Abstract
Aims
As aging is a major risk factor for chronic diseases, strategies to promote healthy aging are essential. Dietary diversity has been reported to be beneficial for human health, however, the role in the biological aging process remains underexplored. Our aim was to analyse the potential link between diet diversity and aging.
Methods
Twenty-two thousand six hundred participants from the National Health and Nutrition Examination Survey (NHANES) were included in this study. Dietary diversity was assessed by the dietary diversity score (DDS), which aggregated data on participants’ self-reported dietary categories for the 5 major food groups (18 subgroups) over 2 rounds. Biological age was determined using the phenotypic age, with the residual between biological age and chronological age, phenotypic age acceleration, representing biological aging advance. Weighted multivariate regressions analysis were used to examine the relationship between DDS and phenotypic age acceleration. Sensitivity, subgroup interaction and mediation analyses were employed for further analysis.
Results
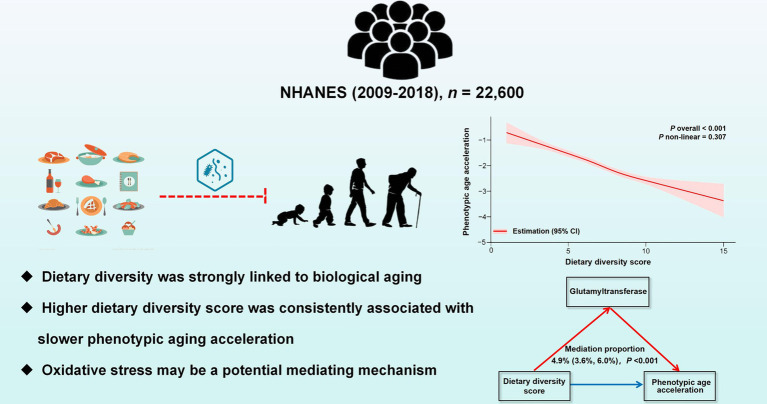
Higher DDS was consistently associated with slower phenotypic age acceleration (β < 0, p < 0.001). Subgroup analyses revealed that the inverse relationship persisted across categories, with minimal interaction effects. Sensitivity analyses confirmed the robustness of results. The oxidative stress indicator glutamyltransferase partially mediated the relationship between DDS and aging [4.9% (3.6, 6.0%), p < 0.001].
Conclusion
Dietary diversity is associated with a slower rate of biological aging, which may be due in part to reduced oxidative stress. These findings underscore the potential of a rich, broad-spectrum diet to promote healthy aging and reduce the burden of age-related diseases.
Keywords: dietary diversity score, biological aging, NHANES, mediation analysis, glutamyltransferase
Graphical abstract
1. Introduction
The accelerated aging of the global population constitutes a major public health issue (1). Aging serves as a key risk factor for numerous chronic diseases, imposing considerable economic burdens on society (2). Therefore, it is crucial to develop and implement preventive strategies and interventions that promote healthy aging.
Several factors, including genetic, environmental, and dietary habits, influence the aging process (3–5). Recently, scholars have established a number of dietary quality assessment tools that have been shown to have strong correlations with chronic disease and aging (6). For example, the Mediterranean diet (MED) (7) and the global diet quality score (QDQS) (8) have been associated with reduced mortality and increased healthy life expectancy. Both greater composite dietary antioxidant index (CDAI) (9) and reduced dietary inflammatory indices (DII) (10) have also been shown to be associated with a low risk of biological aging. However, the assessment of individual foods or nutrients does not take into account the effect of an individual’s overall dietary pattern on their health. There is a need to develop integrated and comprehensive dietary aspects to assess the potential relevance to aging.
In fact, dietary diversity is an essential consideration when assessing dietary quality and includes the intake of a variety of foods that contribute to maintaining optimal nutrient absorption. A nutritionally diverse diet, comprising foods rich in macronutrients, micronutrients, antioxidants, and bioactive compounds, is essential (11). Such dietary diversity fosters a healthier gut microbiota, which is pivotal for overall health, encompassing immune function and metabolic processes (12). Moreover, diverse food sources affect multiple aspects of the organism. A diet high in animal protein, especially red meat with elevated levels of methionine and branched-chain amino acids, may promote age-related diseases (13). On the contrary, increasing the intake of whole grains, vegetables, fruits, nuts, and fish could effectively mitigate inflammaging (14, 15). Thus, dietary diversity may better provide a comprehensive overview of overall dietary patterns than the criteria of individual dietary assessment tools (16).
Several dietary indices have been developed to adequately capture dietary variability, including the food variety score (FVS) (17), dietary diversity score (DDS) (18), and healthy food diversity index (HFDI) (19). Of note, the DDS has been shown to be a simple and effective method of estimating the diversity and balance of dietary intake, originally proposed by Kant et al. (20) and its applicability then validated in various clinical trials. DDS is a measure of counts that assesses the intake of foods or food categories, usually several food macrogroups and subgroups, over a specific timeframe. Previous research had also suggested that the DDS is associated with age-related risk factors, including diabetes mellitus (DM) (21), hypertension (22), and cardiovascular diseases (CVD) (23). All of these studies have shown that higher DDS, is associated with a lower risk of chronic disease. Nevertheless, the specific role of DDS in the biological aging process remains underexplored.
Previously, accurate assessment of organismal aging was not an easy task. The aging process is not uniform due to individual differences, resulting in varied rates of aging and differing susceptibilities to mortality and disease among populations (24). Recently, biological age, calculated from several parameters, has been reported to be useful in studying differences in aging rates (25, 26). Representative measurements of biological aging such as phenotypic age and Klemera–Doubal method (KDM) biological age, which are based on composite clinical biomarkers, have been proposed and validated according to previous studies (27). Compared to chronological age, these two novel biological indicators of aging provide a more accurate picture of organismal aging and can be more closely associated with poor prognosis such as all-cause mortality.
In short, the DDS is capable of estimating food richness, while representative novel indicators of biological age can effectively assess the rate of aging. Given that there are gaps in the link between food diversity and aging, we conducted this experiment using these practical evaluation tools. Furthermore, data from the National Health and Nutrition Examination Survey (NHANES), a practical database reflecting the nutritional status of populations in relation to disease (28, 29), was employed in this study.
2. Materials and methods
2.1. Study population
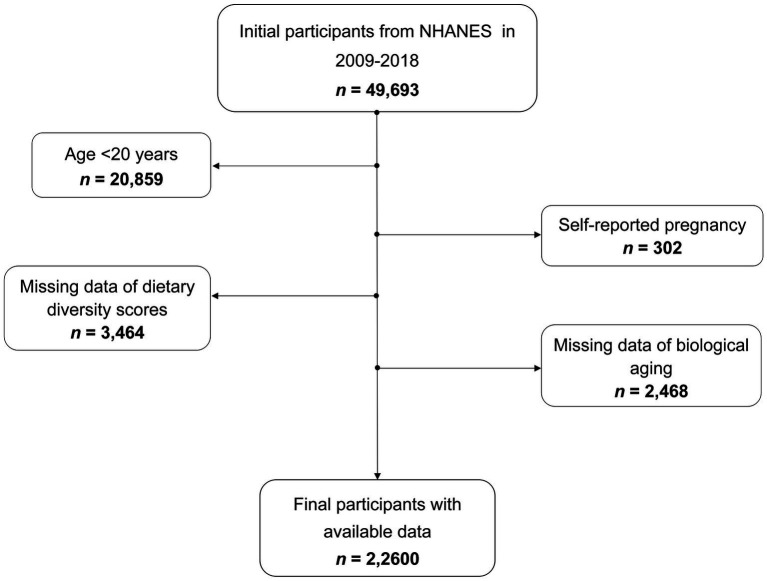
The NHANES database is a comprehensive cross-sectional survey that conducted in the United States, comprised both health interview survey and physical health examination.1 Data collection from NHANES participants has received approval from the ethics review board of the National Center for Health Statistics (NCHS). For our study, we integrated data from five NHANES cycles spanning from 2009 to 2018, initially including 49,693 participants. Due to the age <20 years or self-reported pregnancy, 21,161 participants were eliminated. We further excluded 3,464 individuals whose DDS information was not available. Additionally, 2,468 people with no biological age data were ruled out. After applying these exclusion criteria, the final study population comprised 22,600 participants (11,173 males and 11,427 females), flow chart for participant selection was presented in Figure 1.
Figure 1.
Flow chart for participant selection.
2.2. Data collection
2.2.1. Exposure variable: DDS
The DDS in this study was constructed based on the number of dietary categories in five major food groups (18 subgroups in total), according to the Food and Agriculture Organization of the United Nations food category classification guidelines and similar studies (30, 31). These include grains (whole grains, non-whole/refined grains), vegetables (dark green leafy, vitamin A-rich), fruits (citrus, vitamin A-rich), meat and protein alternatives (red meat, fish and seafood, poultry, organ meat, eggs, legumes and nuts), and dairy products (milks, concentrated dairy products, and solid dairy products). The DDS calculated the total score for all 18 subcategories, ensuring that the same food item was not counted more than once within the same subcategory. Detailed data on the foods consumed by the participants were collected based on two 24-h dietary recalls obtained from NHANES. The DDS was then calculated for each of the 2 days and averaged. A higher DDS is generally indicative of a more varied diet and is associated with a broader intake of essential nutrients. Further details on the methodology employed to derive the DDS can be found in the Supplementary material.
2.2.2. Outcome variables: biological aging
Biological age measurements serve as reliable indicators that more accurately reflect biological aging compared to chronological age. The BioAge R package has developed several validated algorithms to measure biological aging using NHANES data 2 (32). Two different measures were employed to evaluate biological age: phenotypic age and KDM biological age, derived from an algorithm that estimates mortality risk in a reference population based on biomarkers. By comparing a participant’s biological age with their chronological age, it is possible to appreciate the health status and risk of developing age-related diseases. This measure offers a more precise assessment of an individual’s biological age and risk for age-related diseases compared to chronological age alone, especially phenotypic age (33, 34).
In this study, we obtained biological age assessment variables from the NHANES dataset and used systolic blood pressure, blood creatinine, urea nitrogen, albumin, total cholesterol, glycosylated hemoglobin A1c (HbA1c), percentage of lymphocytes, mean erythrocyte volume, leukocyte count, and alkaline phosphatase as biomarkers of aging phenotype. To assess the rate of aging, phenotypic age acceleration and KDM biological age acceleration were calculated. This was calculated by taking the linear residuals of the two physiological ages (phenotypic age and KDM biological age) and the actual age, respectively. Positive residuals indicate accelerated biological aging and negative residuals indicate decelerated senescence (32–34). A higher biological aging would indicate a greater susceptibility to age-related disease, disability, and death, and vice versa. Given the superior value of phenotypic age, we used phenotypic age acceleration as the primary outcome analysis variable and KDM biological age acceleration as the sensitivity analysis indicator.
2.2.3. Covariates variables
Covariate data were collected using three primary methods: surveys, physical assessments, and laboratory analyses. The surveys gathered information on age, gender, race, education level, poverty income ratio (PIR), health conditions (DM, hypertension, CVD, and cancer), smoking status, alcohol consumption, metabolic equivalent (MET) and dietary intake. Physical assessments included body mass index (BMI). White blood cell count (WBC), neutrophil-lymphocyte ratio (NLR), albumin, total cholesterol, HbA1c, alkaline phosphatase, serum creatinine, glutamyltransferase (GGT) and serum Klotho were laboratory indicators.
Specifically, race were classified into four groups: Mexican American, non-Hispanic white, non-Hispanic black, and others. Education levels were divided into three categories: below high school, high school, and above high school. CVD was identified in participants with a history of congestive heart failure, coronary artery disease, angina, or myocardial infarction. DM was diagnosed based on previous diagnosis, the use of diabetes medication or insulin, fasting blood glucose of ≥7.0 mmol/L, glucose levels of ≥11.1 mmol/L after oral glucose intake, or HbA1c levels of ≥6.5% (35). Hypertension was recognized in individuals with a systolic pressure of ≥130 mmHg, diastolic pressure of ≥90 mmHg, a medical diagnosis of high blood pressure, or the use of blood pressure-lowering medications. Smoking status was divided into never (fewer than 100 cigarettes in a lifetime), former (over 100 cigarettes in a lifetime but not currently smoking), and current (over 100 cigarettes in a lifetime and currently smoking). Alcohol consumption was categorized as follows: never (fewer than 12 drinks in a lifetime), low-to-moderate (up to one drink per day for women and two for men over the past year), and heavy (more than one drink per day for women and two for men over the past year). A physical activity index was calculated as the sum of the estimated MET values for all activities. The individual physical activity index (MET-min/week) was derived by multiplying the MET value by the frequency per week and the activity duration. The participants were divided into three groups based on their MET score: none or <600 (min/week), 600–3,999 (min/week), and ≥4,000 (min/week) (36). The average daily caloric intake was assessed over a two-day period using data from a food consumption survey. Detailed measurement methodologies are available at https://www.cdc.gov/nchs/nhanes/.
2.3. Statistical analyses
Statistical analyses were conducted in accordance with the guidelines for the analysis of data from the NHANES, and weighted were applied. The baseline characteristics of the study participants were grouped according to the distribution of the DDS quartiles or the distribution of the phenotypic age acceleration tertiles. Continuous variables were presented as adjusted means ± standard deviation or as medians with interquartile ranges. Categorical variables were described using unweighted numbers (weighted percentages). The statistical significance of the observed differences between the groups was evaluated using the chi-square test and one-way analysis of variance (ANOVA). Weighted multiple linear regression was employed to estimate regression coefficients (β) and 95% confidence intervals (95% CI) for DDS in relation to phenotypic age acceleration across three statistical models. Model 1 did not include any confounding variables. Model 2 was adjusted for demographic factors, including age, gender, race, PIR and educational level. Model 3 further incorporated adjustments for CVD, hypertension, DM, cancer, BMI, smoking status, alcohol consumption, dietary energy and MET. Weighted logistic regression models were also employed with biological age acceleration greater than zero as the outcome variable.
Furthermore, subgroup and interaction analyses were conducted to explore the potential interactive effects of various covariates, which were pre-identified as possible effect modifiers. Restricted cubic spline (RCS) regression with four knots was employed to examine the potential non-linear trends between DDS and phenotypic age acceleration. To ensure the robustness of our findings, four sensitivity analyses were also performed using alternative exposure or outcome variables and adjusted datasets. All statistical procedures were carried out using the R software, version 4.3.2 (https://www.R-project.org; R Foundation, Austria). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Participant characteristics
The study covered 22,600 participants. The weighted baseline characteristics of the study population by phenotypic age acceleration tertiles were shown in Table 1. The participants had an average age of 47.8 ± 16.9 years, of whom 11,173 (49.3%) were male. The mean DDS was 6.7 ± 1.9. As phenotypic age acceleration increased, there was a concomitant decrease in BMI, diet energy, WBC, NLR, HbA1c, alkaline phosphatase, serum creatinine, and GGT. Furthermore, the proportion of males, current smokers, and heavy drinkers significantly increased with higher tertiles. Additionally, with increasing phenotypic age acceleration, the prevalence of CVD, hypertension, and DM also increased.
Table 1.
Characteristics of participants by tertiles of the phenotypic aging acceleration, weighted.
| Total n = 22,600 | Phenotypic age acceleration | ||||
|---|---|---|---|---|---|
| Tertile 1 (<−4.40) n = 7,534 | Tertile 2 (−4.40 to −0.65) n = 7,533 | Tertile 3 (≥−0.65) n = 7,533 | p-value | ||
| Age, years | 47.8 ± 16.9 | 48.7 ± 16.0 | 46.0 ± 17.0 | 48.9 ± 17.4 | <0.001 |
| KDM biological age | 45.4 ± 17.3 | 43.4 ± 15.5 | 42.9 ± 16.4 | 50.5 ± 19.1 | <0.001 |
| KDM biological age acceleration | −2.7 (−7.1, 1.7) | −5.4 (−9.5, −1.1) | −3.0 (−6.9, 0.7) | 0.9 (−3.7, 5.8) | <0.001 |
| Phenotypic age | 45.3 ± 17.6 | 41.7 ± 15.9 | 43.4 ± 17.1 | 51.6 ± 18.2 | <0.001 |
| Male, n (%) | 11,173 (49.3) | 2,390 (32.6) | 3,925 (53.6) | 4,858 (63.4) | <0.001 |
| Race, n (%) | <0.001 | ||||
| Mexican American | 3,346 (8.5) | 1,232 (8.8) | 1,136 (8.6) | 978 (8.0) | |
| Non-Hispanic White | 9,388 (67.5) | 2,898 (67.8) | 3,273 (68.5) | 3,217 (66.0) | |
| Non-Hispanic Black | 4,614 (10.3) | 1,205 (7.8) | 1,457 (9.7) | 1,952 (13.9) | |
| Others | 5,252 (13.8) | 2,199 (15.7) | 1,667 (13.2) | 1,386 (13.9) | |
| Poverty income ratio | 3.0 ± 1.6 | 3.2 ± 1.6 | 3.0 ± 1.6 | 2.7 ± 1.6 | <0.001 |
| Education levels, n (%) | <0.001 | ||||
| Less than high school | 2,107 (4.7) | 748 (4.9) | 640 (4.2) | 719 (5.2) | |
| High school | 8,056 (32.4) | 2,283 (26.4) | 2,649 (32.0) | 3,124 (39.6) | |
| More than high school | 12,437 (62.9) | 4,503 (68.8) | 4,244 (63.8) | 3,690 (55.2) | |
| Cardiovascular disease, n (%) | 2,373 (8.5) | 437 (5.0) | 656 (7.4) | 1,280 (13.8) | <0.001 |
| Hypertension, n (%) | 8,186 (32.1) | 2,043 (24.7) | 2,485 (29.4) | 3,658 (43.6) | <0.001 |
| Diabetes mellitus, n (%) | 4,122 (13.8) | 663 (6.4) | 1,059 (10.0) | 2,400 (26.6) | <0.001 |
| Cancer, n (%) | 2,143 (10.5) | 637 (10.7) | 701 (10.0) | 805 (10.7) | 0.098 |
| Body mass index, kg/m2 | 29.1 ± 6.8 | 27.0 ± 5.6 | 29.1 ± 6.3 | 31.7 ± 7.8 | <0.001 |
| Diet energy, kcal/day | 2,095 ± 799 | 1,990 ± 725 | 2,141 ± 789 | 2,163 ± 876 | <0.001 |
| Smoking status, n (%) | <0.001 | ||||
| No | 12,687 (56.1) | 5,032 (64.4) | 4,296 (57.1) | 3,359 (45.5) | |
| Former | 5,432 (25.1) | 1,687 (25.1) | 1,748 (24.0) | 1,997 (26.5) | |
| Current | 4,481 (18.8) | 815 (10.5) | 1,489 (18.9) | 2,177 (28.0) | |
| Alcohol consumption, n (%) | <0.001 | ||||
| No | 6,837 (23.5) | 2,545 (25.1) | 2,083 (20.9) | 2,209 (24.7) | |
| Low-to-moderate | 14,074 (67.5) | 4,537 (67.1) | 4,859 (69.7) | 4,678 (65.5) | |
| Heavy | 1,689 (9.0) | 452 (7.8) | 591 (9.4) | 646 (9.8) | |
| Metabolic equivalent, min/week | <0.001 | ||||
| <600 | 8,763 (34.3) | 2,920 (34.1) | 2,744 (31.5) | 3,099 (37.7) | |
| 600–3,999 | 8,086 (38.5) | 2,986 (43.0) | 2,698 (38.3) | 2,402 (33.7) | |
| ≥4,000 | 5,751 (27.2) | 1,628 (22.9) | 2,091 (30.3) | 2,032 (28.7) | |
| WBC, 1,000 cells/uL | 7.2 ± 2.0 | 6.0 ± 1.4 | 7.1 ± 1.6 | 8.5 ± 2.3 | <0.001 |
| NLR | 2.18 ± 1.13 | 1.78 ± 0.75 | 2.17 ± 0.94 | 2.71 ± 1.45 | <0.001 |
| Albumin, g/L | 42.7 ± 3.3 | 43.7 ± 2.9 | 42.8 ± 3.1 | 41.3 ± 3.4 | <0.001 |
| Total cholesterol, mmol/L | 4.9 ± 1.0 | 5.1 ± 1.0 | 4.9 ± 1.0 | 4.7 ± 1.0 | <0.001 |
| Glycosylated hemoglobin A1c, % | 5.6 ± 0.7 | 5.4 ± 0.4 | 5.0 ± 0.5 | 5.9 ± 1.1 | <0.001 |
| Alkaline phosphatase, U/L | 68.0 ± 21.1 | 62.6 ± 18.2 | 67.3 ± 20.1 | 74.8 ± 23.2 | <0.001 |
| Serum creatinine, umol/L | 77.3 ± 19.5 | 67.9 ± 13.5 | 77.3 ± 15.4 | 88.0 ± 23.5 | <0.001 |
| Glutamyltransferase, U/L | 19 (14, 29) | 16 (12, 24) | 19 (14, 28) | 23 (16, 35) | <0.001 |
| Serum Klotho, (pg/mL) | 844 ± 291 | 872 ± 298 | 832 ± 275 | 818 ± 293 | <0.001 |
| Dietary diversity score | 6.7 ± 1.9 | 7.1 ± 1.9 | 6.7 ± 1.9 | 6.3 ± 1.9 | <0.001 |
n (%): unweighted numbers (weighted percentage). KDM, Klemera–Doubal method; WBC, white blood cell count, NLR, neutrophil-lymphocyte ratio.
Meanwhile, Supplementary Table S1 presented the basic demographic details and covariates of the study population according to DDS quartiles. We found that the biological age acceleration gradually advanced with rising DDS. Furthermore, several prognostic indicators that usually predict a poor prognosis, such as WBC, NLR and GGT increased significantly, whereas the level of the anti-aging indicator Klotho decreased. This suggested a potential negative correlation between DDS and the biological aging process. In addition, several key characteristics of excluded (≥20 years) versus included subjects are briefly reported in Supplementary Table S2.
3.2. Relationship between DDS and biological aging
Weighted linear regression analysis was conducted to examine the association between DDS and phenotypic age acceleration, and the results were summarized in Table 2. In Model 3, after adjusting various confounders, DDS had negative associations with phenotypic age acceleration (β = −0.33; 95% CI: −0.36, −0.30; p < 0.001). Similar results were observed in categories, DDS interquartile groupings were inversely correlated with phenotypic age acceleration (β = −1.58; 95% CI: −1.73, −1.42; p for trend <0.001). The situation was similar for the other 2 models.
Table 2.
The multiple linear regression analysis for the association between dietary diversity score and phenotypic age acceleration, weighted.
| Dietary diversity score | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| Continuous | −0.39 (−0.42, −0.36) | <0.001 | −0.39 (−0.42, −0.35) | <0.001 | −0.33 (−0.36, −0.30) | <0.001 |
| Quintile 1 | Reference | Reference | Reference | |||
| Quintile 2 | −0.78 (−0.96, −0.61) | <0.001 | −0.75 (−0.92, −0.58) | <0.001 | −0.60 (−0.76, −0.45) | <0.001 |
| Quintile 3 | −1.19 (−1.35, −1.04) | <0.001 | −1.19 (−1.33, −1.04) | <0.001 | −0.99 (−1.12, −0.84) | <0.001 |
| Quintile 4 | −1.95 (−2.11, −1.78) | <0.001 | −1.92 (−2.08, −1.76) | <0.001 | −1.58 (−1.73, −1.42) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | |||
β (95% CI): estimate regression coefficients (95% confidence intervals); Model 1:crude model; Model 2: adjusted for age, gender, race, poverty income ratio and educational level; Model 3: adjusted for Model 2 and hypertension, cardiovascular disease, diabetes mellitus, cancer, body mass index, smoking status, alcohol consumption, dietary energy and metabolic equivalent.
We further applied weighted logistic regression, where phenotypic age acceleration greater than 0 indicates accelerated aging (Table 3). In Model 1 (without adjusting for covariates), the continuous variable DDS was significantly inversely associated with accelerated aging (OR: 0.86; 95% CI: 0.83, 0.88; p < 0.001). Further adjustment for demographic variables, diseases, and lifestyle factors (Model 3) did not considerably affect the results (OR: 0.84; 95% CI: 0.82, 0.86; p < 0.001). Consistent with previous results, participants in the highest quartiles of DDS showed an inverse correlation with risk of accelerated aging in all three models.
Table 3.
The results of logistic regression analysis for the association between dietary diversity score and accelerated phenotypic age, weighted.
| Dietary diversity score | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Continuous | 0.86 (0.83, 0.88) | <0.001 | 0.84 (0.82, 0.86) | <0.001 | 0.84 (0.82, 0.86) | <0.001 |
| Quintile 1 | Reference | Reference | Reference | |||
| Quintile 2 | 0.72 (0.64, 0.82) | <0.001 | 0.71 (0.62, 0.80) | <0.001 | 0.72 (0.63, 0.82) | <0.001 |
| Quintile 3 | 0.63 (0.57, 0.70) | <0.001 | 0.60 (0.55, 0.67) | <0.001 | 0.61 (0.55, 0.68) | <0.001 |
| Quintile 4 | 0.45 (0.40, 0.52) | <0.001 | 0.42 (0.37, 0.48) | <0.001 | 0.43 (0.38, 0.49) | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | |||
OR (95% CI): odd ratio (95% confidence intervals); Model 1:crude model; Model 2: adjusted for age, gender, race, poverty income ratio and educational level; Model 3: adjusted for Model 2 and hypertension, cardiovascular disease, diabetes mellitus, cancer, body mass index, smoking status, alcohol consumption, dietary energy and metabolic equivalent.
3.3. RCS analysis of the association between DDS and biological aging
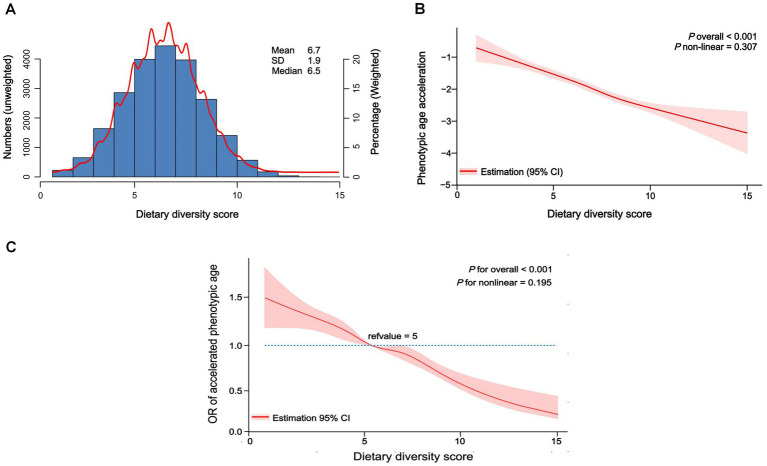
As shown in Figure 2B, the overall relationship between DDS and phenotypic age acceleration was highly significant, implying that DDS had a meaningful effect on phenotypic age acceleration (p for overall <0.001). The results also indicated that the relationship between the two was linear, as showed by p for non-linear = 0.307. The identical result was noted in DDS with accelerated biological aging (p for overall <0.001, p for non-linear = 0.195) (Figure 2C). These may imply that more complex models (non-linear) are not necessary for capturing the effect of DDS on the aging outcome.
Figure 2.
Distribution of dietary diversity score in participants and restricted cubic spline of dietary diversity score and biological aging. (A) Distribution of dietary diversity score in all participants, unweighted. (B) Restricted cubic spline of dietary diversity score and phenotypic age acceleration, weighted adjusted linear regression. (C) Restricted cubic spline of dietary diversity score and accelerated phenotypic aging, weighted adjusted linear regression.
3.4. Subgroup analysis
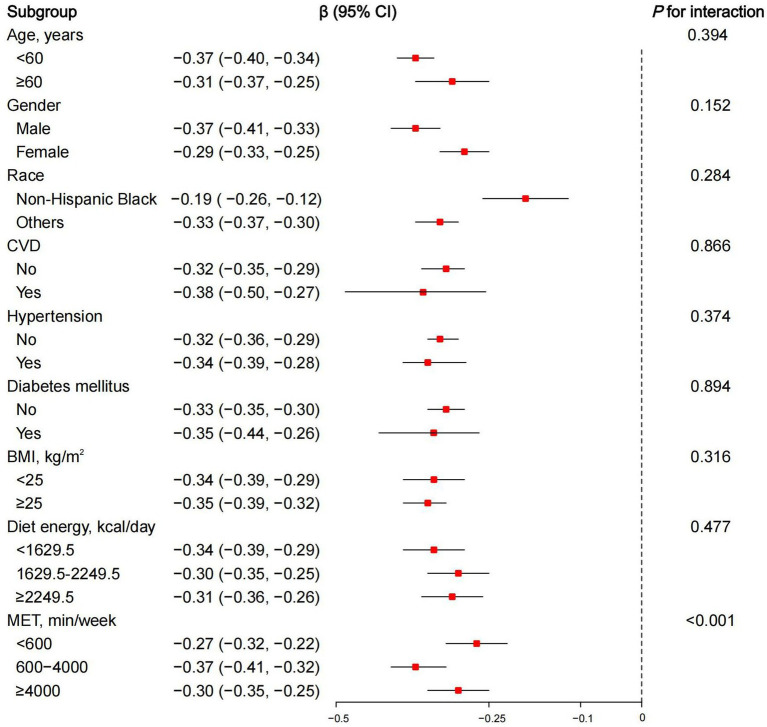
We conducted a subgroup analysis using linear regression to examine the relationship between DDS and phenotypic age acceleration across various variables, including age, gender, race, CVD, hypertension, DM, BMI, diet energy, and MET (Figure 3). Most subgroups exhibit negative β values, indicating a consistent negative effect across all categories. The interaction results suggested that most subgroups do not show significant interaction effects (p for interaction >0.05), implying that the effect is generally consistent. Further, consider that age-related biomarkers are gender-specific. We further summarized the correlations between DDS and biological aging-related markers stratified by gender (Supplementary Table S3). The findings indicated that the correlation between DDS and aging exhibited a general consistency across gender.
Figure 3.
Subgroup analysis of dietary diversity score and phenotypic age acceleration, weighted adjusted linear regression. β (95% CI): regression coefficient (95% confidence interval); CVD, cardiovascular diseases; BMI, body mass index; MET, metabolic equivalent.
3.5. Sensitivity analysis
The sensitivity analysis results presented in Table 4 demonstrate the robustness of the linear regression between DDS and phenotypic age acceleration, adjusting for different factors. First, we employed the data without multiple imputation for further analysis. Secondly, we used another measure of aging acceleration, calculated by subtracting chronological age from KDM biological age. Next, we then screened out participants without major illnesses, such as CVD, hypertension, DM, or cancer. Finally, we modified the calculation of phenotypic age acceleration by adding C-reactive protein (CRP) to the original basis. In summary, the β was consistently negative in all 3 models, suggesting a higher dietary diversity is significantly associated with lower phenotypic age acceleration, regardless of the adjustment methods employed. The results of these data revealed a robust inverse relationship between DDS and biological aging.
Table 4.
Sensitivity analysis of linear regression between dietary diversity score (continuous) and phenotypic age acceleration, weighted.
| Adjustment methods | Model 1 | Model 3 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| No multiple interpolation | −0.39 (−0.42, −0.36) | <0.001 | −0.39 (−0.42, −0.36) | <0.001 | −0.33 (−0.36, −0.30) | <0.001 |
| Alternative age acceleration a | −0.26 (−0.31, −0.21) | <0.001 | −0.13 (−0.18, −0.08) | <0.001 | −0.07 (−0.12, −0.01) | 0.008 |
| Datasets with non-illnesses b | −0.42 (−0.46, −0.39) | <0.001 | −0.33 (−0.37, −0.30) | <0.001 | −0.32 (−0.35, −0.29) | <0.001 |
| Changed age acceleration c | −0.46 (−0.50, −0.42) | <0.001 | −0.46 (−0.50, −0.42) | <0.001 | −0.38 (−0.42, −0.34) | <0.001 |
β (95% CI): regression coefficient (95% confidence interval).
Using the Klemera–Doubal method biological age to calculate age acceleration.
Cardiovascular disease or hypertension or diabetes mellitus or cancer.
Increase C-reactive protein to calculate aging (n = 11,886).
3.6. Mediation analyses
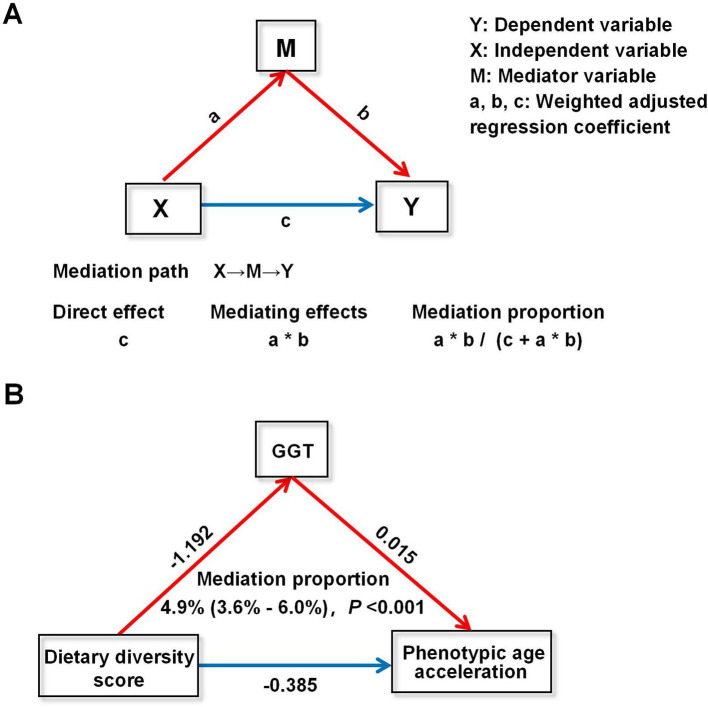
In addition, a parallel mediation analysis was conducted to assess the potential mediating effect of oxidative stress on the association of food diversity with biological aging (Figure 4A). The oxidative stress indicator GGT had a significant mediating effect on the association of DDS and phenotypic age acceleration, with a mediation ratio of 4.9% (3.6, 6.0%) (Figure 4B).
Figure 4.
Mediation analysis of dietary diversity score and phenotypic age acceleration. (A) Schematic conceptualization of mediation analysis. (B) Mediation analysis of dietary diversity score and phenotypic age acceleration. GGT, glutamyltransferase.
4. Discussion
In this study, DDS was employed to assess the food diversity of participants, while a novel integrated biological algorithm was used to generate quantitative biological aging metrics. The overall results of the study indicated that a higher DDS was associated with slower biological aging. This persistent inverse correlation was observed for both continuous and categorical variables, and the results remained reliable after correcting for multiple confounders and accounting for weighting. Moreover, a series of sensitivity analyses were performed utilising disparate aging acceleration metrics, encompassing the KDM biological age acceleration and aging algorithms covering CRP. The results of these analyses indicate a robust correlation between DDS and aging. In conclusion, the data demonstrate an inverse correlation between DDS and the rate of progression of aging. Promoting dietary diversity may facilitate healthy aging, which has significant implications for public health.
Notably, our study also identified a several features related to aging. Participants with higher DDS exhibited higher serum Klotho, a protein with anti-aging properties (37). In contrast, GGT, an indicator of oxidative stress, WBC and NLR, indicators of inflammation, were significantly lower, and levels of albumin, another potential indicator of the anti-inflammatory capacity of the response machine, were higher. It is noteworthy that the baseline data in Table 1 indicate that while a higher DDS was associated with a higher calorie intake, it was also associated with a lower BMI. A suitable explanation, similar studies in the past have demonstrated that populations with more diverse diets tend to consume more plant-based foods and less animal-based foods, which may be associated with a decrease in BMI (38). Moreover, a greater consumption of low-energy-density foods, such as fruits and vegetables, has been linked to a reduced risk of obesity and metabolic syndrome (39). Usually, obesity and metabolic syndrome were also recognized as risk factors associated with aging (40). These findings suggest that food diversity may exhibit multiple aspects of benefit in slowing the aging process.
As previously mentioned, food diversity reduces the risk of age-related chronic diseases such as depression (41) and sarcopenia (42), and is also associated with decreased mortality (43). In terms of aging, an increasing accumulation of clinical evidence is emerging to suggest that dietary diversity plays a pivotal role in the promotion of healthy aging, which is in alignment with the findings of our study. For instance, Zhang and Zhao (44) developed a healthy aging score (HAS) utilising data from the China Health and Nutrition Survey, whereby scores were standardised for physical functional limitations, comorbidities, cognitive functioning and psychological distress. The findings indicated that there was an inverse association between the DDS and the HAS (T3 vs. T1: β −0.16; 95% CI −0.20, −0.11; p for trend <0.001). The results of this study imply that dietary habits may exert an influence on the aging process, and that adherence to a varied diet is associated with healthier aging in older adults. Moreover, data from the Longitudinal Survey of Healthy Longevity indicated that a more diverse diet was associated with superior health outcomes in older adults, with a more pronounced association observed in younger older adults compared to older adults (45). Chalermsri et al. (46) found a correlation between dietary diversity and decreased mortality rates among the elderly, especially those aged 70 years or older and those classified as being underweight. These findings align with our initial conclusion that the richer the dietary variety, the later the phenotypic aging.
Although the exact mechanisms linking food diversity to the reduced aging process remain unclear, several possible explanations have been proposed. First, it is important to note that an important aspect of biological aging is cellular senescence. Oxidative stress and inflammation are key phenotypes of cellular senescence, often referred to as senescence-associated secretory phenotypes (SASP) (47). Studies have shown that a varied diet is usually richer in essential nutrients, antioxidants and anti-inflammatory compounds. This provides a more comprehensive array of vitamins, micronutrients, and phytochemicals to support metabolic health and cellular repair processes (44, 48, 49). Again, this may be a key factor in food diversity to form a healthy diet. As evidenced by our mediation analysis, we found that the oxidative stress indicator GGT played a role in the acceleration of DDS with aging, although the percentage of mediator examples was not particularly high. Nevertheless, the importance of this relationship is clear, as oxidative stress leads to a classical phenotype of cellular senescence. In addition, another important factor contributing to the extensive crosstalk between the gut and other organs is the potential benefits of high dietary diversity. These benefits can be attributed, at least in part, to a more homogeneous microbial diversity of gut bacteria. Reduced microbial diversity is usually indicative of a disrupted gut microbiota, which can lead to congestion of the gut wall, edema, slow colonic transit, and impaired intestinal tight junctions. To increased intestinal permeability, bacterial metabolites cross the intestinal barrier, which in turn elicits an immune response and exacerbates organismal aging and damage (12). In conclusion, dietary diversity can be considered as a potential anti-aging factor as it can influence human health.
There are some unique strengths to our research. It is the first to analyse the relationship between food group quantity and aging. The results for weighted large-sample data can be extended to multi-ethnic and multi-type populations. Some optimized statistical analyses demonstrated the robustness of the results. In addition, mediation analyses explained to some extent the potential associations.
Certain limitations warrant consideration in our study. The cross-sectional nature of the study limits the ability to establish causality. Future longitudinal studies are needed to confirm the causal relationship between dietary diversity and biological aging. Additionally, while our study adjusted for a comprehensive set of covariates, residual confounding cannot be entirely ruled out. Another concern is that the DDS assessment does not take into account an individual’s intake of each food item, which may need to be further optimised, and it may be more convincing to use a diversity assessment that includes a food frequency table.
5. Conclusion
In conclusion, the results of our study provide compelling evidence that a higher dietary diversity is significantly associated with a lower acceleration of phenotypic age. These findings support the hypothesis that a diverse diet may play a key role in slowing the biological aging process. Nevertheless, due to the significant limitation of cross-sectional observational studies in establishing causal relationships, further prospective interventional experiments are required to validate whether dietary diversity can indeed slow the rate of organismal aging. Moreover, further research is required to elucidate the underlying mechanisms.
Acknowledgments
The authors extend their gratitude to the NHANES participants and staff for their invaluable contributions to data collection and for their efforts to make the data publicly available.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Footnotes
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by Ethics Review Board of the National Center for Health Statistics (NCHS). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WL: Writing – original draft. M-yL: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1463569/full#supplementary-material
References
- 1.Giacomello E, Toniolo L. Nutrition, diet and healthy aging. Nutrients. (2021) 14:190. doi: 10.3390/nu14010190, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sierra F, Caspi A, Fortinsky RH, Haynes L, Lithgow GJ, Moffitt TE, et al. Moving geroscience from the bench to clinical care and health policy. J Am Geriatr Soc. (2021) 69:2455–63. doi: 10.1111/jgs.17301, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in aging research to therapeutics for healthy aging. Nature. (2019) 571:183–92. doi: 10.1038/s41586-019-1365-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan H, Pan J, Guo M, Li J, Yu L, Fan L. Dietary strategies with anti-aging potential: dietary patterns and supplements. Food Res Int. (2022) 158:111501. doi: 10.1016/j.foodres.2022.111501, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Le Couteur DG, Raubenheimer D, Solon-Biet S, de Cabo R, Simpson SJ. Does diet influence aging? Evidence from animal studies. J Intern Med. (2024) 295:400–15. doi: 10.1111/joim.13530, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB. Diet strategies for promoting healthy aging and longevity: an epidemiological perspective. J Intern Med. (2024) 295:508–31. doi: 10.1111/joim.13728, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bascuñán KA, Elli L, Vecchi M, Scricciolo A, Mascaretti F, Parisi M, et al. Mediterranean gluten-free diet: is it a fair bet for the treatment of gluten-related disorders? Front Nutr. (2020) 7:583981. doi: 10.3389/fnut.2020.583981, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung TT, Li Y, Bromage S, Bhupathiraju SN, Batis C, Fawzi W, et al. Higher global diet quality score is associated with less 4-year weight gain in US women. J Nutr. (2021) 151:162S–7S. doi: 10.1093/jn/nxab170, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Chen Y. Relationship between composite dietary antioxidant index and aging. Healthcare. (2023) 11:2722. doi: 10.3390/healthcare11202722, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Sarker SK, Cheng L, Dang K, Hu J, Pan S, et al. Association of dietary inflammatory potential, dietary oxidative balance score and biological aging. Clin Nutr. (2024) 43:1–10. doi: 10.1016/j.clnu.2023.11.007, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. (2016) 7:445–54. doi: 10.3945/an.115.011718, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amamoto R, Shimamoto K, Suwa T, Park S, Matsumoto H, Shimizu K, et al. Relationships between dietary diversity and gut microbial diversity in the elderly. Benef Microbes. (2022) 13:453–64. doi: 10.3920/BM2022.0054 [DOI] [PubMed] [Google Scholar]
- 13.Kitada M, Ogura Y, Monno I, Koya D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine. (2019) 43:632–40. doi: 10.1016/j.ebiom.2019.04.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. (2002) 13:3–9. doi: 10.1097/00041433-200202000-00002, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Di Giosia P, Stamerra CA, Giorgini P, Jamialahamdi T, Butler AE, Sahebkar A. The role of nutrition in inflammaging. Aging Res Rev. (2022) 77:101596. doi: 10.1016/j.arr.2022.101596 [DOI] [PubMed] [Google Scholar]
- 16.Drescher LS, Thiele S, Mensink GB. A new index to measure healthy food diversity better reflects a healthy diet than traditional measures. J Nutr. (2007) 137:647–51. doi: 10.1093/jn/137.3.647, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Jang W, Shin Y, Kim Y. Dietary pattern accompanied with a high food variety score is negatively associated with frailty in older adults. Nutrients. (2021) 13:3164. doi: 10.3390/nu13093164, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong WF, Song WQ, Wang XM, Li ZH, Shen D, Liu D, et al. Dietary diversity changes and cognitive frailty in Chinese older adults: a prospective community-based cohort study. Nutrients. (2023) 15:3784. doi: 10.3390/nu15173784, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahromi MK, Daftari G, Tehrani AN, Amirshekari G, Farhadnejad H, Teymoori F, et al. The association of the healthy food diversity index with the risk of non-alcoholic fatty liver disease among the adult population. Clin Nutr ESPEN. (2024) 59:404–11. doi: 10.1016/j.clnesp.2023.12.144, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Kant AK, Schatzkin A, Harris TB, Ziegler RG, Block G. Dietary diversity and subsequent mortality in the first National Health and Nutrition Examination Survey epidemiologic follow-up study. Am J Clin Nutr. (1993) 57:434–40. doi: 10.1093/ajcn/57.3.434, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Zheng G, Cai M, Liu H, Li R, Qian Z, Howard SW, et al. Dietary diversity and inflammatory diet associated with all-cause mortality and incidence and mortality of type 2 diabetes: two prospective cohort studies. Nutrients. (2023) 15:2120. doi: 10.3390/nu15092120, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gicevic S, Gaskins AJ, Fung TT, Rosner B, Tobias DK, Isanaka S, et al. Evaluating pre-pregnancy dietary diversity vs. dietary quality scores as predictors of gestational diabetes and hypertensive disorders of pregnancy. PLoS One. (2018) 13:e0195103. doi: 10.1371/journal.pone.0195103, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalermsri C, Ziaei S, Ekström EC, Muangpaisan W, Aekplakorn W, Satheannopakao W, et al. Dietary diversity associated with risk of cardiovascular diseases among community-dwelling older people: a national health examination survey from Thailand. Front Nutr. (2022) 9:1002066. doi: 10.3389/fnut.2022.1002066, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. (2018) 15:e1002718. doi: 10.1371/journal.pmed.1002718, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A. (2013) 68:667–74. doi: 10.1093/gerona/gls233, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diebel LWM, Rockwood K. Determination of biological age: geriatric assessment vs biological biomarkers. Curr Oncol Rep. (2021) 23:104. doi: 10.1007/s11912-021-01097-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mak JKL, McMurran CE, Kuja-Halkola R, Hall P, Czene K, Jylhävä J, et al. Clinical biomarker-based biological aging and risk of cancer in the UK Biobank. Br J Cancer. (2023) 129:94–103. doi: 10.1038/s41416-023-02288-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun M, Wang L, Wang X, Tong L, Fang J, Wang Y, et al. Interaction between sleep quality and dietary inflammation on frailty: NHANES 2005–2008. Food Funct. (2023) 14:1003–10. doi: 10.1039/d2fo01832b, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Cheng TD, Ferderber C, Kinder B, Wei YJ. Trends in dietary vitamin a intake among US adults by race and ethnicity, 2003–2018. JAMA. (2023) 329:1026–9. doi: 10.1001/jama.2023.0636, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng J, Zhou R, Li F, Chen L, Wu K, Huang J, et al. Association between dietary diversity and cognitive impairment among the oldest-old: findings from a nationwide cohort study. Clin Nutr. (2021) 40:1452–62. doi: 10.1016/j.clnu.2021.02.041, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Liang D, Zhao A. Dietary diversity and the risk of fracture in adults: a prospective study. Nutrients. (2020) 12:3655. doi: 10.3390/nu12123655, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacalini MG, D'Aquila P, Marasco E, Nardini C, Montesanto A, Franceschi C, et al. The methylation of nuclear and mitochondrial DNA in aging phenotypes and longevity. Mech Aging Dev. (2017) 165:156–61. doi: 10.1016/j.mad.2017.01.006, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Kwon D, Belsky DW. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience. (2021) 43:2795–808. doi: 10.1007/s11357-021-00480-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. (2018) 10:573–91. doi: 10.18632/aging.101414, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. (2022) 45:1453–61. doi: 10.2337/dc21-2371, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Diao X, Ling Y, Zeng Y, Wu Y, Guo C, Jin Y, et al. Physical activity and cancer risk: a dose-response analysis for the Global Burden of Disease Study 2019. Cancer Commun. (2023) 43:1229–43. doi: 10.1002/cac2.12488, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraham CR, Li A. Aging-suppressor Klotho: prospects in diagnostics and therapeutics. Ageing Res Rev. (2022) 82:101766. doi: 10.1016/j.arr.2022.101766, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Zhao H, Zhang J, Zhou J, Ma Y. Dietary diversity and possible cataract among Chinese elderly population. Front Nutr. (2024) 11:1342190. doi: 10.3389/fnut.2024.1342190, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salehi-Abargouei A, Akbari F, Bellissimo N, Azadbakht L. Dietary diversity score and obesity: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. (2016) 70:1–9. doi: 10.1038/ejcn.2015.118, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Föhr T, Hendrix A, Kankaanpää A, Laakkonen EK, Kujala U, Pietiläinen KH, et al. Metabolic syndrome and epigenetic aging: a twin study. Int J Obes. (2024) 48:778–87. doi: 10.1038/s41366-024-01466-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Li PW, Zhang D. Association between all aspects of dietary diversity and risk of depressive symptoms in US adults. Food Funct. (2023) 14:9204–11. doi: 10.1039/d3fo00642e, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Zheng G, Xia H, Lai Z, Shi H, Zhang J, Wang C, et al. Dietary inflammatory index and dietary diversity score associated with sarcopenia and its components: findings from a nationwide cross-sectional study. Nutrients. (2024) 16:1038. doi: 10.3390/nu16071038, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi M, Sasazuki S, Shimazu T, Sawada N, Yamaji T, Iwasaki M, et al. Association of dietary diversity with total mortality and major causes of mortality in the Japanese population: JPHC study. Eur J Clin Nutr. (2020) 74:54–66. doi: 10.1038/s41430-019-0416-y, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Zhao A. Dietary diversity and healthy aging: a prospective study. Nutrients. (2021) 13:1787. doi: 10.3390/nu13061787, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y, An Q, Rao J. The effects of dietary diversity on health status among the older adults: an empirical study from China. BMC Public Health. (2024) 24:674. doi: 10.1186/s12889-024-18172-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalermsri C, Rahman SM, Ekström EC, Ziaei S, Aekplakorn W, Satheannopakao W, et al. Dietary diversity predicts the mortality among older people: data from the fifth Thai national health examination survey. Arch Gerontol Geriatr. (2023) 110:104986. doi: 10.1016/j.archger.2023.104986, PMID: [DOI] [PubMed] [Google Scholar]
- 47.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Deledda A, Annunziata G, Tenore GC, Palmas V, Manzin A, Velluzzi F. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxidants. (2021) 10:708. doi: 10.3390/antiox10050708, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stromsnes K, Correas AG, Lehmann J, Gambini J, Olaso-Gonzalez G. Anti-inflammatory properties of diet: role in healthy aging. Biomedicines. (2021) 9:922. doi: 10.3390/biomedicines9080922, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.