Abstract
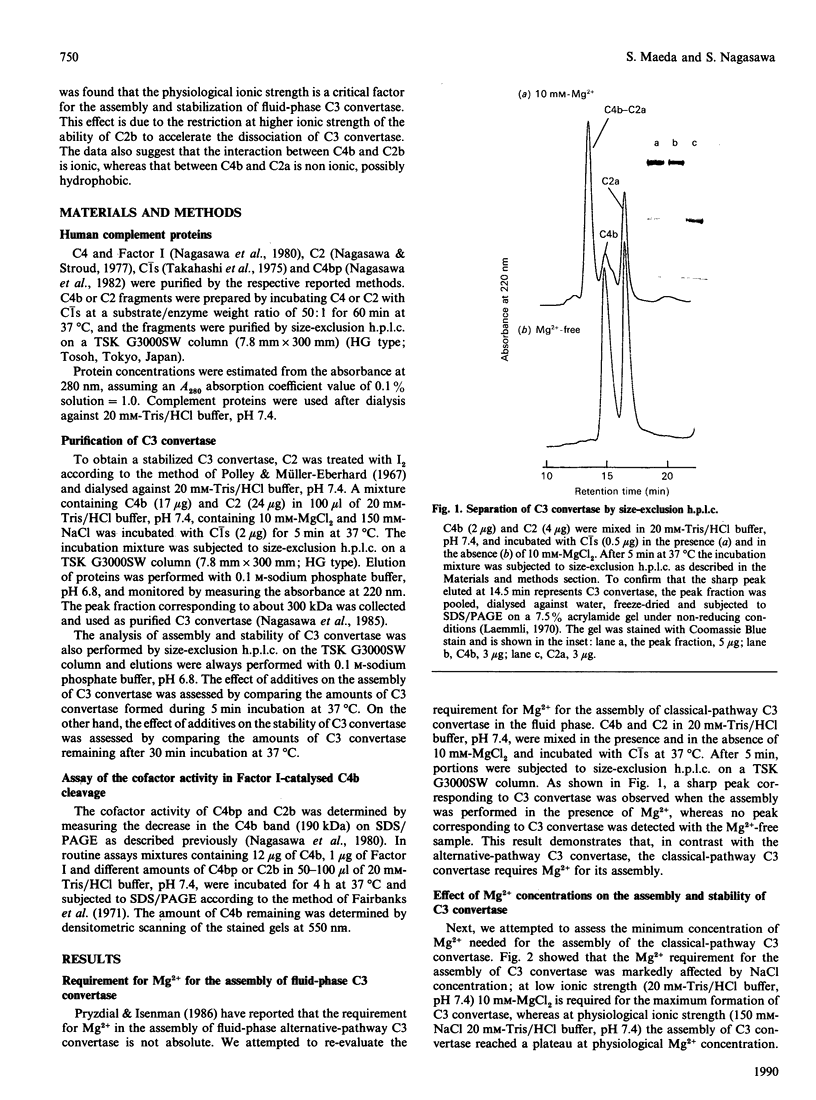
The assembly of the classical-pathway C3 convertase from C4 and I2-treated C2 by the action of C1s is an Mg2(+)-dependent reaction. The Mg2+ concentration necessary for the assembly of C3 convertase in the fluid phase was found to be dependent on NaCl concentration. In the absence of NaCl more than 5 mM-MgCl2 was found to be required, whereas 0.5 mM-MgCl2 was adequate for the assembly of C3 convertase in the presence of 150 mM-NaCl. The C3 convertase assembled in a low-ionic-strength buffer was extremely labile compared with that assembled in buffer of physiological ionic strength, and the stability of C3 convertase was improved with the increase in NaCl concentration. It was found that the stabilizing effect of NaCl on C3 convertase was due to inhibition of the dissociating activity of C2b, which was formed during the assembly of C3 convertase. In addition to the dissociation-accelerating effect, C2b inhibited the assembly of C3 convertase in low-ionic-strength buffer, and this effect also was diminished with increase in NaCl concentration. An increase in NaCl concentration to more than 200 mM resulted in a decrease in the assembly of C3 convertase. This effect was not due to the lability of the assembled C3 convertase but due rather to the inhibition of C2 cleavage by C1s. Purified C3 convertase itself is stable in dilute medium or high-ionic-strength medium such as 500 mM-NaCl, suggesting that the interactions between C4b and C2a are hydrophobic. In these respects C2b seemed to be functionally similar to C4bp, but C2b failed to act as a cofactor for the Factor I-catalysed C4b cleavage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. R. Primary structure of human complement component C2. Homology to two unrelated protein families. Biochem J. 1986 Oct 15;239(2):339–345. doi: 10.1042/bj2390339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fishelson Z., Müller-Eberhard H. J. C3 convertase of human complement: enhanced formation and stability of the enzyme generated with nickel instead of magnesium. J Immunol. 1982 Dec;129(6):2603–2607. [PubMed] [Google Scholar]
- Gigli I., Fujita T., Nussenzweig V. Modulation of the classical pathway C3 convertase by plasma proteins C4 binding protein and C3b inactivator. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6596–6600. doi: 10.1073/pnas.76.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara C., Nakamura T., Nagasawa S., Koyama J. Monoclonal anti-human C4b antibodies: stabilization and inhibition of the classical-pathway C3 convertase. Mol Immunol. 1986 Feb;23(2):151–157. doi: 10.1016/0161-5890(86)90037-4. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Functional properties of membrane-associated complement receptor CR1. J Immunol. 1983 Apr;130(4):1876–1880. [PubMed] [Google Scholar]
- Kerr M. A. The human complement system: assembly of the classical pathway C3 convertase. Biochem J. 1980 Jul 1;189(1):173–181. doi: 10.1042/bj1890173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley B. J., Campbell R. D. Internal homologies of the Ba fragment from human complement component Factor B, a class III MHC antigen. EMBO J. 1984 Jan;3(1):153–157. doi: 10.1002/j.1460-2075.1984.tb01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Nagasawa S., Kobayashi C., Maki-Suzuki T., Yamashita N., Koyama J. Purification and characterization of the C3 convertase of the classical pathway of human complement system by size exclusion high-performance liquid chromatography. J Biochem. 1985 Feb;97(2):493–499. doi: 10.1093/oxfordjournals.jbchem.a135083. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Mizuguchi K., Ichihara C., Koyama J. Limited chymotryptic cleavage of human C4-binding protein: isolation of a carbohydrate-containing core domain and an active fragment. J Biochem. 1982 Oct;92(4):1329–1332. doi: 10.1093/oxfordjournals.jbchem.a134052. [DOI] [PubMed] [Google Scholar]
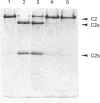
- Nagasawa S., Stroud R. M. Cleavage of C2 by C1s into the antigenically distinct fragments C2a and C2b: demonstration of binding of C2b to C4b. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2998–3001. doi: 10.1073/pnas.74.7.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Oglesby T. J., Accavitti M. A., Volanakis J. E. Evidence for a C4b binding site on the C2b domain of C2. J Immunol. 1988 Aug 1;141(3):926–931. [PubMed] [Google Scholar]
- Parkes C., Gagnon J., Kerr M. A. The reaction of iodine and thiol-blocking reagents with human complement components C2 and factor B. Purification and N-terminal amino acid sequence of a peptide from C2a containing a free thiol group. Biochem J. 1983 Jul 1;213(1):201–209. doi: 10.1042/bj2130201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzdial E. L., Isenman D. E. A reexamination of the role of magnesium in the human alternative pathway of complement. Mol Immunol. 1986 Jan;23(1):87–96. doi: 10.1016/0161-5890(86)90175-6. [DOI] [PubMed] [Google Scholar]
- Pryzdial E. L., Isenman D. E. Alternative complement pathway activation fragment Ba binds to C3b. Evidence that formation of the factor B-C3b complex involves two discrete points of contact. J Biol Chem. 1987 Feb 5;262(4):1519–1525. [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Nagasawa S., Koyama J. The NH-2-terminal sequences of a subunit of the first component of human complement, C1s, and its activated form, C1s. FEBS Lett. 1975 Feb 15;50(3):330–333. doi: 10.1016/0014-5793(75)80521-7. [DOI] [PubMed] [Google Scholar]
- Villiers M. B., Thielens N. M., Colomb M. G. Soluble C3 proconvertase and convertase of the classical pathway of human complement. Conditions of stabilization in vitro. Biochem J. 1985 Mar 1;226(2):429–436. doi: 10.1042/bj2260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt W., Hinsch B., Schmidt G., von Zabern I. Function of the activated fourth component of complement (C4b) in activation of C2. FEBS Lett. 1982 Aug 2;144(2):195–198. doi: 10.1016/0014-5793(82)80636-4. [DOI] [PubMed] [Google Scholar]