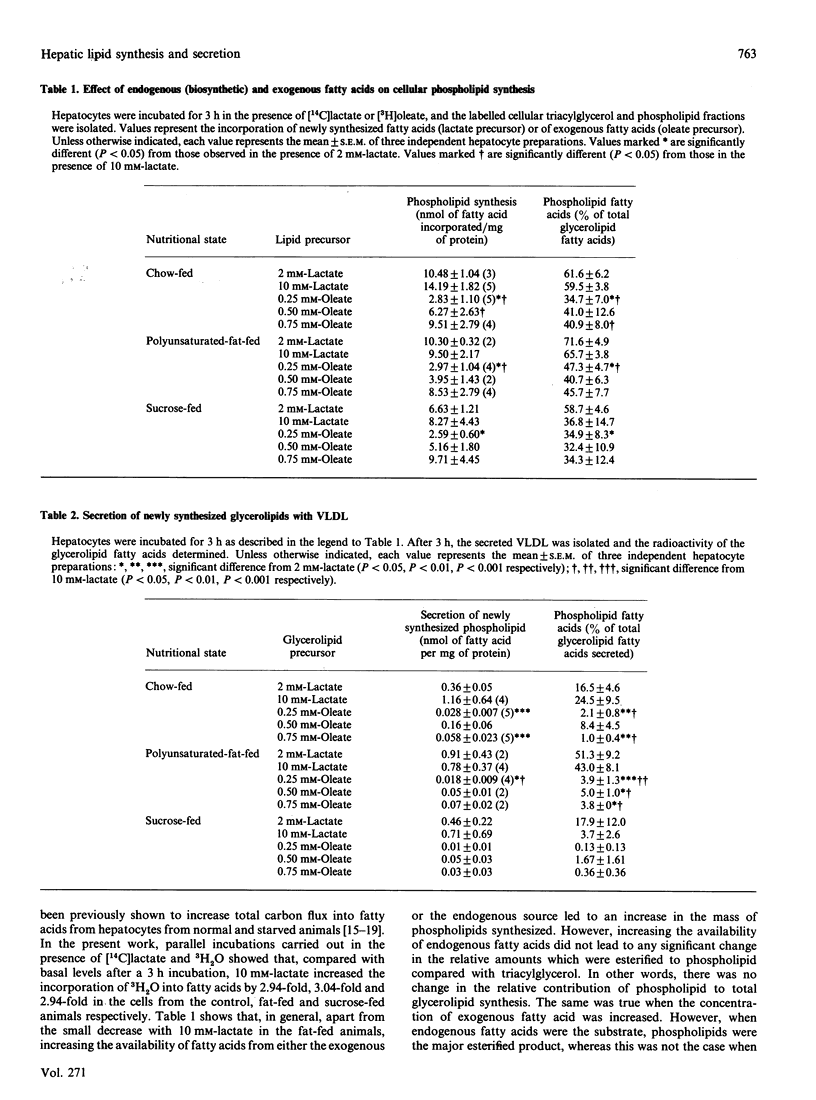
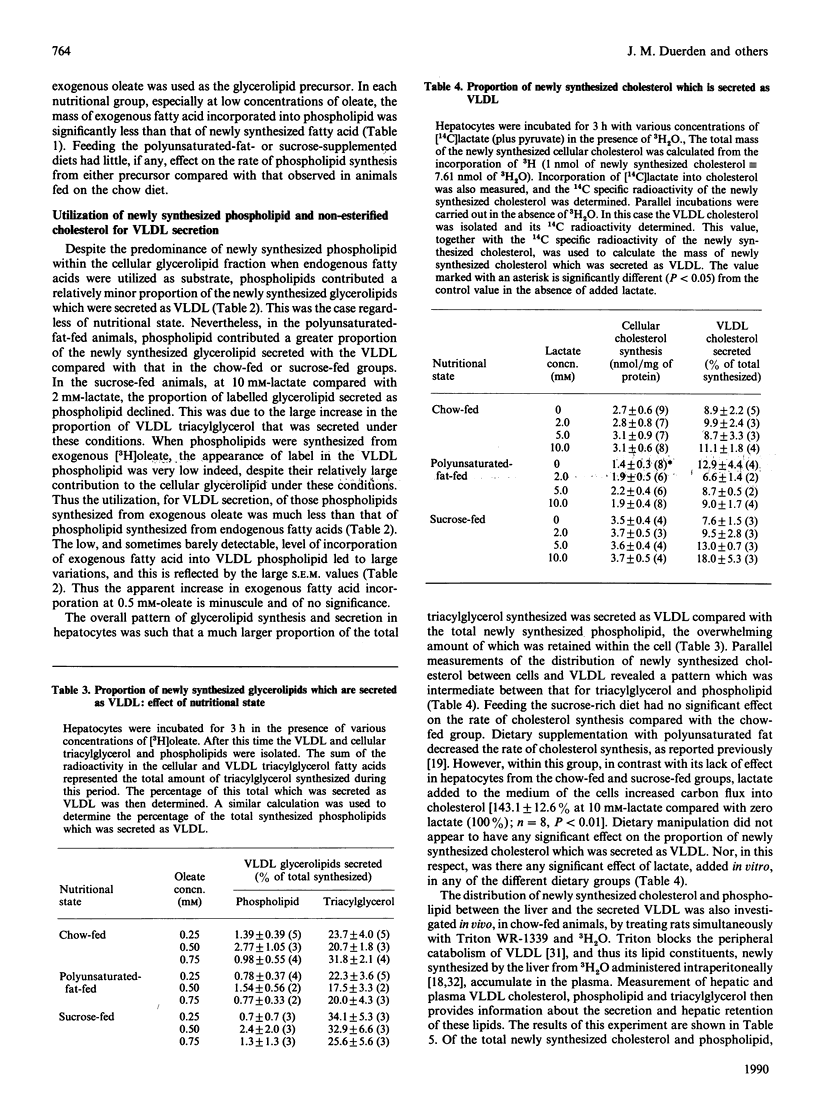
Abstract
The distribution of newly synthesized and exogenous fatty acids and of newly synthesized cholesterol between cellular and very-low-density lipoprotein (VLDL) lipids was studied in hepatocytes derived from animals fed on a normal diet or on diets supplemented with polyunsaturated fat or sucrose. Phospholipid synthesis from either exogenous or endogenous (biosynthetic) fatty acids was unaffected by nutritional state. Cholesterol synthesis was decreased in the fat-fed animals, but sucrose feeding had no significant effect. In all nutritional states, newly synthesized rather than exogenous fatty acids were better substrates for phospholipid synthesis. In all groups, compared with newly synthesized triacylglycerol, smaller proportions of newly synthesized phospholipid and cholesterol were secreted as VLDLs. This was confirmed in intact animals by using Triton WR-1339. Newly synthesized phospholipid formed a greater proportion of the VLDL glycerolipid in the fat-fed than in the normal or sucrose-fed animals. In all groups, phospholipids labelled from endogenous fatty acids were secreted in preference to those labelled from exogenous fatty acids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Blackshear P. J., Williamson D. H. Rates of triacylglycerol entry into the circulation in the lactating rat. Biochem J. 1981 May 15;196(2):637–640. doi: 10.1042/bj1960637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azain M. J., Fukuda N., Chao F. F., Yamamoto M., Ontko J. A. Contributions of fatty acid and sterol synthesis to triglyceride and cholesterol secretion by the perfused rat liver in genetic hyperlipemia and obesity. J Biol Chem. 1985 Jan 10;260(1):174–181. [PubMed] [Google Scholar]
- Boogaerts J. R., Malone-McNeal M., Archambault-Schexnayder J., Davis R. A. Dietary carbohydrate induces lipogenesis and very-low-density lipoprotein synthesis. Am J Physiol. 1984 Jan;246(1 Pt 1):E77–E83. doi: 10.1152/ajpendo.1984.246.1.E77. [DOI] [PubMed] [Google Scholar]
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao F. F., Stiers D. L., Ontko J. A. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. J Lipid Res. 1986 Nov;27(11):1174–1181. [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Secretion and storage of newly synthesized hepatic triacylglycerol fatty acids in vivo in different nutritional states and in diabetes. Biochem J. 1988 Nov 1;255(3):929–935. doi: 10.1042/bj2550929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A. G., Louis L. N., Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973 Jan;76(1):41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Francone O. L., Kalopissis A. D., Griffaton G. Contribution of cytoplasmic storage triacylglycerol to VLDL-triacylglycerol in isolated rat hepatocytes. Biochim Biophys Acta. 1989 Mar 14;1002(1):28–36. doi: 10.1016/0005-2760(89)90060-x. [DOI] [PubMed] [Google Scholar]
- Fukuda N., Azain M. J., Ontko J. A. Altered hepatic metabolism of free fatty acids underlying hypersecretion of very low density lipoproteins in the genetically obese Zucker rats. J Biol Chem. 1982 Dec 10;257(23):14066–14072. [PubMed] [Google Scholar]
- Gibbons G. F. Assembly and secretion of hepatic very-low-density lipoprotein. Biochem J. 1990 May 15;268(1):1–13. doi: 10.1042/bj2680001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Attwell Thomas C. P., Pullinger C. R. The metabolic route by which oleate is converted into cholesterol in rat hepatocytes. Biochem J. 1986 Apr 1;235(1):19–24. doi: 10.1042/bj2350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R., Björnsson O. G. Changes in the sensitivity of lipogenesis in rat hepatocytes to hormones and precursors over the diurnal cycle and during longer-term starvation of donor animals. J Lipid Res. 1984 Dec 1;25(12):1358–1367. [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Diurnal variations in the effects of an unsaturated-fat-containing diet on fatty acid and cholesterol synthesis in rat hepatocytes. Biochem J. 1986 Nov 1;239(3):617–623. doi: 10.1042/bj2390617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Pullinger C. R. Regulation of hepatic very-low-density lipoprotein secretion in rats fed on a diet high in unsaturated fat. Biochem J. 1987 Apr 15;243(2):487–492. doi: 10.1042/bj2430487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb S., Barber T. A., Pariza M. W., Pugh T. D. Lipid synthesis and ultrastructure of adult rat hepatocytes during their first twenty-four hours in culture. Exp Cell Res. 1978 Nov;117(1):39–46. doi: 10.1016/0014-4827(78)90425-1. [DOI] [PubMed] [Google Scholar]
- Groener J. E., van Golde L. M. Effect of fasting and feeding a high-sucrose, fat-free diet on the synthesis of hepatic glycerolipids in vivo and in isolated hepatocytes. Biochim Biophys Acta. 1977 Apr 26;487(1):105–114. doi: 10.1016/0005-2760(77)90047-9. [DOI] [PubMed] [Google Scholar]
- Groener J. E., van Golde L. M. Utilization of exogenously added and endogenously synthesized fatty acids for glycerolipids synthesis in isolated rat hepatocytes. Biochim Biophys Acta. 1978 Apr 28;529(1):88–95. doi: 10.1016/0005-2760(78)90106-6. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Fieldsend J. K. Phosphatidylcholine synthesis for incorporation into membranes or for secretion as plasma lipoproteins by Golgi membranes of rat liver. J Lipid Res. 1987 Mar;28(3):268–278. [PubMed] [Google Scholar]
- Higgins J. A., Hutson J. L. The roles of Golgi and endoplasmic reticulum in the synthesis and assembly of lipoprotein lipids in rat hepatocytes. J Lipid Res. 1984 Dec 1;25(12):1295–1305. [PubMed] [Google Scholar]
- Holub B. J. Differential utilization of 1-palmitoyl and 1-stearoyl homologues of various unsaturated 1,2-diacyl-sn-glycerols for phosphatidylcholine and phosphatidylethanolamine synthesis in rat liver microsomes. J Biol Chem. 1978 Feb 10;253(3):691–696. [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Kalopissis A. D., Griglio S., Malewiak M. I., Rozen R., Liepvre X. L. Very-low-density-lipoprotein secretion by isolated hepatocytes of fat-fed rats. Biochem J. 1981 Aug 15;198(2):373–377. doi: 10.1042/bj1980373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrup J. Metabolism of palmitate in perfused rat liver. Isolation of subcellular fractions containing triacylglycerol. Biochem J. 1979 Oct 15;184(1):63–71. doi: 10.1042/bj1840063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S. O., Bjursell G., Boström K., Carlsson P., Elovson J., Protter A. A., Reuben M. A., Bondjers G. Apolipoprotein B: structure, biosynthesis and role in the lipoprotein assembly process. Atherosclerosis. 1987 Nov;68(1-2):1–17. doi: 10.1016/0021-9150(87)90088-8. [DOI] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967 May;190(2):321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Pritchard P. H., Brindley D. N., Vance D. E. Fatty acids promote translocation of CTP:phosphocholine cytidylyltransferase to the endoplasmic reticulum and stimulate rat hepatic phosphatidylcholine synthesis. J Biol Chem. 1983 Jun 10;258(11):6782–6788. [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of oleate and compactin on the metabolism and secretion of cholesterol and cholesteryl ester by rat hepatocytes. Biochim Biophys Acta. 1982 Nov 12;713(2):323–332. doi: 10.1016/0005-2760(82)90250-8. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. The relationship between the rate of hepatic sterol synthesis and the incorporation of [3H]water. J Lipid Res. 1983 Oct;24(10):1321–1328. [PubMed] [Google Scholar]
- Salmon D. M., Bowen N. L., Hems D. A. Synthesis of fatty acids in the perused mouse liver. Biochem J. 1974 Sep;142(3):611–618. doi: 10.1042/bj1420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R., Akesson B., Nilsson A. Effect of different fatty acids on glycerolipid synthesis in isolated rat hepatocytes. J Biol Chem. 1974 Aug 25;249(16):5102–5107. [PubMed] [Google Scholar]
- Thomas G., Loriette C., Pepin D., Chambaz J., Bereziat G. Selective channelling of arachidonic and linoleic acids into glycerolipids of rat hepatocytes in primary culture. Biochem J. 1988 Dec 1;256(2):641–647. doi: 10.1042/bj2560641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijburg L. B., Geelen M. J., van Golde L. M. Regulation of the biosynthesis of triacylglycerol, phosphatidylcholine and phosphatidylethanolamine in the liver. Biochim Biophys Acta. 1989 Jul 17;1004(1):1–19. doi: 10.1016/0005-2760(89)90206-3. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. Does rat liver Golgi have the capacity to synthesize phospholipids for lipoprotein secretion? J Biol Chem. 1988 Apr 25;263(12):5898–5909. [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. The role of phosphatidylcholine biosynthesis in the secretion of lipoproteins from hepatocytes. Can J Biochem Cell Biol. 1985 Aug;63(8):870–881. doi: 10.1139/o85-108. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yamamoto I., Tanaka Y., Ontko J. A. Fatty acid metabolism and lipid secretion by perfused livers from rats fed laboratory stock and sucrose-rich diets. J Lipid Res. 1987 Oct;28(10):1156–1165. [PubMed] [Google Scholar]
- Yao Z. M., Vance D. E. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988 Feb 25;263(6):2998–3004. [PubMed] [Google Scholar]


