Abstract
A beta-lactamase from Mycobacterium fortuitum D316 was purified and some physico-chemical properties and substrate profile determined. On the basis of its N-terminal sequence and of its sensitivity to beta-iodopenicillanate inactivation, the enzyme appeared to be a class A beta-lactamase, but its substrate profile was quite unexpected, since nine cephalosporins were among the eleven best substrates. The enzyme also hydrolysed ureidopenicillins and some so-called 'beta-lactamase-stable' cephalosporins.
Full text
PDF


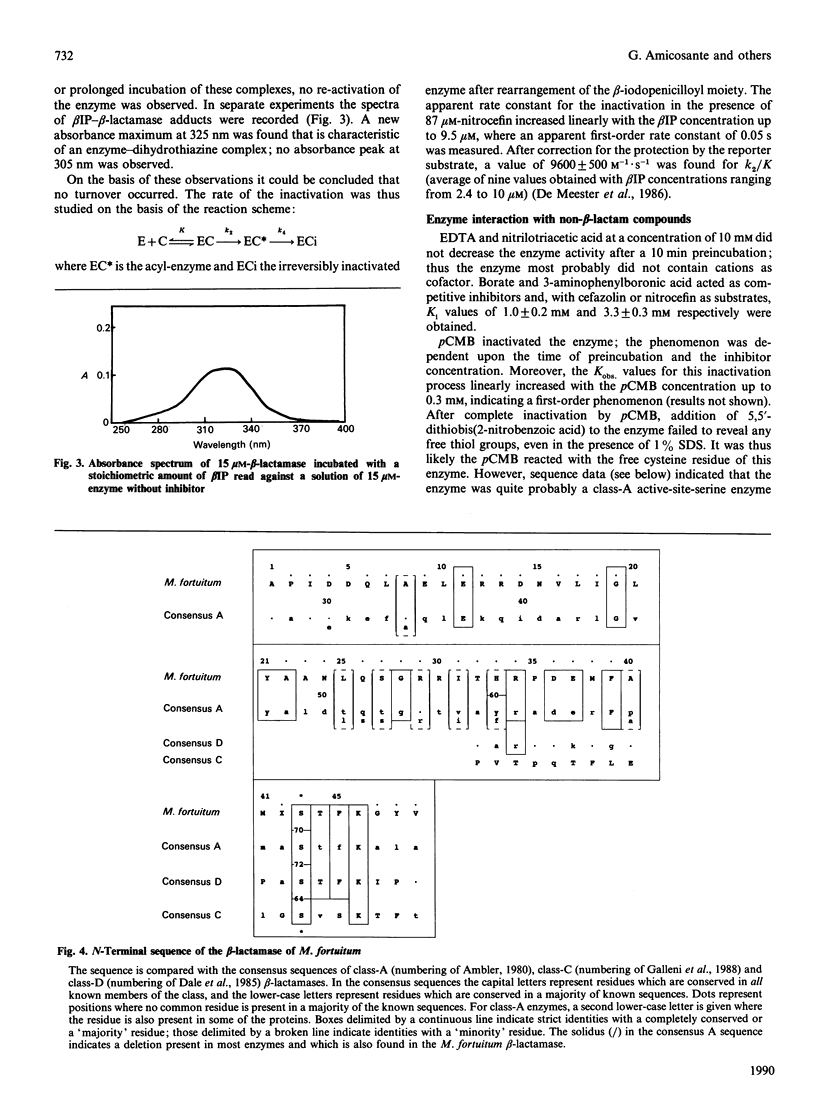


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Amicosante G., Oratore A., Franceschini N., Maccarrone M., Strom R., Galleni M., Frère J. M. Citrobacter diversus ULA-27 beta-lactamases. Improved purification and general properties. Biochem J. 1988 Sep 15;254(3):885–890. doi: 10.1042/bj2540885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989 Mar;33(3):264–270. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini D., Graziani M. T., Dupré S. Determination of disulphide groups in proteins. Nature. 1966 Oct 15;212(5059):294–295. doi: 10.1038/212294a0. [DOI] [PubMed] [Google Scholar]
- Choubey D., Gopinathan K. P. Characterization of beta-lactamase from Mycobacterium butyricum ATCC 19979. Biochem Int. 1986 Feb;12(2):207–214. [PubMed] [Google Scholar]
- Cynamon M. H., Palmer G. S. In vitro susceptibility of Mycobacterium fortuitum to N-formimidoyl thienamycin and several cephamycins. Antimicrob Agents Chemother. 1982 Dec;22(6):1079–1081. doi: 10.1128/aac.22.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynamon M. H., Palmer G. S. In vitro susceptibility of Mycobacterium fortuitum to amoxicillin or cephalothin in combination with clavulanic acid. Antimicrob Agents Chemother. 1983 Jun;23(6):935–937. doi: 10.1128/aac.23.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J. W., Godwin D., Mossakowska D., Stephenson P., Wall S. Sequence of the OXA2 beta-lactamase: comparison with other penicillin-reactive enzymes. FEBS Lett. 1985 Oct 21;191(1):39–44. doi: 10.1016/0014-5793(85)80989-3. [DOI] [PubMed] [Google Scholar]
- De Meester F., Frère J. M., Waley S. G., Cartwright S. J., Virden R., Lindberg F. 6-beta-Iodopenicillanate as a probe for the classification of beta-lactamases. Biochem J. 1986 Nov 1;239(3):575–580. doi: 10.1042/bj2390575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester F., Joris B., Reckinger G., Bellefroid-Bourguignon C., Frère J. M., Waley S. G. Automated analysis of enzyme inactivation phenomena. Application to beta-lactamases and DD-peptidases. Biochem Pharmacol. 1987 Jul 15;36(14):2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- Fattorini L., Fiorentino D., Amicosante G., Franceschini N., Oratore A., Orefici G. Beta-lactamase production and biological characteristics in nitrosoguanidine induced Mycobacterium fortuitum mutants. Acta Leprol. 1989;7 (Suppl 1):44–47. [PubMed] [Google Scholar]
- Galleni M., Lindberg F., Normark S., Cole S., Honore N., Joris B., Frere J. M. Sequence and comparative analysis of three Enterobacter cloacae ampC beta-lactamase genes and their products. Biochem J. 1988 Mar 15;250(3):753–760. doi: 10.1042/bj2500753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., De Meester F., Galleni M., Frère J. M., Van Beeumen J. The K1 beta-lactamase of Klebsiella pneumoniae. Biochem J. 1987 Apr 15;243(2):561–567. doi: 10.1042/bj2430561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda S., Yabu K. Purification and some properties of beta-lactamase from Mycobacterium smegmatis. Microbiol Immunol. 1983;27(2):191–193. doi: 10.1111/j.1348-0421.1983.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Kasik J. E., Peacham L. Properties of beta-lactamases produced by three species of mycobacteria. Biochem J. 1968 May;107(5):675–682. doi: 10.1042/bj1070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matagne A., Misselyn-Bauduin A. M., Joris B., Erpicum T., Granier B., Frère J. M. The diversity of the catalytic properties of class A beta-lactamases. Biochem J. 1990 Jan 1;265(1):131–146. doi: 10.1042/bj2650131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Harris A. M. Identification of beta-lactamases by analytical isoelectric focusing: correlation with bacterial taxonomy. J Gen Microbiol. 1976 May;94(1):55–67. doi: 10.1099/00221287-94-1-55. [DOI] [PubMed] [Google Scholar]
- Oliva B., Marinucci M. C., Amicosante G., Oratore A., Bennett P. M. Citrobacter diversus ULA27 produces two forms of a chromosomal beta-lactamase. J Antimicrob Chemother. 1987 Jul;20(1):23–35. doi: 10.1093/jac/20.1.23. [DOI] [PubMed] [Google Scholar]
- Ricci G., Nardini M., Chiaraluce R., Duprè S., Cavallini D. Interaction of pantetheinase with sulfhydryl reagents and disulfides. Biochim Biophys Acta. 1986 Mar 7;870(1):82–91. doi: 10.1016/0167-4838(86)90011-7. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Nash D. R., Udou T., Steingrube V. A., Steele L. C., Swenson J. M., Silcox V. A. Isoelectric focusing of beta-lactamases in Mycobacterium fortuitum. Association of a single enzyme pattern with cefoxitin resistance. Am Rev Respir Dis. 1985 Nov;132(5):1093–1097. doi: 10.1164/arrd.1985.132.5.1093. [DOI] [PubMed] [Google Scholar]
- Woodruff H. B., Foster J. W. Microbiological Aspects of Penicillin: VII. Bacterial Penicillinase. J Bacteriol. 1945 Jan;49(1):7–17. doi: 10.1128/jb.49.1.7-17.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu K., Kaneda S., Ochiai T. Relationship between beta-lactamase activity and resistance to beta-lactam antibiotics in Mycobacterium smegmatis. Microbiol Immunol. 1985;29(9):803–809. doi: 10.1111/j.1348-0421.1985.tb00883.x. [DOI] [PubMed] [Google Scholar]



