Abstract
Introduction The endoscopic endonasal route has demonstrated to be the approach of choice for a large majority of clival chordomas (CCs). However, its results in elderly patients are under-evaluated in the literature. The aim of this study is to assess the surgical outcome for these patients, determining the factors associated with a larger tumor resection in this population.
Materials and Methods Our institutional database of CC has been retrospectively reviewed, to identify all cases over 65 years old, operated through an endoscopic endonasal approach (EEA). Preoperative clinical and radiological features were considered, as well as surgical results, morbidity, and patients' outcome at follow-up.
Results Out of our series of 143 endoscopic surgical procedures for CC, 34 (23.8%) were in patients older than 65 and 10 in older than 75 (7.0%). Gross tumor removal was achieved in 22 cases (64.7%). Complications consisted of 2 (5.9%) postoperative cerebrospinal leaks, 1 (2.9%) meningitis, 1 (2.9%) permanent cranial nerve VI palsy, 1 (2.9%) pneumonia, and 2 (5.9%) urinary infections. In 39.1% of cases, the preoperative ophthalmoplegia improved or resolved. Twenty-seven patients (79.4%) underwent radiation therapy. At follow-up (37.7 ± 44.9 months), 13 patients (38.2%) showed a recurrence/progression and 13 (38.3%) deceased.
Conclusion EEA can be a useful approach in elderlies, balancing the large tumor removal with an acceptable morbidity rate, even if higher than that for general CC population. However, patient selection remains crucial. A multidisciplinary evaluation is important to assess not only their medical conditions, but also their social and familiar conditions.
Keywords: chordomas, endoscopic endonasal approach, clivus, skull base, elderly, radiation therapy, outcome, complications, recurrences
Introduction
Chordomas have peculiar biological features: usually, they are slowly growing tumors, but they are also characterized by a local aggressiveness, often causing heavy neurological symptoms, with frequent local recurrences, potentially central nervous system (CSF) dissemination or even extra-CNS metastases. 1 Skull base and cranio-vertebral junction represent their most common locations, accounting for the 38.7% of all chordomas. 2 Nowadays, the current standard of treatment of clival chordomas (CCs) is a paradigm constituted by gross total surgical resection, when possible, followed by radiation therapy. 3 4 The approach of choice for a large majority of CCs is the endoscopic endonasal route, a direct, minimally invasive, extracranial and extradural corridor, which has demonstrated effectiveness and solid results for these cases. 3 5 6 7 8 9
A recent epidemiological investigation has demonstrated that the incidence of CC progressively increases with the patients age from a nadir between 0 and 19 years to a peak in the group of patients between 65 and 74, with a slight prevalence in men than in women. 2 Conversely, spinal chordomas tend to be diagnosed a decade later. 2 Therefore, CCs should be considered as common tumors also in the elderly and, thanks to the increasing life expectancy of the population and capillary diffusion of neuroradiological facilities, their diagnosis is becoming more and more frequent in patients older than 65 years. 2
However, the impact of comorbidities and of the fragilities typical of the elderly population on the current treatment paradigm for CC has been poorly considered in the literature, as well as the results and the risk of complications of the endoscopic endonasal approach (EEA). Indeed, most of the studies are focusing on adult patients with CC with a mean age between 40 and 58 years, not specifically considering the elderly patients. 10 Moreover, also the clinical manifestations of this subgroup of CC, their most common symptoms, biological behavior, or response to adjuvant treatment have not been specifically analyzed. The aim of this study is to review our series of CCs to analyze the clinical, radiological, and biological characteristics of those tumors diagnosed in patients older than 65 years and to assess their surgical and oncological outcome.
Materials and Methods
All consecutive CCs operated through an EEA in our center from 1998 (when the first clivus CC was operated with this technique at our Institution) to December 2022 have been retrospectively considered. Inclusion criteria were the histological confirmation of chordoma and the availability of clinical and radiological information both at diagnosis and at follow-up. Patients with a follow-up shorter than 3 months were excluded.
Patients older than 65 years old were considered for this study, while all the endoscopic endonasal procedures performed for younger patients were used as the control group. Elderly patients were divided into two groups: older than 65 (elderlies) and older than 75 (late elderlies).
All patients underwent a preoperative evaluation, including the collection of medical history, previous surgical or radiation treatment, and assessment of Karnofsky performance score (KPS). A neurological physical examination was performed, as well as biochemical tests for the pituitary function (basal in all patients, with stimulation or inhibition tests whenever required) and an ophthalmological assessment, including the visual field determination, in case respectively of tumors involving the sellar region or the opto-chiasmatic cistern. Preoperative radiological assessment consisted of a brain magnetic resonance imaging (MRI) with gadolinium contrast medium, a brain computed tomography (CT) with angiographic sequences (CTA), and a body CT-scan to exclude metastases. The preoperative MRI and CTA were used to define the tumor localization in the clivus (upper, middle, or lower clivus), and the presence of intra-dural extension, and they were merged and imported in the intra-operative neuronavigation system.
Our surgical technique has been already described elsewhere. 10 11 Surgical complications were retrieved from medical records. Gross total removal (GTR) was defined as absence of visible tumor remnants at 3 months follow-up MRI with gadolinium contrast medium, otherwise it was considered as subtotal if the residual tumor was less than 20% of the original mass, and partial if the tumor remnant was greater than 20% of the original neoplasm.
Neurological, endocrinological, and ophthalmological evaluations were repeated, according to the aforementioned criteria, after 3, 6, and 12 months and then annually to determine the patients' clinical outcome. All patients underwent a multidisciplinary discussion before surgery to plan the treatment protocol and the adjuvant treatments. MRIs were repeated at regular intervals (3–6 months) to assess tumor recurrence or progression. Quality of life (QOL) was evaluated at the last follow-up, according to Katz score. 12
Statistical Analysis
The primary outcome of the study was to assess the rate of GTR achieved with EEA in elderly and late elderly patients with CC, comparing its results with the control group of patients younger than 65 years old. Secondary outcomes were the analysis of the prognostic role of preoperative clinical, radiological, and histological factors on the tumor removal extent in elderly cohort.
Direct comparisons were performed with a Pearson's Chi square test or a Fisher's exact test. For the analysis of the predictors of GTR in elderly patients, a backward-stepwise approach analysis was used: sex, KPS at surgery, referral symptoms, previous treatments, preoperative symptoms, tumor size and location in the clivus, intra-/extra-dural extension, and histological features were weighted as predictors of tumor extension for use in the final multiple logistic regression analysis.
Continuous variables were expressed as mean ± standard deviation, while categorical variables as absolute ( n ) and relative frequency (%). A p -value <0.05 was considered statistically significant.
Statistical analysis was performed using Stata (StataCorp. 2017, Stata Statistical Software: Release 15. College Station, Texas, United States: StataCorp LLC).
Results
Thirty-four patients older than 65 (27.6%) are included in this serie, out of an overall of 123 cases of CC, undergone 143 surgical procedures in our center in the considered timespan ( Fig. 1 ). Ten (8.1%) were late elderlies (over 75 years old). Males were 18 (52.9%). Nineteen cases (55.9%) were naive for surgery and the remaining had been already treated with surgery (7, 20.6%) or surgery and radiotherapy (8, 23.5%) ( Table 1 ).
Fig. 1.
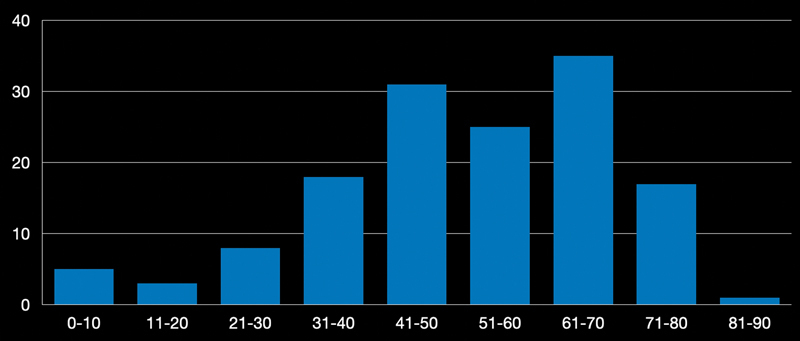
Graph representing the age distribution of our series of CC operated through an EEA. The peak in the 7th and 8th decades is visible. CC, clival chordoma; EEA, endoscopic endonasal approach.
Table 1. Demographics and preoperative clinical, neuroradiological, and histological features of the series and of the control group.
| Older than 65 | Older than 75 | Younger than 65 | p a | ||
|---|---|---|---|---|---|
| Sex | Males | 18 (52.9%) | 5 (50%) | 55 (50.4%) | 0.80 |
| Females | 16 (47.1%) | 5 (50%) | 54 (49.6%) | ||
| Previous treatment | Naive | 19 (55.9%) | 7 (70%) | 59 (54.1%) | 0.94 |
| Surgery | 7 (20.6%) | 1 (10%) | 23 (21.1%) | ||
| Surgery and radiotherapy | 8 (23.5%) | 2 (20%) | 26 (23.9%) | ||
| Only radiotherapy | 0 (0%) | 0 (0%) | 1 (0.9%) | ||
| Aim of surgery | Resective | 29 (85.3%) | 9 (90%) | 94 (86.2%) | 0.89 |
| Palliative | 5 (14.7%) | 1 (1%) | 15 (13.8%) | ||
| Pre-op KPS | 84 ± 8 | 85 ± 7 | 85 ± 9 | 0.85 | |
| Referral symptom | Incidental finding | 5 (14.7%) | 1 (10%) | 23 (21.1%) | 0.80 |
| Recurrence/progression at follow-up | 13 (38.2%) | 3 (30%) | 48 (44.0%) | ||
| Endocrinologic disturbances | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Visual deficit | 1 (2.9%) | 0 (0%) | 4 (3.7%) | ||
| Neurological deficit | 15 (44.2%) | 6 (60%) | 34 (31.2%) | ||
| Preoperative endocrinological symptoms | None | 32 (94.1%) | 10 (100%) | 98 (89.9%) | 0.65 |
| Part anterior hypopituitarism | 2 (5.9%) | 0 (0%) | 9 (8.3%) | ||
| Panhypopituitarism | 0 (0%) | 0 (0%) | 2 (1.8%) | ||
| Panhypopituitarism and DI | 0 (0%) | 0 (0%) | 0 (0%) | ||
| DI | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Preoperative visual symptoms | None | 30 (88.3%) | 10 (100%) | 93 (85.4%) | 0.86 |
| Quadrantanopia (1 or less quadrant) | 0 (0.0%) | 0 (0%) | 1 (0.9%) | ||
| Incomplete bitemporal hemianopia | 4 (11.7%) | 0 (0%) | 7 (6.4%) | ||
| Complete bitemporal hemianopia | 0 (0%) | 0 (0%) | 5 (4.6%) | ||
| Quadrantanopia (more than 2 quadrants) | 0 (0%) | 0 (0%) | 3 (2.7%) | ||
| Blindness | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Preoperative neurological symptoms | None | 8 (23.5%) | 2 (20%) | 39 (35.8%) | 0.35 |
| Oculomotor palsy | 23 (67.6%) | 7 (70%) | 61 (56.0%) | 0.23 | |
| Trigeminal neuralgia | 2 (5.9%) | 1 (10%) | 9 (8.2%) | 0.65 | |
| Dysphagia/dysphonia | 2 (5.9%) | 0 (0%) | 5 (4.6%) | 0.93 | |
| Facial palsy | 1 (2.9%) | 1 (10%) | 3 (2.7%) | 0.78 | |
| Hemiparesis | 1 (2.9%) | 1 (10%) | 6 (5.5%) | 0.53 | |
| CN XII palsy | 3 (8.8%) | 1 (10%) | 2 (1.8%) | 0.54 | |
| Intracranial hypertension | 0 (0%) | 0 (0%) | 1 (0.9%) | 0.73 | |
| Hypoacusis | 2 (5.8%) | 1 (10%) | 1 (0.9%) | 0.51 | |
| Seizures | 0 (0%) | 0 (0%) | 1 (0.9%) | 0.73 | |
| Size | <3 cm | 6 (17.7%) | 1 (10%) | 26 (23.9%) | 0.45 |
| >3 cm | 28 (82.3%) | 9 (90%) | 83 (76.1%) | ||
| Location | Upper clivus | 5 (14.7%) | 0 (0%) | 8 (7.3%) | 0.51 |
| Upper/middle clivus | 8 (23.5%) | 3 (30%) | 43 (39.5%) | ||
| Middle clivus | 5 (14.7%) | 2 (20%) | 14 (12.8%) | ||
| Middle/lower clivus | 3 (8.9%) | 1 (10%) | 12 (11.0%) | ||
| Lower clivus | 5 (14.7%) | 2 (20%) | 11 (10.1%) | ||
| Entire Clivus Extension | 8 (23.5%) | 2 (20%) | 21 (19.3%) | ||
| Dural infiltration | Complete extradural | 6 (17.7%) | 0 (0%) | 25 (22.9%) | 0.29 |
| Extradural with dural infiltration | 18 (52.9%) | 5 (50%) | 37 (33.9%) | ||
| Intradural extension through a pit hole | 8 (23.5%) | 4 (40%) | 37 (33.9%) | ||
| Large intradural extension | 2 (5.9%) | 1 (10%) | 6 (5.6%) | ||
| Complete intradural | 0 (0%) | 0 (0%) | 4 (3.7%) | ||
| Histology | Chondroid c | 1 (2.9%) | 0 (0%) | 9 (8.3%) | 0.44 |
| Conventional c. | 32 (94.2%) | 9 (90%) | 91 (83.5%) | ||
| Dedifferentiated c. | 1 (2.9%) | 1 (10%) | 7 (6.4%) | ||
| Undifferentiated c. | 0 (0%) | 0 (0%) | 2 (1.8%) |
Abbreviation: CN, cranial nerve.
Statistical analysis between elderly patient group (over 65) and control group (under 65).
Clinical Presentation and Tumor Characteristics
The most common presenting symptom leading to the CC diagnosis in over 65 and over 75 year populations (respectively 15, 44.2% and 6, 60%) was represented by neurological disturbances, mainly represented by diplopia and ophthalmoplegia. Five (14.7%) cases were incidental findings in asymptomatic patients who underwent MRI for other causes and 13 (38.2%) were recurrences or progression at follow-up of previously treated tumors.
As reported in Table 1 , at preoperative evaluation, campimetric visual deficits were observed in four cases (11.8%) and anterior hypopituitarism in two cases (5.9%). Oculomotor nerve palsy was the most common symptom (22, 64.7%), followed by cranial nerve (CN) XII palsy (3, 8.8%) and trigeminal neuralgia (2, 5.9%).
At preoperative MRI, the tumor was located in the upper clivus in 5 cases (14.7%), in the middle in other 5 (14.7%), and in the upper and middle third in 8 (23.5%), while in 8 it was interestingly in the entire clivus (23.5%). Lower clivus was involved in other 8 cases (23.5%). Most of the tumors were completely extradural (24, 70.6%) and only in 10 (29.4%) cases an intradural extension was detected.
Histologically, the majority of tumors histologically resulted in conventional chordomas (32, 94.2%), 1 (2.9%) was a chondroid subform and 1 (2.9%) a dedifferentiated type.
Surgical Results, Complications, and Follow-Up
GTR was achieved in 22 cases (64.7%), as reported in Table 2 . Complications consisted of 2 (5.9%) cases of postoperative CSF leak, 1 (2.9%) developing a meningitis, 4 (11.8%) transient and 1 (2.9%) permanent CN VI palsies. One 77-year-old patient developed a pneumonia (2.9%), which was treated with intravenous antibiotic therapy ( Figs. 2 3 4 ), and 2 (5.9%) presented a urinary infection, resolved with oral administration of fosfomycin. One patient presented an episode of delirium at the first postoperative night, resolved in 36 hours and treated with olanzapine and constant presence of the caregiver. At 3 months postoperative evaluation (before radiotherapy), no additional endocrinological or visual deficit was assessed ( Table 3 ). In nine patients (39.1%), the preoperative ophthalmoplegia improved or resolved, trigeminal neuralgia was stable in all cases as well as CN VII palsy, hemiparesis. Two patients with rhinogenic hypoacusis, one with dysphagia, and one with CN XII palsy improved after surgery ( Table 3 ).
Table 2. Surgical results, complications, and follow-up of the series and of the control group (under 65 years old).
| Older than 65 | Older than 75 | Younger than 65 | p | ||
|---|---|---|---|---|---|
| Tumor resection | GTR | 22 (64.7%) | 6 (60%) | 65 (59.6%) | 0.58 |
| STR | 10 (29.4%) | 3 (30%) | 39 (35.8%) | ||
| PTR | 2 (5.8%) | 1 (10%) | 5 (4.6%) | ||
| Morbidity | Overall | 11 (32.3%) | 3 (30%) | 14 (12.8%) | 0.07 |
| CSF leak | 2 (5.9%) | 0 (0%) | 4 (3.7%) | ||
| Hemorrhage | 0 (0%) | 0 (0%) | 2 (1.8%) | ||
| Ischemia | 0 (0%) | 0 (0%) | 1 (0.9%) | ||
| Epistaxis | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Carotid rupture | 0 (0%) | 0 (0%) | 2 (1.8%) | ||
| Transient de novo CN palsy | 4 (11.8%) | 2 (20%) | 2 (1.9%) | ||
| Permanent de novo palsy | 1 (2.9%) | 0 (0%) | 1 (0.9%) | ||
| Meningitis | 1 (2.9%) | 0 (0%) | 2 (1.8%) | ||
| Pneumonia | 1 (2.9%) | 1 (10%) | 0 (0%) | ||
| Urinary infection | 2 (5.9%) | 0 (0%) | 0 (0%) | ||
| Adjuvant treatment | Radiation therapy | 27 (79.4%) | 8 (80%) | 72 (66.0%) | 0.62 |
| Chemotherapy | 0 (0%) | 0 (0%) | 4 (3.7%) | ||
| Radiation and chemotherapy | 0 (0%) | 0 (0%) | 1 (0.9%) | ||
| None | 7 (20.6%) | 2 (20%) | 32 (29.4%) | ||
| QOL at follow-up | Re-integrated at same level | 17 (80.9%) | 3 (60%) | 59 (86.7%) | 0.39 |
| Re-integrated at lower level | 2 (9.5%) | 1 (20%) | 8 (11.8%) | ||
| Semi-dependent | 1 (4.8%) | 0 (0%) | 0 (0%) | ||
| Dependent | 1 (4.7%) | 1 (20%) | 1 (1.5%) | ||
| Recurrence/progression | 13 (38.2%) | 3 (30%) | 45 (41.3%) | 0.68 | |
| Mean recurrence/progression time | 38.2 ± 46.6 | 23.5 | 40.2 | ||
| Deceased | 13 (38.2%) | 5 (50%) | 40 (36.7%) | 0.87 | |
| Mean survival time | 66.3 ± 64.7 | 76.3 | 47.9 |
Abbreviations: GTR, gross total removal; PTR, partial removal; QOL, quality of life; STR, subtotal removal.
Statistical analysis between elderly patient group (over 65) and control group (under 65).
Fig. 2.
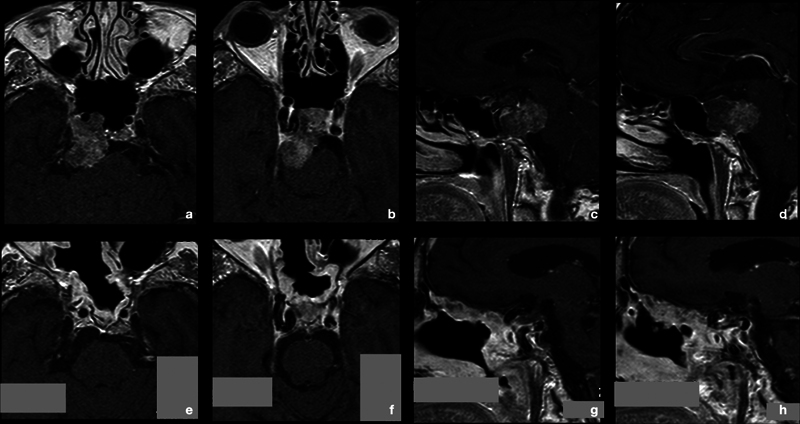
A 77-year-old woman came to our attention for multiple episodes of transient diplopia, occurred in the previous months. She had a history of chronic cardiac failure and chronic obstructive pulmonary disease. At hospital admission, she was neurologically intact and the preoperative MRI (a–d) showed an intra/extradural tumor, erosive of the upper third of clivus up the dorsum sellae and compressing the brainstem. Patient underwent an endoscopic endonasal surgery for the chordoma (see Figs. 2 and 3 ). Extubation was performed immediately after the awakening. At second postoperative day, she presented fever, blood and urinary cultures were negative, she did not show rhinorrhea, and CSF bio-chemical tests excluded a meningitis. Chest radiography showed a bronchopneumonic area and an intravenous therapy with antibiotic was started. General parameters remained stable, and therapy was discontinued after 7 days with complete clinical and radiological resolution of the lung infection. Histological diagnosis confirmed the suspect of conventional chordoma. At 3 months MRI, the GTR was confirmed and she underwent proton beam therapy (e–h) . At 132 months follow-up, she is still alive with no local recurrence of the tumor. CSF, cerebrospinal fluid; GTR, gross total removal; MRI, magnetic resonance imaging.
Fig. 3.
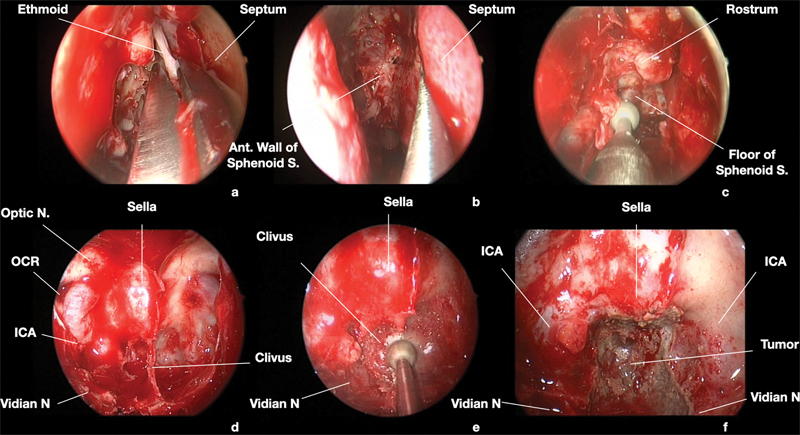
Surgical endoscopic endonasal images of the case presented in Fig. 1 . 0° scope. A right transpterygoid-transclival approach was adopted to remove the tumor. Surgery started with right middle turbinectomy, followed by ipsilateral ethmoidectomy (a). Afterwards, the posterior third of nasal septum was removed (b). The anterior wall of sphenoid sinus was opened, and its floor progressively drilled out (c). The anatomical landmarks of the posterior sphenoidal wall were identified and confirmed by neuronavigation, particularity the vidian nerves were used to localize the intra-petrosal and paraclival junction of ICA (d). The clival area inferior to the sella and comprised between the ICAs was drilled out to expose the extra-dural component of the tumor (e, f). Ant., anterior; ICA, internal carotid artery; N, nerve ; OCR, optic carotid recess; S., sinus.
Fig. 4.
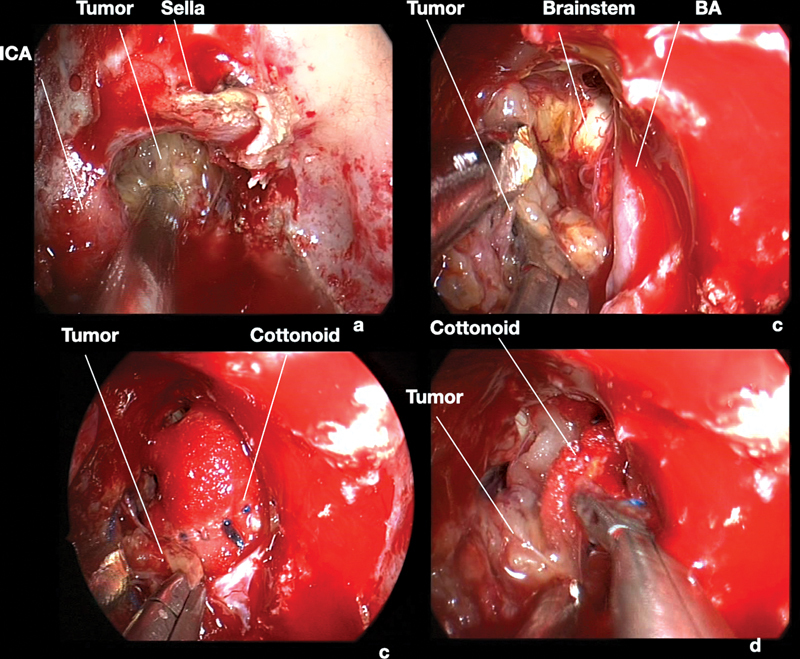
Surgical endoscopic endonasal images of the case presented in Fig. 1 . 0° scope. The extra-dural portion of the tumor was removed with curettes and suction (a) . After dura incision, the intra-dural part of the mass was cleaved by the basilar artery and by the brainstem, respecting the arachnoidal plane (b–d) . BA, basilar artery; CN, cranial nerve; ICA, internal carotid artery; SCA, superior cerebellar artery.
Table 3. Postoperative clinical results (at early postsurgical evaluation before radiotherapy).
| Older than 65 | Older than 76 | ||||||
|---|---|---|---|---|---|---|---|
| Norm/impr | Unch. | Wors. | Norm/impr | Unch. | Wors. | ||
| Endocrinological symptoms | None | 0 | 32 | 0 | 0 | 10 | 0 |
| Part. anterior hypopituitarism. | 1 | 1 | 0 | 0 | 0 | 0 | |
| Visual symptoms | None | 0 | 30 | 0 | 0 | 10 | 0 |
| Incomplete bitemporal hemianopia | 0 | 4 | 0 | 0 | 0 | 0 | |
| Neurological symptoms | None | 0 | 7 | 1 | 0 | 1 | 1 |
| Ocular palsy | 9 | 14 | 0 | 3 | 4 | 0 | |
| Trigeminal neuralgia | 0 | 2 | 0 | 0 | 1 | 0 | |
| Dysphagia/dysphonia | 1 | 1 | 0 | 0 | 0 | 0 | |
| Facial palsy | 0 | 1 | 0 | 0 | 1 | 0 | |
| Hemiparesis | 0 | 1 | 0 | 0 | 1 | 0 | |
| CN XII palsy | 1 | 2 | 0 | 1 | 0 | 0 | |
| Hypoacusis | 2 | 0 | 0 | 1 | 0 | 0 | |
Abbreviation: CN, cranial nerve.
Most of patients (27, 79.4%) underwent radiation therapy, while 7 cases, who had been already irradiated, were just followed up (one patient, who had been already treated with radiation, was considered suitable for re-irradiation due to the location of the recurrence and the time passed since the first treatment) ( Table 2 ). Mean follow-up was 37.7 ± 44.9 months. During this time, 13 (38.2%) recurrences/progressions were observed after a mean time of 38.2 ± 46.6 months and 13 patients (38.2%) deceased due to tumor progression after a mean of 66.3 ± 64.7 months since surgery ( Table 2 ). Among living patients at follow-up, a normal QOL was preserved or restored in 17 (80.9%) elderlies and 3 (60%) late elderlies.
Statistical Analysis
Comparing the extent of tumor removal achieved in the series of elderlies (over 65) and late elderlies (over 75) with the control group, no statistical relevant differences were observed ( p > 0.05) ( Table 2 ).
Similarly, no differences were observed for preoperative demographic data, referral symptoms, previous treatment, endocrinological, visual and neurological deficits, and tumor size, location, dural infiltration and histological histotype between the elderly cohort and the control group ( Table 1 ).
Finally, previous treatments ( p < 0.001) and tumor size ( p : 0.004) were significantly associated with the tumor resection extension in logistic regression analysis for elderly patients ( Table 4 ). For the low numerosity, it was not possible to perform a multivariate analysis for the late elderly cohort.
Table 4. Logistic regression to assess the predictors of tumor removal extent in elderly population a .
| Odds ratio | Std. error | z | p | 95% Conf. Interval | ||
|---|---|---|---|---|---|---|
| Sex | 1.2 | 0.5 | 0.5 | 0.622 | 0.5 | 2.7 |
| Previous treatment | 2.8 | 0.7 | 4.1 | 0.001 | 1.7 | 4.6 |
| Size | 4.9 | 2.7 | 2.8 | 0.004 | 1.6 | 14.7 |
| KPS | 2.0 | 0.8 | 1.7 | 0.088 | 0.9 | 4.5 |
Variables excluded at stepwise analysis are not reported.
Discussion
Our study is the first to be completely focused on the analysis of the surgical and oncological management of CC in elderlies and late elderlies, presenting a large series of 34 patients over 65 years and 10 over 75 years old. We have demonstrated that in this cohort of patients, characterized for their multiple comorbidities and fragilities, EEA can be a useful approach, balancing the possibility to achieve a GTR (obtained in 64.7% of elderlies and in 60% of late elderlies) with an acceptable morbidity rate of 32.3% in over 65 and 30% in over 75 years old patients.
Particularly, although the overall morbidity of these patients is indubitably higher than that of younger patients, it should be considered that the main surgical complication was represented by two cases of postoperative CSF leak (5.9%), which were promptly re-operated through the same EEA. The only permanent postoperative sequela was represented by a case of persistent CN VI palsy, while the other four deficits were transient, with full recovery within 3 months since surgery. Moreover, infective complications were more frequent than in younger patients, with 1 (2.9%) case of meningitis, 1 (2.9%) of pneumonia, and 2 (5.8%) of urinary infection, treated with antibiotic therapies and resolved with no consequences or subsequent neurological deficit. Indeed, one of the advantages of EEA is its minimal invasiveness with prompt patient recovery. 3 5 9 10 11 13 14 15 In comparison to other transcranial or transfacial (including the transoral for the lower clivus) approaches for the clival region, EEA would allow patients to restore spontaneous breathing immediately after awakening from anesthesia and the oral feeding after 6 hours from surgery, usually not needing of a preventive tracheostomy or prolonged intubation. 15 16 For these same reasons, during our 25 years' experience, we have progressively avoided the use of postoperative intensive care unit (ICU) also for elderly and even late elderly patients, preferring to keep them under observation in the recovery room for few hours after surgery and allowing a caregiver to stay with them for the following time and eventually during the first postoperative day. We think that this management could have contributed to reduce the risk of ICU-related infection, and to prevent possible episodes of delirium, which is not infrequent in elderly patients after general anesthesia, as we have observed in a single case in our experience. 17 18 Moreover, we should remark that the EEA allows the surgeon to adopt a fully extracranial corridor, therefore with no brain manipulation or retraction. 3 5 6 7 8 10 11 13 14 15 19 This could be a further element, fastening the patient recovery and avoiding the need of prolonged hospitalization, with all the possible subsequent detrimental consequences in elderlies. 20 In our series, the great majority of CCs were completely extradural (70.6%); therefore, EEA allows the surgeon to resect these tumors, with no transgression of the dural plane. As a consequence, the patient can be mobilized on the same day of surgery, avoiding prolonged immobilization and bed staying. We consider that early mobilization could be a further factor to prevent possible complications and to avoid the need for bladder catheterization, with possible development of urinary fever or even sepsis. 20 21 Indeed, our two cases, who have developed a cystitis, were both patients needing a urinary catheter. In these cases, the substitution of the device and a target oral therapy with fosfomycin was sufficient to control the infection.
Comparing the GTR rate of elderly population with younger patients, we found no statistically relevant differences. We argue that this could be due both to the good tolerability of such approach (which allows the surgeon to extend as much as possible the tumor resection also in this cohort of patients) and to a careful multidisciplinary patient selection. 3 5 6 7 8 9 10 11 12 13 14 15 We have also observed that the prognostic factors, affecting the tumor resection rate in elderly patients, are the same as that we had already observed in the general population of CC, namely the primary surgery ( p < 0.001) and the size of the tumor ( p : 0.004). 1 11 12 The better surgical outcome that we have observed for naive patients confirms that the first surgery has better odds to achieve the GTR, while the following surgeries are usually negatively affected by the presence of surgical scars and postoperative or post-actinic fibrous tissue. 10 11 In elderly patients, we have not observed that the resection rate is related to tumor location in the clivus, or its intradural extension, as reported for the general CC population, probably for low numerosity of the study sample. Similarly, no analysis was possible to assess the outcome of the different histological subtypes for their low number. 22
Moreover, our series confirms also that for elderly patients. EEA is valid and effective in the restoration of compressive neurological symptoms due to CC. 3 5 6 7 8 9 10 11 12 13 14 15 Indeed, we observed a 39.1% rate of preoperative ophthalmoplegia regression, and a clinical improvement also in two cases of rhinogenic hypoacusis, in one with mixed CN palsy and in one with a CN XII deficit. This could have contributed to the preservation or restoration of an acceptable QOL, which is crucial for the subsequent adjuvant therapies. 23 Indeed, in living patients at follow-up, QOL was fully preserved in 80.9% of elderlies and 60% of late elderlies, similarly to results achieved by younger patients. In our study, we have observed that clinical and radiological characteristics of CC are not dissimilar between elderlies and younger patients, and also their biological behavior at follow-up is similar to a recurrence rate of 38.2% in elderlies and 30% of late elderlies, not statistically different from the results observed in under 65 year old cases. Considering this significant recurrence rate also in elderlies, we can suggest that postoperative radiation therapy should be advised also in this population to prevent early progression and increase their overall survival. 24 25 26 It has been reported that radiotherapy could increase the risk of delayed CSF leak in patients already operated trough an EEA, therefore we used to fill the surgical field with abdominal fat, covered with mucoperiosteum even whether no intra-operative leak is observed. 24 25 26 This maneuver could also be particularly useful in elderlies, which could be more severely affected by the development of delayed leak with meningitis.
For this specific cohort of patients, the management of loco-regional recurrences remains particularly challenging. 10 27 The good tolerability of a second surgery should be balanced with their general conditions, KPS, and life expectancy, to avoid potentially harmful over-treatments. In general, patient selection represents a cornerstone of this surgery, particularly in elderlies. 10 27 Indeed, it is important not only to assess the preoperative clinical and general conditions of each case, but also to explore her/his potential adherence to postoperative prescriptions and her/his collaboration degree to the entire treatment plan. Moreover, a further element to be considered in the preoperative evaluation of each case is the familiar and/or social network, to assess the presence of one or more caregivers able to assist and help the patient during the hospitalization and the follow-up. 28 Therefore, a comprehensive multidisciplinary evaluation of all patients before each treatment step (i.e., surgery, radiotherapy, follow-up) is of paramount importance to orientate their management. 5 For this aim, our local board is composed not only of different medical specialties involved in these patients cure (neurosurgeons, neuroradiologists, neuropathologists, medical and radiation oncologists, palliative care specialists) but also of dedicated staff members, such as nurses who will take care of the patients during the hospitalization and assist their caregivers in the following period. We think that the rate (27.6%) of elderly patients included in our general CC series is lower than the expected considering the incidence recently reported in the literature (38.7%), as a consequence of this selection bias, which has led us to prefer a palliative nonsurgical management for those few cases with medical, neurological, or general conditions, which make them not suitable for surgery. 2
The main strength of this study is its focus on elderly and late elderly patients, who are usually a neglected population in the surgical series of CC, and the long follow-up available. This has allowed us to draw specific considerations on this cohort of patients, which are becoming more and more frequent. The main limitation consists of low numerosity of the series, even if it represents one of the largest in the literature especially for late elderlies. This could have limited the statistical analysis. A further limit is due to the selection bias of the study, which included only patients undergone surgery and eventually radiotherapy, not considering the cases managed conservatively. An additional drawback is constituted by the lack of data of patients' comorbidities, the analysis of which was beyond the aim of this study.
Conclusions
Our study demonstrated that in selected elderly patients, EEA can be a useful approach, balancing the possibility to achieve a GTR with an acceptable morbidity rate. For its characteristics, the EEA, which allows to approach these tumors with an extracranial, extradural approach, avoiding brain manipulation or retraction and with a very quick patient recovery, without the need for a prolonged hospitalization or immobilization at bed, is very well tolerated also by elderly or late elderly patients.
As a consequence, EEA represents an ideal route for this challenging population, constituting the first choice in our center. However, patient selection remains crucial, and we suggest to tailor the choice of treatments to each case. A multidisciplinary comprehensive evaluation is important to assess not only the medical conditions of the patients but also her/his social and familiar network.
Larger prospective studies are needed to determine the long-term outcome of these population of patients with CC.
Footnotes
Conflict of Interest None declared.
References
- 1.Labidi M, Watanabe K, Bouazza S et al. Clivus chordomas: a systematic review and meta-analysis of contemporary surgical management. J Neurosurg Sci. 2016;60(04):476–484. [PubMed] [Google Scholar]
- 2.Das P, Soni P, Jones J et al. Descriptive epidemiology of chordomas in the United States. J Neurooncol. 2020;148(01):173–178. doi: 10.1007/s11060-020-03511-x. [DOI] [PubMed] [Google Scholar]
- 3.Cavallo L M, Mazzatenta D, d'Avella E et al. The management of clival chordomas: an Italian multicentric study. J Neurosurg. 2020;135(01):93–102. doi: 10.3171/2020.5.JNS20925. [DOI] [PubMed] [Google Scholar]
- 4.Yaniv D, Soudry E, Strenov Y, Cohen M A, Mizrachi A. Skull base chordomas review of current treatment paradigms. World J Otorhinolaryngol Head Neck Surg. 2020;6(02):125–131. doi: 10.1016/j.wjorl.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell R G, Prevedello D M, Ditzel Filho L, Otto B A, Carrau R L. Contemporary management of clival chordomas. Curr Opin Otolaryngol Head Neck Surg. 2015;23(02):153–161. doi: 10.1097/MOO.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 6.Frank G, Sciarretta V, Calbucci F, Farneti G, Mazzatenta D, Pasquini E.The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas Neurosurgery 200659(1, Suppl 1):ONS50–ONS57., discussion ONS50–ONS57 [DOI] [PubMed] [Google Scholar]
- 7.George B, Bresson D, Herman P, Froelich S. Chordomas: a review. Neurosurg Clin N Am. 2015;26(03):437–452. doi: 10.1016/j.nec.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Chordoma Global Consensus Group . Stacchiotti S, Sommer J. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16(02):e71–e83. doi: 10.1016/S1470-2045(14)71190-8. [DOI] [PubMed] [Google Scholar]
- 9.Stippler M, Gardner P A, Snyderman C H, Carrau R L, Prevedello D M, Kassam A B.Endoscopic endonasal approach for clival chordomas Neurosurgery 20096402268–277., discussion 277–278 [DOI] [PubMed] [Google Scholar]
- 10.Zoli M, Guaraldi F, Gori D et al. Endoscopic endonasal approach for loco-regional recurrent clivus chordomas. Brain Spine. 2022;2:100918. doi: 10.1016/j.bas.2022.100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoli M, Milanese L, Bonfatti R et al. Clival chordomas: considerations after 16 years of endoscopic endonasal surgery. J Neurosurg. 2018;128(02):329–338. doi: 10.3171/2016.11.JNS162082. [DOI] [PubMed] [Google Scholar]
- 12.Katz S, Ford A B, Moskowitz R W, Jackson B A, Jaffe M W. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185(185):914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 13.Baldassarre B M, Di Perna G, Portonero I et al. Craniovertebral junction chordomas: case series and strategies to overcome the surgical challenge. J Craniovertebr Junction Spine. 2021;12(04):420–431. doi: 10.4103/jcvjs.jcvjs_87_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceylan S, Emengen A, Caklili M et al. Operative nuances and surgical limits of the endoscopic approach to clival chordomas and chondrosarcomas: a single-center experience of 72 patients. Clin Neurol Neurosurg. 2021;208:106875. doi: 10.1016/j.clineuro.2021.106875. [DOI] [PubMed] [Google Scholar]
- 15.Schnurman Z, Benjamin C G, Miceli M, Sen C. Clival chordomas in the endoscopic endonasal era: comparison with management with open skull base approaches. Neurosurgery. 2023;92(04):756–761. doi: 10.1227/neu.0000000000002286. [DOI] [PubMed] [Google Scholar]
- 16.Van Abel K M, Mallory G W, Kasperbauer J L et al. Transnasal odontoid resection: is there an anatomic explanation for differing swallowing outcomes? Neurosurg Focus. 2014;37(04):E16. doi: 10.3171/2014.7.FOCUS14338. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti D, Bharadwaj S, Akash V S et al. Postoperative delirium after intracranial neurosurgery: a prospective cohort study from a developing nation. Acta Neurochir (Wien) 2023;165(06):1473–1482. doi: 10.1007/s00701-023-05610-w. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Sha Z, Fan Y et al. Evaluating the efficiency of a nomogram based on the data of neurosurgical intensive care unit patients to predict pulmonary infection of multidrug-resistant Acinetobacter baumannii. Front Cell Infect Microbiol. 2023;13:1.152512E6. doi: 10.3389/fcimb.2023.1152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang E W, Zanation A M, Gardner P Aet al. ICAR: endoscopic skull-base surgery Int Forum Allergy Rhinol 20199(S3):S145–S365. [DOI] [PubMed] [Google Scholar]
- 20.Najjar E, Hassanin M A, Komaitis S, Karouni F, Quraishi N. Complications after early versus late mobilization after an incidental durotomy: a systematic review and meta-analysis. Eur Spine J. 2023;32(03):778–786. doi: 10.1007/s00586-023-07526-6. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Li Y, Huang Y et al. Prediction of catheter-associated urinary tract infections among neurosurgical intensive care patients: a decision tree analysis. World Neurosurg. 2023;170:123–132. doi: 10.1016/j.wneu.2022.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Asioli S, Zoli M, Guaraldi F et al. Peculiar pathological, radiological and clinical features of skull-base de-differentiated chordomas. Results from a referral centre case-series and literature review. Histopathology. 2020;76(05):731–739. doi: 10.1111/his.14024. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro-Neto C, Rowan N R, Celda M P, Mukherjee D, Gompel J JV, Choby G. Optimizing quality of life and minimizing morbidity through nasal preservation in endoscopic skull base surgery: a contemporary review. J Neurol Surg B Skull Base. 2022;83(06):602–610. doi: 10.1055/s-0042-1749654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bin-Alamer O, Mallela A N, Palmisciano P et al. Adjuvant stereotactic radiosurgery with or without postoperative fractionated radiation therapy in adults with skull base chordomas: a systematic review. Neurosurg Focus. 2022;53(05):E5. doi: 10.3171/2022.8.FOCUS22239. [DOI] [PubMed] [Google Scholar]
- 25.Chen A TC, Hong C BC, Narazaki D K et al. High dose image-guided, intensity modulated radiation therapy (IG-IMRT) for chordomas of the sacrum, mobile spine and skull base: preliminary outcomes. J Neurooncol. 2022;158(01):23–31. doi: 10.1007/s11060-022-04003-w. [DOI] [PubMed] [Google Scholar]
- 26.Iannalfi A, D'Ippolito E, Riva G et al. Proton and carbon ion radiotherapy in skull base chordomas: a prospective study based on a dual particle and a patient-customized treatment strategy. Neuro-oncol. 2020;22(09):1348–1358. doi: 10.1093/neuonc/noaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacchiotti S, Gronchi A, Fossati P et al. Best practices for the management of local-regional recurrent chordoma: a position paper by the Chordoma Global Consensus Group. Ann Oncol. 2017;28(06):1230–1242. doi: 10.1093/annonc/mdx054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Qiu X, Jin Q et al. Influencing factors of home exercise adherence in elderly patients with stroke: A multiperspective qualitative study. Front Psychiatry. 2023;14:1.157106E6. doi: 10.3389/fpsyt.2023.1157106. [DOI] [PMC free article] [PubMed] [Google Scholar]


