Abstract
This case series highlights chronic subdural hematoma in previously healthy young and middle-aged patients, where symptoms persisted despite initial surgical intervention. Subsequent diagnosis revealed spontaneous intracranial hypotension through computed tomography myelography. All patients experienced symptom relief after undergoing epidural blood patch. In conclusion, spontaneous intracranial hypotension should be considered in chronic subdural hematoma cases without trauma or underlying disease, with epidural blood patch recommended before surgical intervention if spontaneous intracranial hypotension is suspected.
Keywords: Headache, Subdural hematoma, Intracranial hypotension, Epidural blood patch, Case reports
INTRODUCTION
Headache is a common complaint among patients visiting the emergency room. Chronic subdural hematoma (CSDH) is one of the causes of headache. The annual reported incidence of CSDH is approximately 2.0 to 20.6 cases per 100,000 population [1]. CSDH is predominantly unilateral in the elderly. Minor head trauma is the most common cause of CSDH and can also occur in conditions such as coagulopathy or brain atrophy. CSDH with spontaneous intracranial hypotension (SIH) has been reported in several recent studies. Treatment options vary according to the presence or absence of SIH in patients with CSDH [2,3]. Therefore, it is very important to suspect SIH as the cause of CSDH at the beginning of diagnosis.
The patients in this series of case reports were young and middle-aged patients who were previously healthy. Though they were diagnosed with SDH and underwent surgery, there was no significant improvements in symptoms. Subsequently, SIH was suspected and subsequently diagnosed by computed tomography (CT) myelography. Symptoms were improved after performing an epidural blood patch (EBP).
CASE REPORTS
Case 1
A previously healthy 46-year-old, male patient who was 182 cm tall and 78 kg in weight (body mass index [BMI], 23.5 kg/m2) visited the emergency room because of worsening of the headaches over a month. The headache, which felt like a pounding at the back of the head persisted with a numeric rating scale (NRS) ≥5. There was a history of repetitive blunt trauma to head over the previous month. A brain CT scan revealed bilateral SDH (Fig. 1A). The patient was referred to the neurosurgery department with a diagnosis of CSDH, and burr hole trephination and SDH removal were performed on the left side under general anesthesia (Fig. 1B).
Fig. 1.
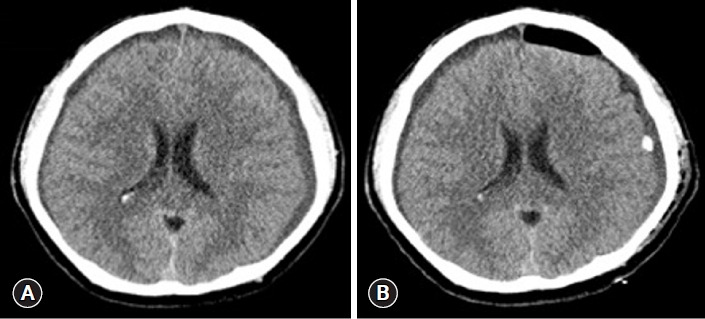
Brain computed tomography images of case 1. (A) Left chronic subdural hemorrhage shown on day 1 of hospitalization. (B) After the first surgery (burr hole trephination and subdural hematoma removal), subdural hematoma was evacuated.
On the postoperative day (POD) 3, his headache worsened, and a follow-up brain CT was performed which showed that the SDH on right side had increased. SIH was suspected and CT myelography was performed. The CT myelography revealed a cerebrospinal fluid (CSF) leakage at C7, C8, T1, and T8 levels. He was referred to pain clinic for an EBP. An EBP (10 mL) was performed at the right C7/T1 area under fluoroscopic guidance.
On POD 7, the size of the right-sided hematoma increased on a brain CT. A right lateral burr hole trephination and catheter insertion for SDH removal was performed under general anesthesia on POD 8. After the second operation, the patient continued to experience intermittent headaches. Therefore, EBP (10 mL, C7/T1 area) was performed once more under fluoroscopic guidance. Subsequently, the headache improved, and the patient was discharged on the POD 14.
Case 2
A 39-year-old previously healthy male patient, 171 cm tall and weighing 59.8 kg (BMI, 20.5 kg/m2) was seen in the outpatient neurology department, complaining of headache for 2 months. The headache symptoms consisted of a pounding pain starting from the back of the neck and going all the way to the forehead. It decreased on lying flat and worsened on standing. The NRS score was 3 to 4 points when weak and 10 points when severe. He occasionally woke up at night due to the headache associated with emesis with any movement. He was admitted to the neurology ward, and a brain magnetic resonance imaging (MRI) showed uncal herniation and SDH with a midline shift. On day 5 hospitalization, a left lateral burr hole trephination and SDH removal were performed under general anesthesia (Fig. 2A, B).
Fig. 2.
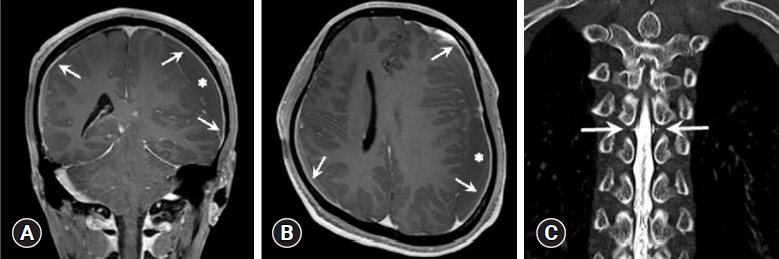
Case 2 images. (A, B) Gadolinium-enhanced T1-weighted magnetic resonance images of the brain showing diffuse pachymeningeal enhancement (arrows) and subdural left hemispheric chronic hematoma (asterisks). (A) Coronal view. (B) Axial view. (C) Computed tomography myelography coronal view showing leakage of contrast material (arrows) at T5 level.
On POD 1 and 2, headache with an NRS score of 3 to 6 was present and showed no improvement. SIH was suspected and CT myelography was performed. The spine CT myelography was showed contrast leakage at both T5 and T6 levels (Fig. 2C).
An EBP (10 mL) was performed at the T9/T10 area under fluoroscopic guidance. After the EBP, the headache improved, and a follow-up brain CT on POD 6 showed the improvement. The patient was discharged on POD 10.
Case 3
A previously healthy 52-year-old female patient, 163 cm tall and weighing 59 kg (BMI, 22.1 kg/m2) was evaluated for headaches that started 2 months previously. It consisted of a throbbing pain with a NRS of 4 in both the parietal lobes, accompanied by nausea while eating. The symptoms improved on medication prescribed. However, she was admitted to the emergency room a month later with worsening headaches. Following intravenous medication, her symptoms improved, and she was discharged. The next day, she was seen as an outpatient in the neurology department and a brain CT was performed, which showed bilateral CSDH. A bilateral burr hole trephination and SDH removal were performed under local anesthesia (Fig. 3A).
Fig. 3.
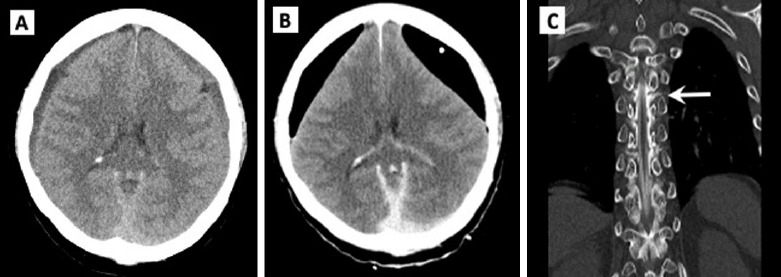
Case 3 images. (A) Brain computed tomography (CT) showing bilateral chronic subdural hemorrhage on day 1 of hospitalization. (B) Postoperative CT scans demonstrating extensive pneumocephalus in the subdural space in both frontal areas. (C) CT myelography coronal view showing leakage of contrast material (arrow) at T6 level.
Following the surgery, the patient lost consciousness and pneumocephalus, in an area larger than the previous hematoma, was observed on follow-up brain CT (Fig. 3B). SIH was suspected, and a spine CT myelography was performed. Contrast leakage was observed in T4–T6 levels (Fig. 3C).
An EBP (10 mL) was performed at the T10/T11 level on the right under fluoroscopic guidance (Fig. 4). Her headaches improved and a follow-up brain CT showed the improvement. The patient was discharged on POD 12.
Fig. 4.
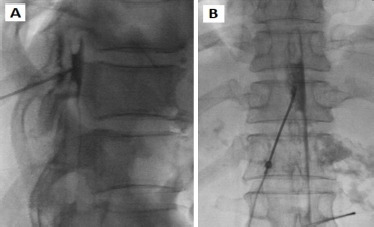
Epidural blood patch performed at T10/11 area. (A) Lateral view. (B) Anteroposterior view.
Ethics statement
Informed consents for publication of the research details and clinical images were obtained from the patients.
DISCUSSION
SIH is caused by a decrease in the volume and pressure of the CSF due to continuous leakage into the spiral axis dural defect. The incidence of SIH is estimated to be two to five in 100,000 [4], with age of onset between 40 to 50 years. The typical symptom of SIH is an orthostatic headache that worsens over time, accompanied by symptoms including neck stiffness, nausea, vomiting, tinnitus, and dizziness. The major complications of SIH are brain sagging, cerebral venous sinus thrombosis, and coma due to SDH. The exact pathogenesis of SDH is unknown but is suspected to be due to tearing of the bridge veins within the dural border cell layer, usually developing into CSDH. The incidence of CSDH in patients with SIH has been reported to be between 20% to 40% [2,3]. Only a few SIH patients with CSDH tend to complain of orthostatic headaches because of abnormally low intracranial pressure or volume correction by CSDH. Therefore, if CSDH is diagnosed in young and middle-aged patients with no history of trauma or hematological disease, SIH should be suspected, and caution should be exercised, even if it is a nonorthostatic headache.
Based on clinical symptoms, and brain MRI, radionuclide cisternography, and CT myelography may be performed to make diagnosis of SIH [2]. Characteristic brain MRI findings in SIH include extensive meningeal contrast enhancement, downward displacement of brain, subdural effusion, cerebral venous sinus thrombosis, and hyperemia of the pituitary gland. Radionuclide cisternography and CT myelography may be helpful in identifying of CSF leakage site [2].
Though many studies have investigated whether to initially proceed with EBP or SDH drainage when CSDH is caused by SIH, it remains controversial. A study by de Noronha et al. [5] suggested performing surgical drainage first and then proceeding with EBP. In contrast, some studies have reported that surgery before EBP is deleterious [6–9]. SDH drainage can lead to a rapid decrease in intracranial pressure, traction and destruction of the bridging veins, and acute bleeding, leading to recurrence, persistent symptoms, and sometimes worsened clinical course. Chung et al. [6] reported that surgery should be only performed after treating the site of CSF leakage with EBP. Schievink [7] suggested that SDH was spontaneously absorbed with EBP alone, even with a mass effect. Loya et al. [8] reported that EBP successfully improved coma in 85%. Kelley and Johnson [9] reported that surgery may increase the risk of brain herniation. Takahashi et al. [10] recommended that if the thickness of hematoma is more than 15 mm on CT or MRI, surgery should be performed immediately after EBP. Other similar cases are summarized in Table 1 [1,2,10,11].
Table 1.
Summary of cases of spontaneous intracranial hypotension with chronic subdural hematoma
| Study | No. of cases | Age (yr) | Sex | Clinical feature | Initial treatment | Next treatment | No. of EBPs | Initial treatment |
|---|---|---|---|---|---|---|---|---|
| Takahashi et al. [10] (2016) | 2 | 49 | Male | Headache | Burr hole drainage | Burr hole drainage, blind lumbar EBP | 2 | Favorable |
| 40 | Male | Orthostatic headache | Burr hole drainage + target EBP | Burr hole drainage | 1 | Favorable | ||
| Zhang and Pan [11] (2016) | 1 | 43 | Male | Orthostatic headache | Burr hole drainage | Target EBP | 1 | Favorable |
| Rettenmaier et al. [2] (2017) | 1 | 62 | Female | Orthostatic headaches associated with nausea, emesis, neck pain | Blind lumbar EBP (L3, L4) | Target EBP | 2 | Favorable |
| Osada et al. [1] (2020) | 4 | 51 | Male | Headache, coma | Burr hole drainage | Blind EBP (T1, T12) | 1 | Poor |
| 64 | Male | Headache, vomiting | Burr hole drainage | Blind EBP (T5, T11) | 2 | Favorable | ||
| 53 | Female | Headache | Burr hole drainage | Blind EBP (T3, T10) | 1 | Favorable | ||
| 44 | Male | Orthostatic headache | Burr hole drainage + target EBP (T4) | 1 | Favorable |
EBP, epidural blood patch.
The goal of SIH treatment is to stop CSF leak and increase the CSF volume. Conservative treatment, including bed rest, fluid therapy, caffeine, and steroids, usually relieves symptoms; however, if symptoms persist or worsen, EBP should be considered, and the clinical course is generally good [2]. There is no consensus regarding the puncture area and the blood volume to be injected during EBP. Although the blind lumbar EBP is sometimes successful in treatment of SIH and targeted EBP has a larger risk profile, a targeted EBP has superior efficacy and a smaller proportion of patients require a second EBP [2], or cervical, thoracic, or lumbar vertebrae. The optimal volume of blood to be injected during EBP is not yet known; however, most studies suggest a 15 mL injection volume.
The three patients in our series were previously healthy young or middle-aged individuals who were diagnosed with SDH. Although the patients underwent surgery, their symptoms did not improve significantly. Therefore, SIH was suspected, and was diagnosed by CT myelography. Symptoms improved after undergoing EBP. However, case 1 showed an increase in SDH on CT and underwent reoperation. EBP was repeated as there were no significant improvement symptoms. In the case of SIH with CSDH, surgical drainage is not the primary treatment modality. Therefore, EBP should be performed before SDH surgery. If there is thick bleeding and worsening of symptoms, surgery should be performed immediately after EBP.
In conclusion, SIH is an important diagnosis to be considered in young middle-aged patents with CSDH, without history of trauma, underlying disease, or bilateral hematoma. If CSDH accompanied by SIH is suspected, the location of the CSF leakage should be radiographically identified and EBP should be performed, by an anesthesiologist, prior to surgical treatment.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
The authors received no financial support for this study.
Author contributions
Conceptualization: YSC; Methodology: all authors; Project administration: YSC; Visualization: ARL; Writing–original draft: YSC; Writing–review & editing: YSC. All authors read and approved the final manuscript.
Data availability
Data sharing is not applicable as no new data were created or analyzed in this study.
REFERENCES
- 1.Osada Y, Shibahara I, Nakagawa A, et al. Unilateral chronic subdural hematoma due to spontaneous intracranial hypotension: a report of four cases. Br J Neurosurg. 2020;34:632–7. doi: 10.1080/02688697.2019.1667482. [DOI] [PubMed] [Google Scholar]
- 2.Rettenmaier LA, Park BJ, Holland MT, et al. Value of targeted epidural blood patch and management of subdural hematoma in spontaneous intracranial hypotension: case report and review of the literature. World Neurosurg. 2017;97:27–38. doi: 10.1016/j.wneu.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 3.Shin HS, Lee SH, Ko HC, Koh JS. Extended pneumocephalus after drainage of chronic subdural hematoma associated with intracranial hypotension: case report with pathophysiologic consideration. J Korean Neurosurg Soc. 2016;59:69–74. doi: 10.3340/jkns.2016.59.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schievink W, Roiter V. Epidemiology of cervical artery dissection. Front Neurol Neurosci. 2005;20:12–5. doi: 10.1159/000088125. [DOI] [PubMed] [Google Scholar]
- 5.de Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CA. Subdural haematoma: a potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatry. 2003;74:752–5. doi: 10.1136/jnnp.74.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung SJ, Lee JH, Kim SJ, Kwun BD, Lee MC. Subdural hematoma in spontaneous CSF hypovolemia. Neurology. 2006;67:1088–9. doi: 10.1212/01.wnl.0000237338.44702.61. [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI. Stroke and death due to spontaneous intracranial hypotension. Neurocrit Care. 2013;18:248–51. doi: 10.1007/s12028-012-9800-3. [DOI] [PubMed] [Google Scholar]
- 8.Loya JJ, Mindea SA, Yu H, Venkatasubramanian C, Chang SD, Burns TC. Intracranial hypotension producing reversible coma: a systematic review, including three new cases. J Neurosurg. 2012;117:615–28. doi: 10.3171/2012.4.JNS112030. [DOI] [PubMed] [Google Scholar]
- 9.Kelley GR, Johnson PL. Sinking brain syndrome: craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology. 2004;62:157. doi: 10.1212/wnl.62.1.157. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Mima T, Akiba Y. Chronic subdural hematoma associated with spontaneous intracranial hypotension: therapeutic strategies and outcomes of 55 cases. Neurol Med Chir (Tokyo) 2016;56:69–76. doi: 10.2176/nmc.oa.2015-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Pan KH. Epidural blood patch for spontaneous intracranial hypotension with chronic subdural haematoma: A case report and literature review. International medical reserch. 2016;44:976–981. doi: 10.1177/0300060516645955. [DOI] [PMC free article] [PubMed] [Google Scholar]


