Abstract
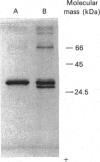
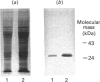
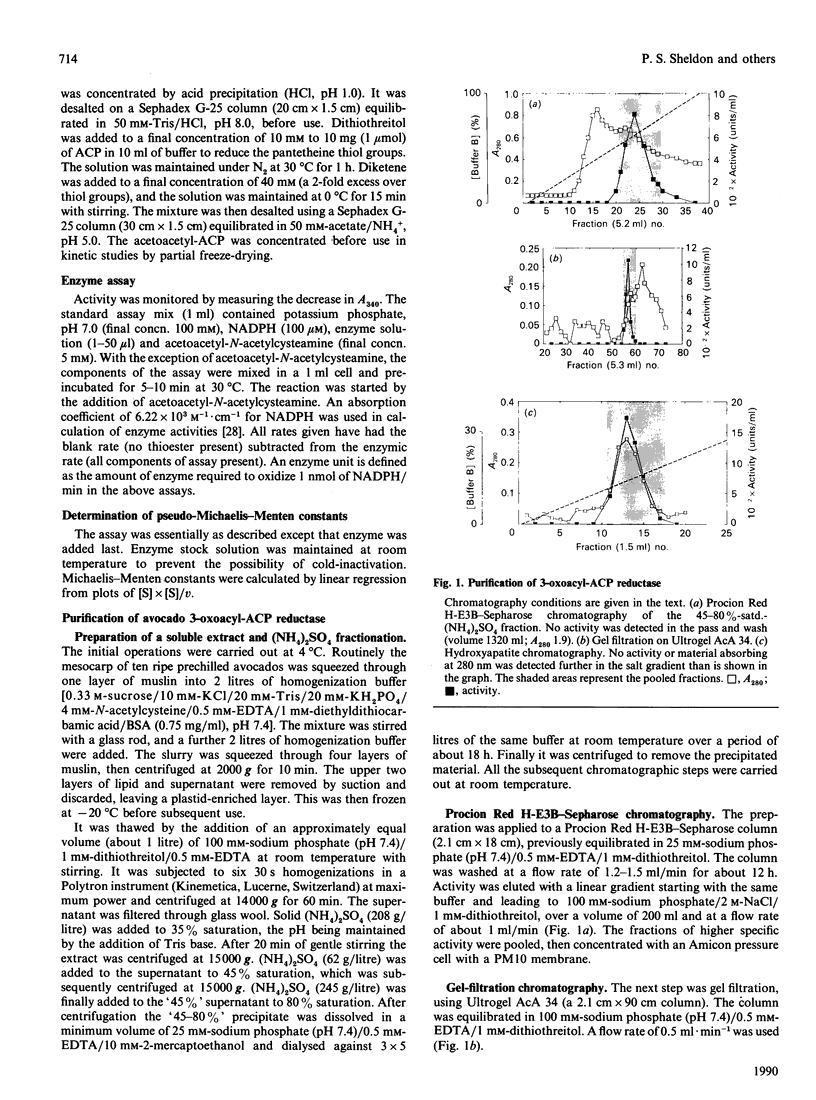
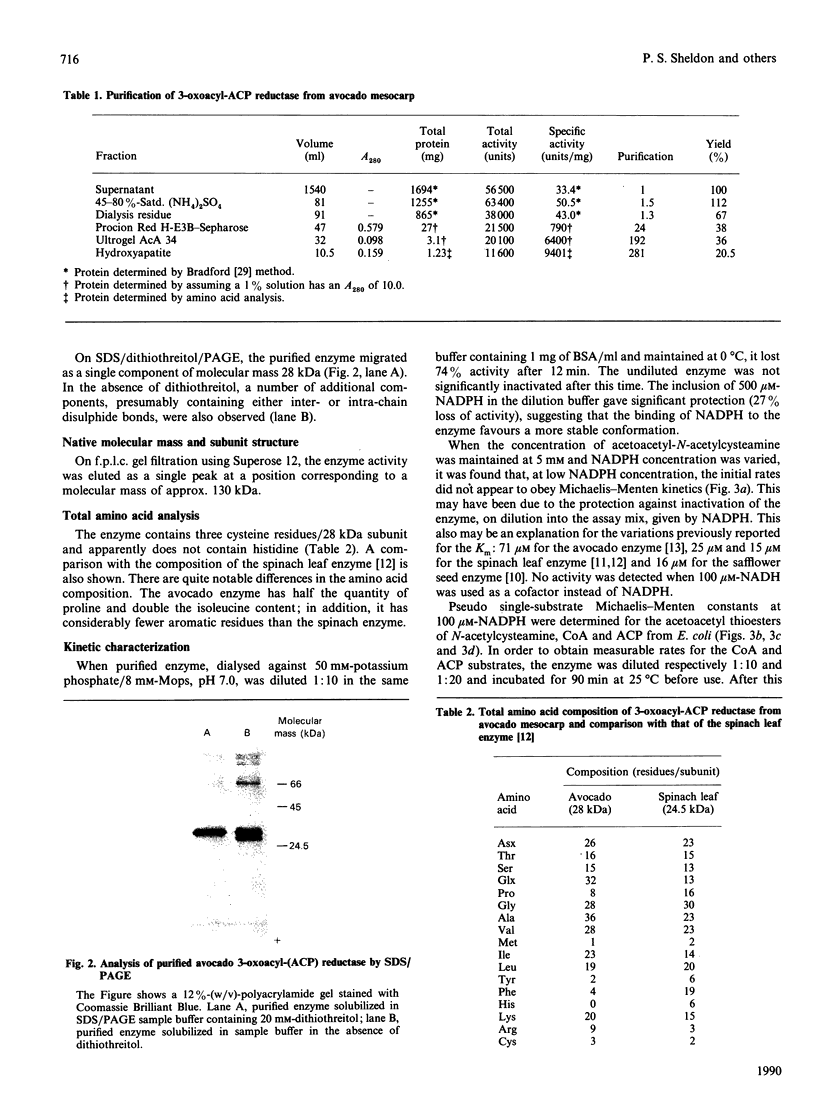
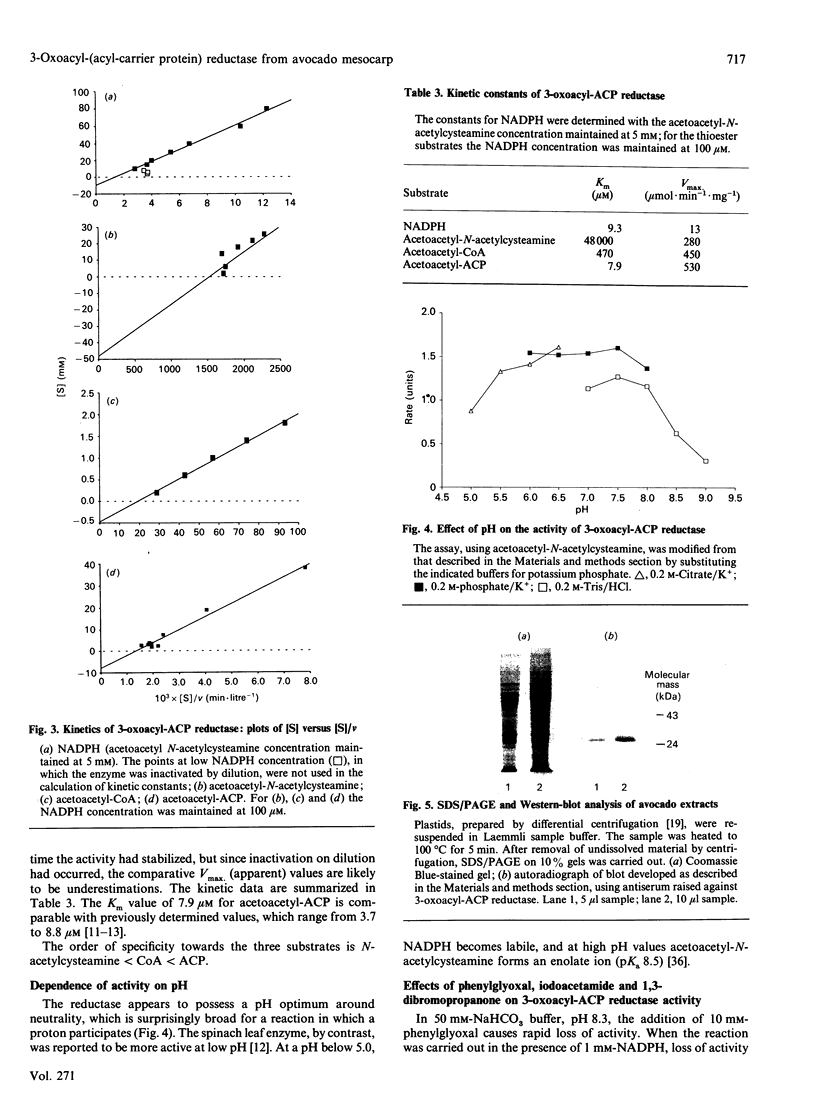
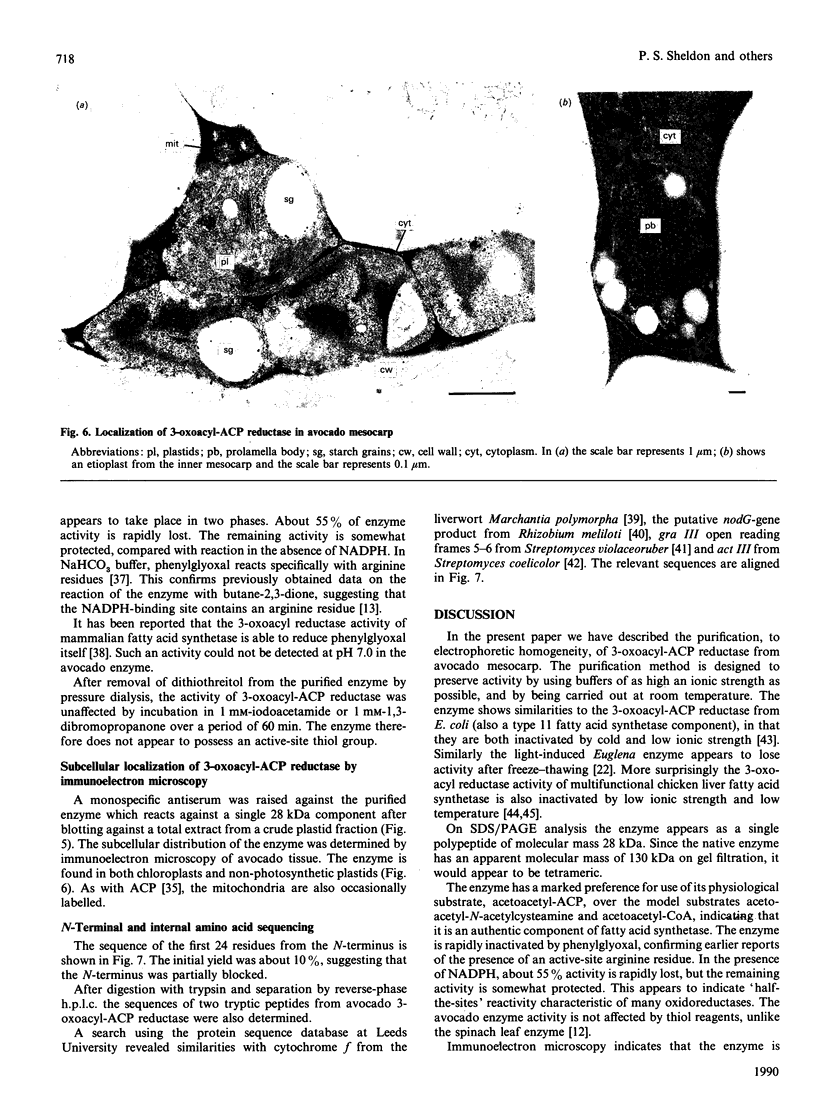
The NADPH-linked 3-oxoacyl-(acyl-carrier protein) (ACP) reductase (EC 1.1.1.100), also known as 'beta-ketoacyl-ACP reductase', has been purified from the mesocarp of mature avocado pears (Persea americana). The enzyme is inactivated by low ionic strength and low temperature. On SDS/PAGE under reducing conditions, purified 3-oxoacyl-ACP reductase migrated as a single polypeptide giving a molecular mass of 28 kDa. Gel-filtration chromatography gave an apparent native molecular mass of 130 kDa, suggesting that the enzyme is tetrameric. The enzyme is inactivated by dilution, but some protection is afforded by the presence of NADPH. Kinetic constants have been determined using synthetic analogues as well as the natural ACP substrate. It exhibits a broad pH optimum around neutrality. Phenylglyoxal inactivates the enzyme, and partial protection is given by 1 mM-NADPH. Antibodies have been raised against the protein, which were used to localize it using immunogold electron microscopy. It is localized in plastids. N-Terminal amino-acid-sequence analysis was performed on the enzyme, and it shows close structural similarity with cytochrome f. Internal amino-acid-sequence data, derived from tryptic peptides, shows similarity with the putative gene products encoded by the nodG gene from the nitrogen-fixing bacterium Rhizobium meliloti and the gra III act III genes from Streptomyces spp.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Biró S., Motamedi H., Collins J. F., Hutchinson C. R. Analysis of the nucleotide sequence of the Streptomyces glaucescens tcmI genes provides key information about the enzymology of polyketide antibiotic biosynthesis. EMBO J. 1989 Sep;8(9):2727–2736. doi: 10.1002/j.1460-2075.1989.tb08414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K., Vance D. Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem. 1977;46:263–298. doi: 10.1146/annurev.bi.46.070177.001403. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caughey I., Kekwick R. G. The characteristics of some components of the fatty acid synthetase system in the plastids from the mesocarp of avocado (Persea americana) fruit. Eur J Biochem. 1982 Apr;123(3):553–561. doi: 10.1111/j.1432-1033.1982.tb06568.x. [DOI] [PubMed] [Google Scholar]
- Cheung S. T., Fonda M. L. Reaction of phenylglyoxal with arginine. The effect of buffers and pH. Biochem Biophys Res Commun. 1979 Oct 12;90(3):940–947. doi: 10.1016/0006-291x(79)91918-1. [DOI] [PubMed] [Google Scholar]
- Debellé F., Sharma S. B. Nucleotide sequence of Rhizobium meliloti RCR2011 genes involved in host specificity of nodulation. Nucleic Acids Res. 1986 Sep 25;14(18):7453–7472. doi: 10.1093/nar/14.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst-Fonberg M. L. An NADH-Dependent Acetoacetyl-CoA Reductase from Euglena gracilis: Purification and Characterization, Including Inhibition by Acyl Carrier Protein. Plant Physiol. 1986 Dec;82(4):978–984. doi: 10.1104/pp.82.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fulco A. J. Fatty acid metabolism in bacteria. Prog Lipid Res. 1983;22(2):133–160. doi: 10.1016/0163-7827(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Guy P., Law S., Hardie G. Mammalian fatty acid synthetase: evidence for subunit identity and specific removal of the thioesterase component using elastase digestion. FEBS Lett. 1978 Oct 1;94(1):33–37. doi: 10.1016/0014-5793(78)80900-4. [DOI] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Hendren R. W., Bloch K. Fatty acid synthetases from Euglena gracilis. Separation of component activities of the ACP-dependent fatty acid synthetase and partial purification of the beta-ketoacyl-ACP synthetase. J Biol Chem. 1980 Feb 25;255(4):1504–1508. [PubMed] [Google Scholar]
- Johnson D. M., Gagnon J., Reid K. B. Factor D of the alternative pathway of human complement. Purification, alignment and N-terminal amino acid sequences of the major cyanogen bromide fragments, and localization of the serine residue at the active site. Biochem J. 1980 Jun 1;187(3):863–874. doi: 10.1042/bj1870863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem M. A., Hammes G. G. Correlation of enzymatic activities and aggregation state in chicken liver fatty acid synthase. Biochim Biophys Acta. 1988 Aug 31;956(1):39–48. doi: 10.1016/0167-4838(88)90295-6. [DOI] [PubMed] [Google Scholar]
- LYNEN F., OCHOA S. Enzymes of fatty acid metabolism. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):299–314. doi: 10.1016/0006-3002(53)90149-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Lowe C. R., Pearson J. C. Affinity chromatography on immobilized dyes. Methods Enzymol. 1984;104:97–113. doi: 10.1016/s0076-6879(84)04085-4. [DOI] [PubMed] [Google Scholar]
- McCarthy A. D., Hardie D. G. The multifunctional polypeptide chains of rabbit-mammary fatty-acid synthase. Stoichiometry of active sites and active-site mapping using limited proteolysis. Eur J Biochem. 1983 Jan 17;130(1):185–193. doi: 10.1111/j.1432-1033.1983.tb07135.x. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose A. J., Kolattukudy P. E. Enzymatic reduction of phenylglyoxal and 2,3-butanedione, two commonly used arginine-modifying reagents, by the ketoacyl reductase domain of fatty acid synthase. Int J Biochem. 1986;18(9):807–812. doi: 10.1016/0020-711x(86)90057-1. [DOI] [PubMed] [Google Scholar]
- Schulz H., Wakil S. J. Studies on the mechanism of fatty acid synthesis. XXV. On the mechanism of beta-ketoacylacyl carrier protein reductase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1895–1901. [PubMed] [Google Scholar]
- Schweizer E., Werkmeister K., Jain M. K. Fatty acid biosynthesis in yeast. Mol Cell Biochem. 1978 Nov 1;21(2):95–107. doi: 10.1007/BF00240280. [DOI] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. H., Malpartida F., Bibb M. J., Kieser H. M., Bibb M. J., Hopwood D. A. Structure and deduced function of the granaticin-producing polyketide synthase gene cluster of Streptomyces violaceoruber Tü22. EMBO J. 1989 Sep;8(9):2717–2725. doi: 10.1002/j.1460-2075.1989.tb08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Fatty Acid Synthetase of Spinacia oleracea Leaves. Plant Physiol. 1982 Jun;69(6):1257–1262. doi: 10.1104/pp.69.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Purification and characterizations of beta-Ketoacyl-[acyl-carrier-protein] reductase, beta-hydroxyacyl-[acyl-carrier-protein] dehydrase, and enoyl-[acyl-carrier-protein] reductase from Spinacia oleracea leaves. Arch Biochem Biophys. 1982 Oct 1;218(1):77–91. doi: 10.1016/0003-9861(82)90323-x. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. The procaryotic nature of the fatty acid synthetase of developing Carthamus tinctorius L. (Safflower) seeds. Arch Biochem Biophys. 1982 Aug;217(1):144–154. doi: 10.1016/0003-9861(82)90488-x. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Arslanian M. J., Oh Y. H., Aune K. C., Vanaman T. C., Wakil S. J. Presence of two polypeptide chains comprising fatty acid synthetase. Proc Natl Acad Sci U S A. 1975 May;72(5):1940–1944. doi: 10.1073/pnas.72.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Fatty Acid synthesis in endosperm of young castor bean seedlings. Plant Physiol. 1978 Aug;62(2):173–178. doi: 10.1104/pp.62.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Saturated fatty acid biosynthesis and its regulation. Annu Rev Biochem. 1973;42:21–60. doi: 10.1146/annurev.bi.42.070173.000321. [DOI] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. Y., Hammes G. G. Fluorescence studies of chicken liver fatty acid synthase. Segmental flexibility and distance measurements. J Biol Chem. 1986 Oct 15;261(29):13643–13651. [PubMed] [Google Scholar]