Abstract
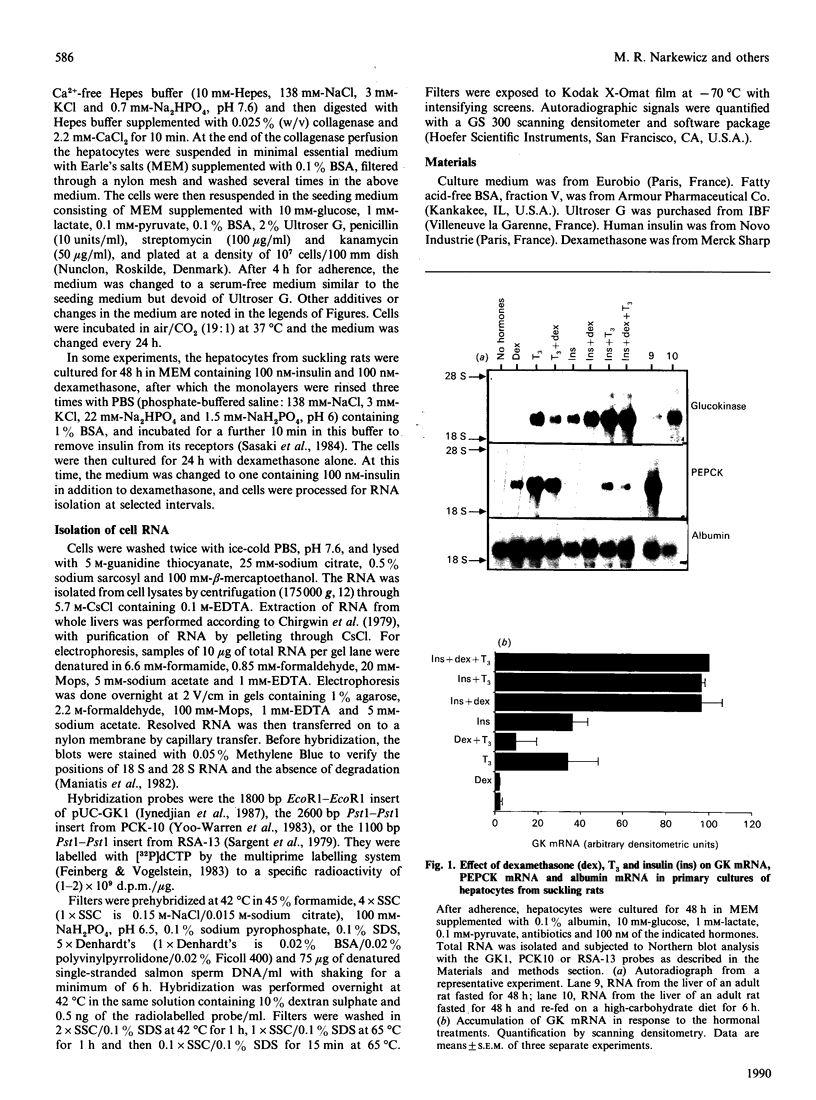
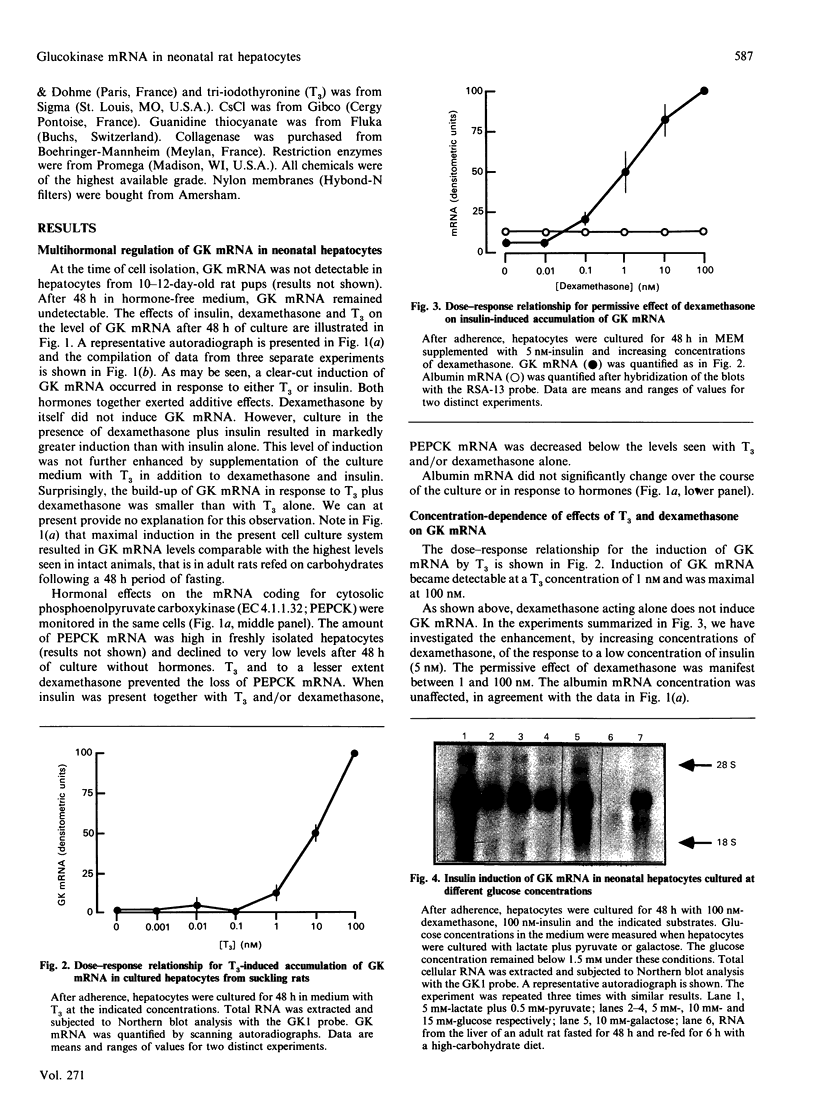
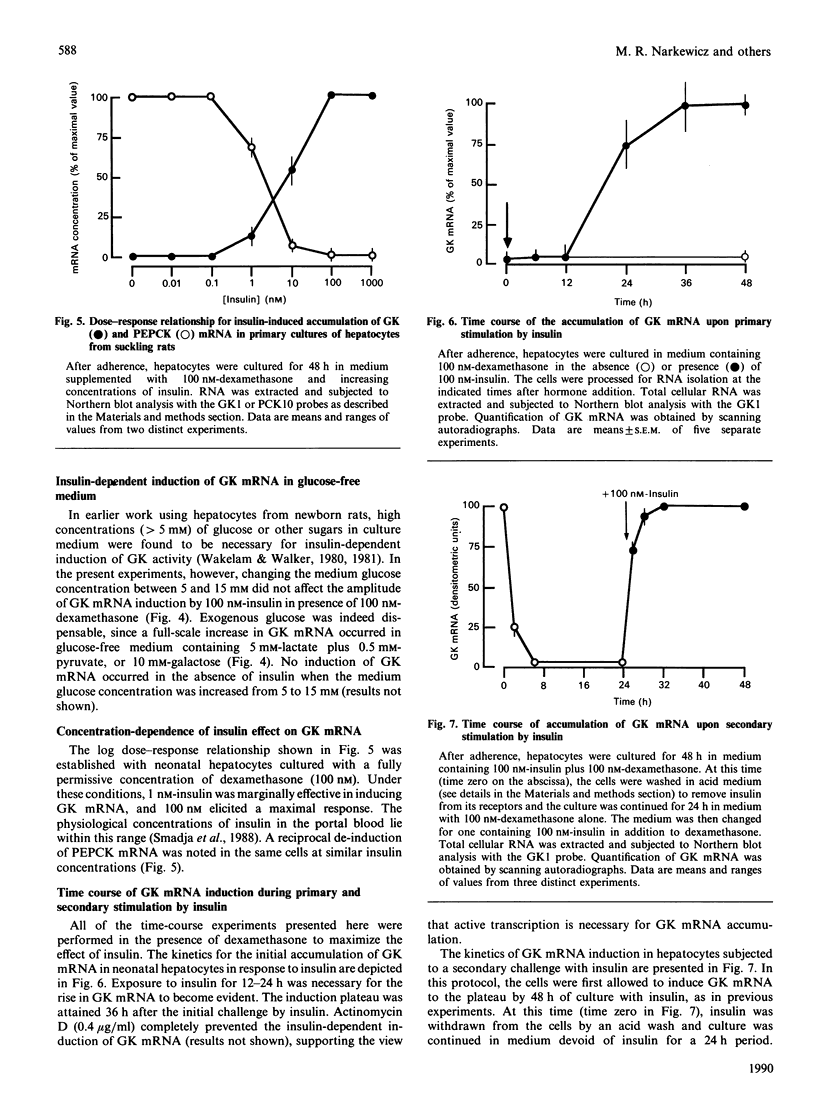
Glucokinase (EC 2.7.1.2) first appears in the liver of the rat 2 weeks after birth and increases after weaning on to a high-carbohydrate diet. We investigated the hormonal regulation of glucokinase (GK) mRNA in primary cultures of hepatocytes from 10-12-day-old suckling rats. GK mRNA was undetectable in such cells after 48 h of culture in serum-free medium devoid of hormones. Addition of insulin or tri-iodothyronine (T3) to the medium resulted in induction of GK mRNA. The effects of insulin and T3 were dose-dependent and additive. Dexamethasone alone did not induce GK mRNA, but enhanced the response to insulin and decreased the response to T3. Induction of GK mRNA by insulin was not affected when the medium glucose concentration was varied between 5 and 15 mM, nor when culture was conducted in glucose-free medium supplemented with lactate and pyruvate or galactose. The time course of initial accumulation of GK mRNA in response to insulin was characterized by a lag of 12 h and an induction plateau reached after 36 h. If hepatocytes were then withdrawn from insulin for 24 h and subsequently subjected to a secondary stimulation by insulin, GK mRNA re-accumulated with much faster kinetics and reached the fully induced level within 8 h. Both primary and secondary responses to insulin were abolished by actinomycin D. These results provide insight into the role of hormonal stimuli in the ontogenic development of hepatic glucokinase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. Dietary regulation of gene expression: enzymes involved in carbohydrate and lipid metabolism. Annu Rev Nutr. 1987;7:157–185. doi: 10.1146/annurev.nu.07.070187.001105. [DOI] [PubMed] [Google Scholar]
- Haney P. M., Estrin C. R., Caliendo A., Patel M. S. Precocious induction of hepatic glucokinase and malic enzyme in artificially reared rat pups fed a high-carbohydrate diet. Arch Biochem Biophys. 1986 Feb 1;244(2):787–794. doi: 10.1016/0003-9861(86)90647-8. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978 Nov;235(5):E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Gjinovci A., Renold A. E. Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J Biol Chem. 1988 Jan 15;263(2):740–744. [PubMed] [Google Scholar]
- Iynedjian P. B., Jotterand D., Nouspikel T., Asfari M., Pilot P. R. Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon-cAMP system. J Biol Chem. 1989 Dec 25;264(36):21824–21829. [PubMed] [Google Scholar]
- Iynedjian P. B., Ucla C., Mach B. Molecular cloning of glucokinase cDNA. Developmental and dietary regulation of glucokinase mRNA in rat liver. J Biol Chem. 1987 May 5;262(13):6032–6038. [PubMed] [Google Scholar]
- Jamdar S. C., Greengard O. Premature formation of glucokinase in developing rat liver. J Biol Chem. 1970 Jun 10;245(11):2779–2783. [PubMed] [Google Scholar]
- Magnuson M. A., Andreone T. L., Printz R. L., Koch S., Granner D. K. Rat glucokinase gene: structure and regulation by insulin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4838–4842. doi: 10.1073/pnas.86.13.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Aoyama K., Ichihara A. Precocious induction of glucokinase in primary cultures of postnatal rat hepatocytes. Biochem Biophys Res Commun. 1979 Nov 28;91(2):515–520. doi: 10.1016/0006-291x(79)91552-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Partridge N. C., Hoh C. H., Weaver P. K., Oliver I. T. Premature induction of glucokinase in the neonatal rat by thyroid hormone. Eur J Biochem. 1975 Feb 3;51(1):49–54. doi: 10.1111/j.1432-1033.1975.tb03905.x. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Smadja C., Morin J., Ferré P., Girard J. Metabolic fate of a gastric glucose load in unrestrained rats bearing a portal vein catheter. Am J Physiol. 1988 Apr;254(4 Pt 1):E407–E413. doi: 10.1152/ajpendo.1988.254.4.E407. [DOI] [PubMed] [Google Scholar]
- Spence J. T. Levels of translatable mRNA coding for rat liver glucokinase. J Biol Chem. 1983 Aug 10;258(15):9143–9146. [PubMed] [Google Scholar]
- Wakelam M. J., Walker D. G. De novo synthesis of glucokinase in hepatocytes isolated from neonatal rats. FEBS Lett. 1980 Feb 25;111(1):115–119. doi: 10.1016/0014-5793(80)80774-5. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Walker D. G. The separate roles of glucose and insulin in the induction of glucokinase in hepatocytes isolated from neonatal rats. Biochem J. 1981 May 15;196(2):383–390. doi: 10.1042/bj1960383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Eaton S. W. Regulation of development of hepatic glucokinase in the neonatal rat by the diet. Biochem J. 1967 Nov;105(2):771–777. doi: 10.1042/bj1050771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Holland G. The development of hepatic glucokinase in the neonatal rat. Biochem J. 1965 Dec;97(3):845–854. doi: 10.1042/bj0970845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P., Dubois J. D., Dussault J. H. Free thyroid hormone concentrations during postnatal development in the rat. Pediatr Res. 1980 Mar;14(3):247–249. doi: 10.1203/00006450-198003000-00014. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]