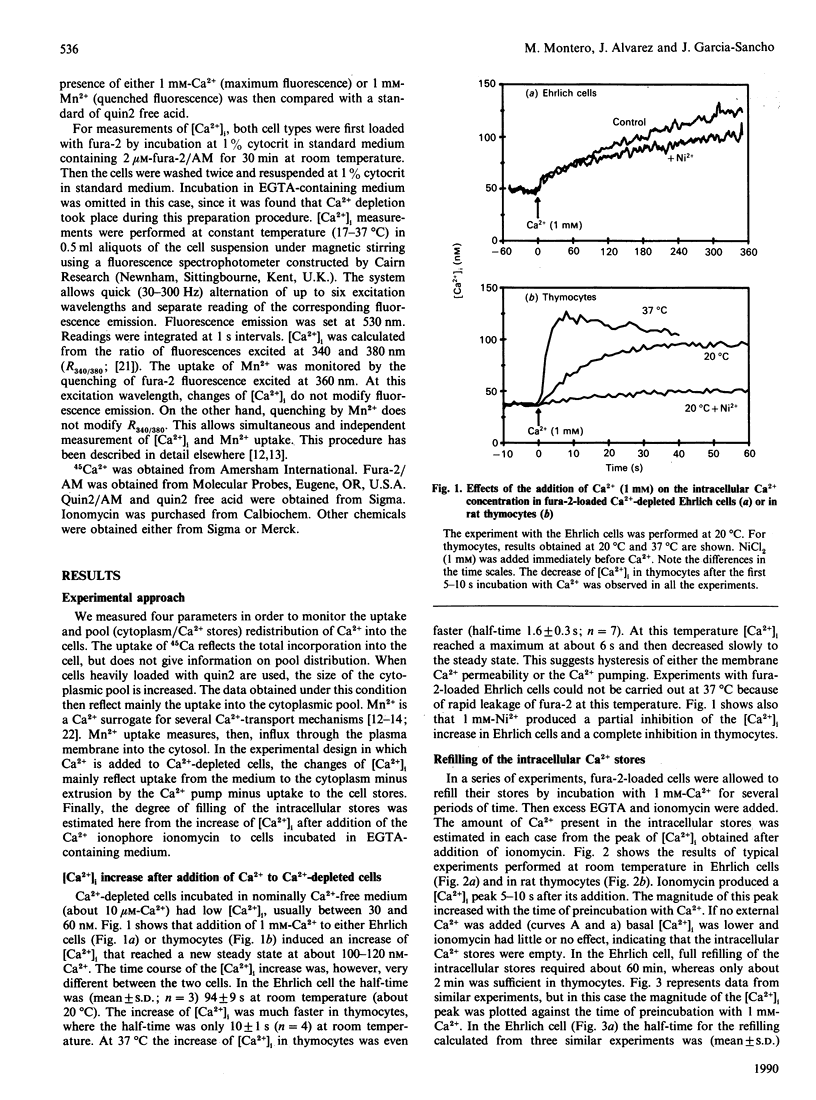
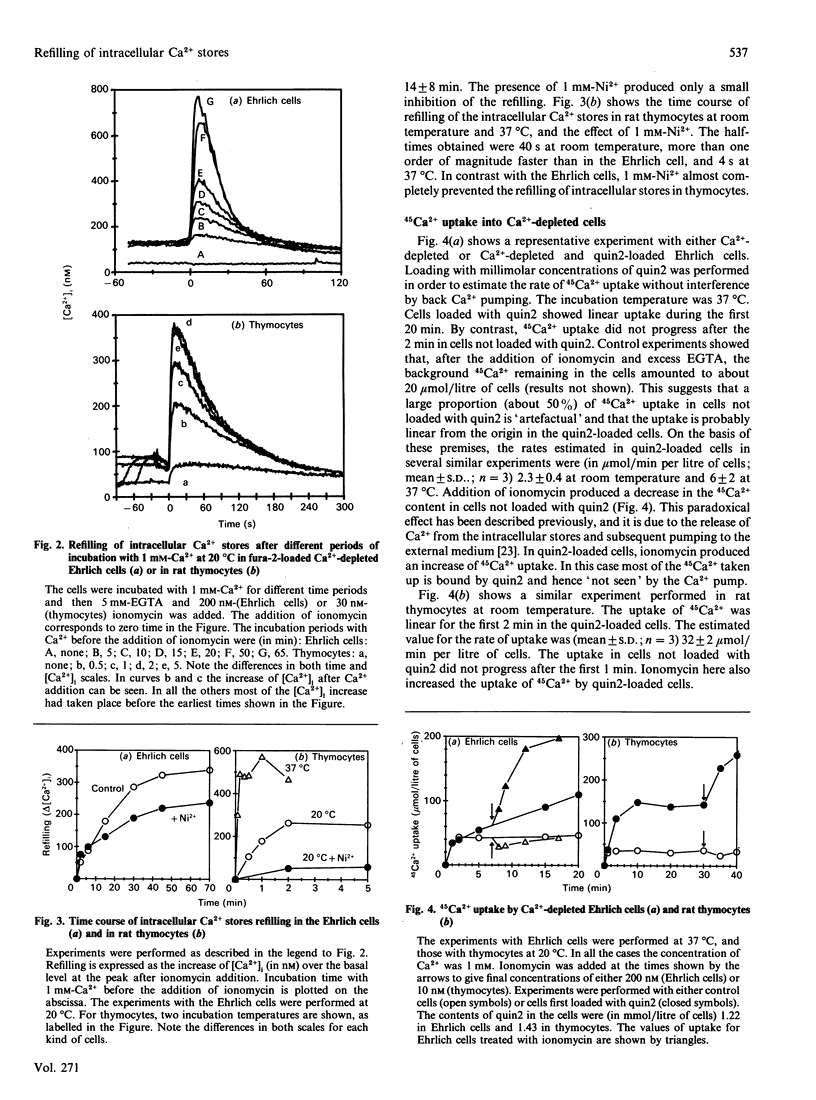
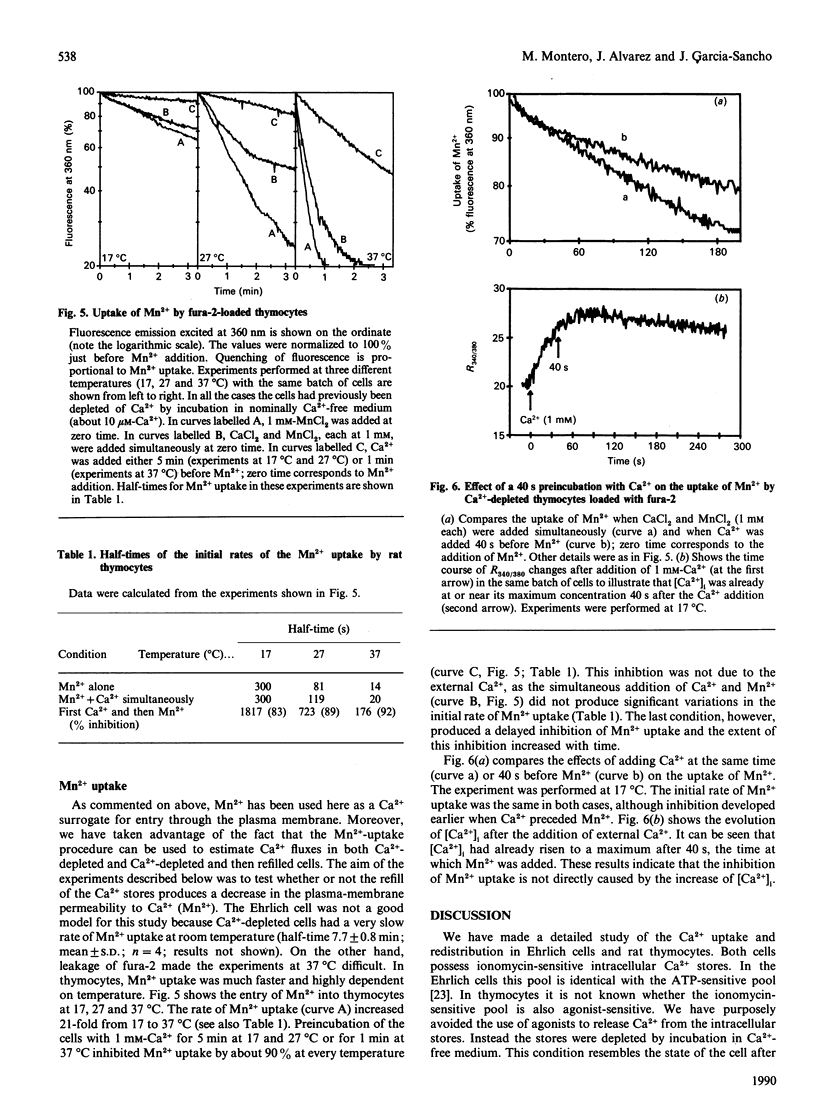
We have studied the uptake of Ca2+ and its redistribution between the cytoplasm and the intracellular stores in Ehrlich-ascites-tumour cells and rat thymocytes previously depleted of Ca2+ by incubation in Ca2(+)-free medium. Measurements included changes of the cytoplasmic Ca2+ concentration ([Ca2+]i), uptake of 45Ca2+ and uptake of Mn2+, a Ca2+ surrogate for Ca2+ channels. Refilling of the Ca2+ stores in thymocytes was very fast (half-filling time: 4 s at 37 degrees C) and very sensitive to temperature (10 times slower at 20 degrees C). It was always preceded by increase of [Ca2+]i. In the Ehrlich cell, both refilling and increase of [Ca2+]i were about one order of magnitude slower. The increase of [Ca2+]i and the refilling of the intracellular stores were both almost completely blocked by Ni2+ in thymocytes, but only partially in the Ehrlich cell. The rates of 45Ca2+ and Mn2+ uptake varied consistently with temperature and the kind of cell. These results suggest that the intracellular stores are refilled by Ca2+ taken up from the cytoplasm. We also find that filling of the Ca2+ stores decreases by about 90% the rate of Mn2+ uptake in thymocytes. This is direct evidence of modulation of the plasma-membrane Ca2+ entry by the degree of filling of the intracellular stores. This modulation occurs in the absence of agonists, suggesting some kind of signalling between the intracellular stores and the Ca2+ entry pathways of the plasma membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. T., Sanchez A., García-Sancho J. Monitoring of the activation of receptor-operated calcium channels in human platelets. Biochem Biophys Res Commun. 1989 Jul 14;162(1):24–29. doi: 10.1016/0006-291x(89)91956-6. [DOI] [PubMed] [Google Scholar]
- Artalejo A. R., García-Sancho J. Mobilization of intracellular calcium by extracellular ATP and by calcium ionophores in the Ehrlich ascites-tumour cell. Biochim Biophys Acta. 1988 Jun 7;941(1):48–54. doi: 10.1016/0005-2736(88)90212-x. [DOI] [PubMed] [Google Scholar]
- Aub D. L., McKinney J. S., Putney J. W., Jr Nature of the receptor-regulated calcium pool in the rat parotid gland. J Physiol. 1982 Oct;331:557–565. doi: 10.1113/jphysiol.1982.sp014391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran D. T., Peck W. A., Frengley P. A., Lichtman M. A. Cortisol-induced inhibition of amino acid transport in thymic lymphocytes: kinetic parameters; relation to ATP levels and protein synthesis; and specificity. Biochim Biophys Acta. 1973 May 25;307(3):627–639. doi: 10.1016/0005-2736(73)90307-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Drapeau P., Nachshen D. A. Manganese fluxes and manganese-dependent neurotransmitter release in presynaptic nerve endings isolated from rat brain. J Physiol. 1984 Mar;348:493–510. doi: 10.1113/jphysiol.1984.sp015121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 1988 Aug;2(11):2670–2676. doi: 10.1096/fasebj.2.11.2456243. [DOI] [PubMed] [Google Scholar]
- Garcia-Sancho J., Alonso M. T., Sanchez A. Receptor-operated calcium channels in human platelets. Biochem Soc Trans. 1989 Dec;17(6):980–982. doi: 10.1042/bst0170980. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui Y., Christensen H. N. Discrimination of single transport systems. The Na plus-sensitive transport of neutral amino acids in the Ehrlich cell. J Gen Physiol. 1966 Sep;50(1):203–224. doi: 10.1085/jgp.50.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Time-dependent restoration of the trigger pool of calcium after termination of angiotensin II action in adrenal glomerulosa cells. J Biol Chem. 1987 Apr 5;262(10):4557–4563. [PubMed] [Google Scholar]
- Merritt J. E., Hallam T. J. Platelets and parotid acinar cells have different mechanisms for agonist-stimulated divalent cation entry. J Biol Chem. 1988 May 5;263(13):6161–6164. [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Muallem S. Calcium transport pathways of pancreatic acinar cells. Annu Rev Physiol. 1989;51:83–105. doi: 10.1146/annurev.ph.51.030189.000503. [DOI] [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Release and reloading of intracellular Ca stores after cholinergic stimulation of the parietal cell. Am J Physiol. 1988 Apr;254(4 Pt 1):C498–C504. doi: 10.1152/ajpcell.1988.254.4.C498. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Guesdon F., Claret M. A regulatory calcium-binding site for calcium channel in isolated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3289–3294. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Takemura H., Hughes A. R., Horstman D. A., Thastrup O. How do inositol phosphates regulate calcium signaling? FASEB J. 1989 Jun;3(8):1899–1905. doi: 10.1096/fasebj.3.8.2542110. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. Kinetic differences between thrombin-induced and ADP-induced calcium influx and release from internal stores in fura-2-loaded human platelets. Biochem Biophys Res Commun. 1986 May 14;136(3):1124–1129. doi: 10.1016/0006-291x(86)90450-x. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Takemura H., Putney J. W., Jr Capacitative calcium entry in parotid acinar cells. Biochem J. 1989 Mar 1;258(2):409–412. doi: 10.1042/bj2580409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Monck J. R. Hormone effects on cellular Ca2+ fluxes. Annu Rev Physiol. 1989;51:107–124. doi: 10.1146/annurev.ph.51.030189.000543. [DOI] [PubMed] [Google Scholar]


