Abstract
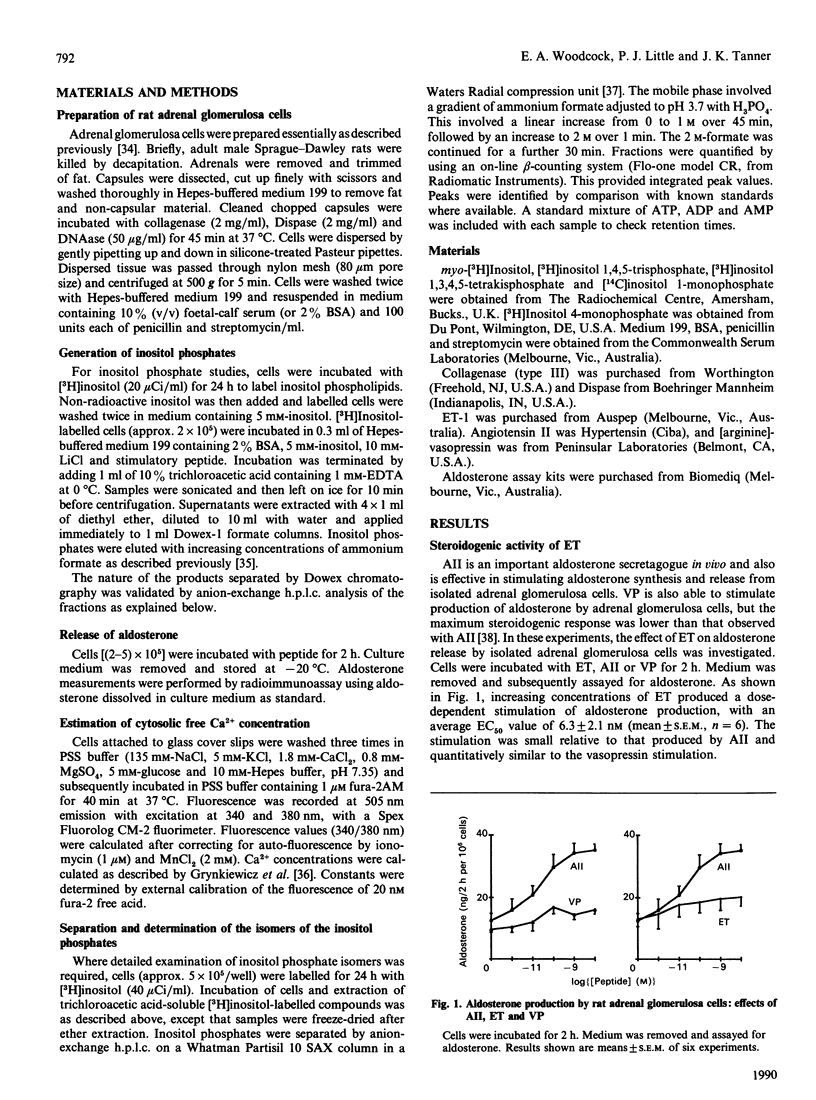
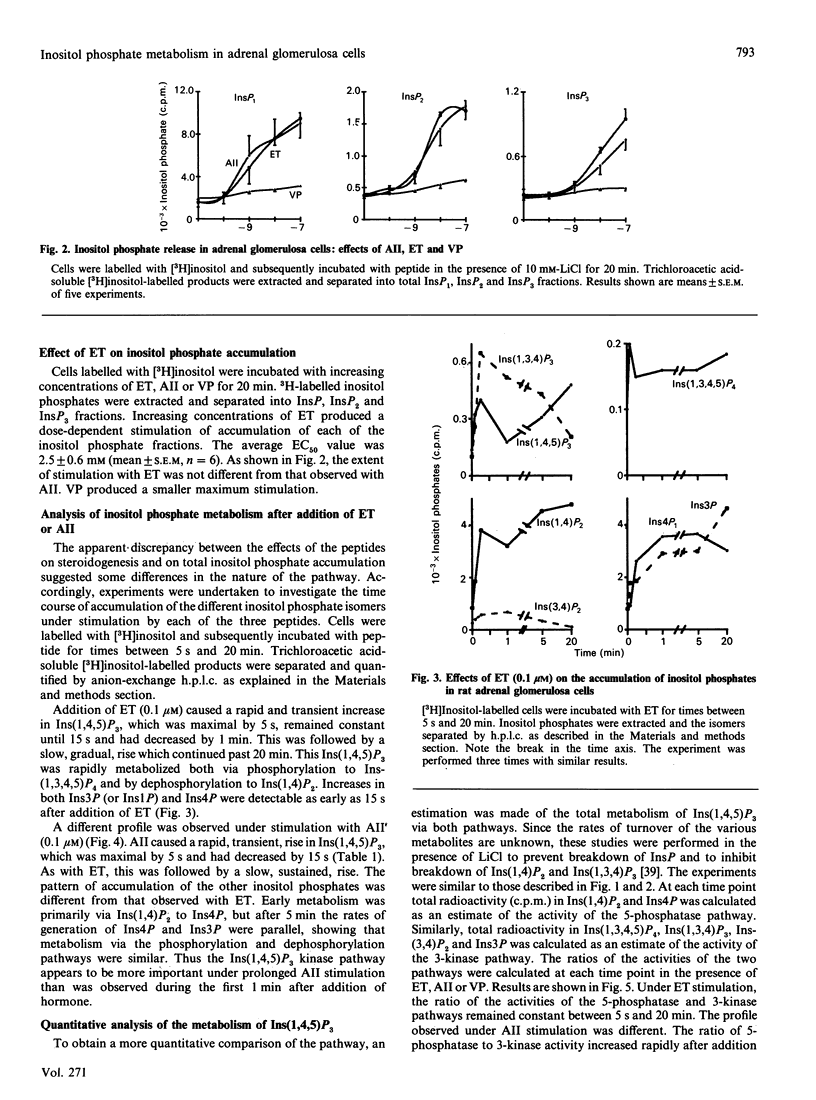
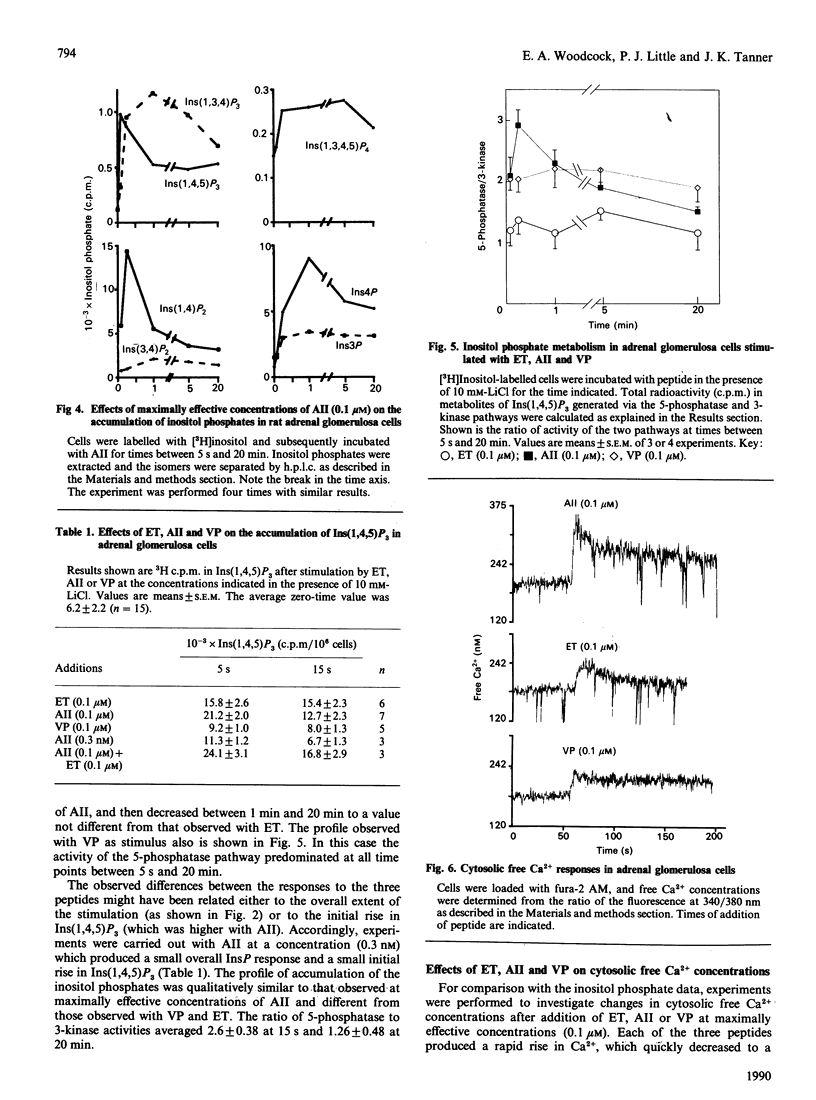
Endothelin has steroidogenic activity in adrenal glomerulosa cells, as do two other vasoconstrictor peptides, angiotensin II and vasopressin. The steroidogenic activities of angiotensin II and vasopressin are probably mediated via the phosphatidylinositol-turnover pathway and associated changes in cytosolic Ca2+ concentration. Endothelin caused a steroidogenic response, which was small compared with that to angiotensin II and quantitatively similar to the vasopressin response. Cytosolic free Ca2+ responses were similarly higher to angiotensin II than to either of the other two peptides. However, total inositol phosphate responses to endothelin and angiotensin II were similar when these were measured over 20 min, and were quantitatively greater than the vasopressin response. A detailed study has been made of the phosphatidylinositol-turnover response to endothelin in comparison with responses to angiotensin II and vasopressin. Each of the three peptides produced a rapid and transient rise in Ins(1,4,5)P3 (max. 5-15 s), followed by a slow sustained rise. Ins(1,4,5)P3 was metabolized by both dephosphorylation and phosphorylation pathways, but the relative importance of the two metabolic pathways was different under stimulation by each of the three peptides. These findings show that adrenal glomerulosa cells can distinguish between the stimulation of phosphatidylinositol turnover by three different effectors. These differences in the pathway may be associated with the observed different steroidogenic and Ca2+ responses to the three peptides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Catt K. J. Participation of voltage-dependent calcium channels in the regulation of adrenal glomerulosa function by angiotensin II and potassium. Endocrinology. 1986 Jan;118(1):112–118. doi: 10.1210/endo-118-1-112. [DOI] [PubMed] [Google Scholar]
- Aiyar N., Nambi P., Stassen F. L., Crooke S. T. Vascular vasopressin receptors mediate phosphatidylinositol turnover and calcium efflux in an established smooth muscle cell line. Life Sci. 1986 Jul 7;39(1):37–45. doi: 10.1016/0024-3205(86)90435-2. [DOI] [PubMed] [Google Scholar]
- Ambler S. K., Thompson B., Solski P. A., Brown J. H., Taylor P. Receptor-mediated inositol phosphate formation in relation to calcium mobilization: a comparison of two cell lines. Mol Pharmacol. 1987 Sep;32(3):376–383. [PubMed] [Google Scholar]
- Baukal A. J., Balla T., Hunyady L., Hausdorff W., Guillemette G., Catt K. J. Angiotensin II and guanine nucleotides stimulate formation of inositol 1,4,5-trisphosphate and its metabolites in permeabilized adrenal glomerulosa cells. J Biol Chem. 1988 May 5;263(13):6087–6092. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Quantitative analysis of the cytosolic-free-Ca2+-dependency of aldosterone production in bovine adrenal glomerulosa cells. Different requirements for angiotensin II and K+. Biochem J. 1987 Oct 15;247(2):335–340. doi: 10.1042/bj2470335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate and inositol 1,4,5-trisphosphate act by different mechanisms when controlling Ca2+ in mouse lacrimal acinar cells. FEBS Lett. 1989 Jul 17;251(1-2):43–48. doi: 10.1016/0014-5793(89)81425-5. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Cozza E. N., Gomez-Sanchez C. E., Foecking M. F., Chiou S. Endothelin binding to cultured calf adrenal zona glomerulosa cells and stimulation of aldosterone secretion. J Clin Invest. 1989 Sep;84(3):1032–1035. doi: 10.1172/JCI114226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guillon G., Gallo-Payet N., Balestre M. N., Lombard C. Cholera-toxin and corticotropin modulation of inositol phosphate accumulation induced by vasopressin and angiotensin II in rat glomerulosa cells. Biochem J. 1988 Aug 1;253(3):765–775. doi: 10.1042/bj2530765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. D., Dean N. M., Boynton A. L. Inositol 1,3,4,5-tetrakisphosphate induces Ca2+ sequestration in rat liver cells. Science. 1988 Nov 25;242(4882):1176–1178. doi: 10.1126/science.2847317. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Yoshimi H., Takata S., Watanabe T. X., Kumagai S., Nakajima K., Sakakibara S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):868–875. doi: 10.1016/0006-291x(88)90220-3. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Takemura H., Putney J. W., Jr Formation and metabolism of [3H]inositol phosphates in AR42J pancreatoma cells. Substance P-induced Ca2+ mobilization in the apparent absence of inositol 1,4,5-trisphosphate 3-kinase activity. J Biol Chem. 1988 Oct 25;263(30):15297–15303. [PubMed] [Google Scholar]
- Hu J. R., Berninger U. G., Lang R. E. Endothelin stimulates atrial natriuretic peptide (ANP) release from rat atria. Eur J Pharmacol. 1988 Dec 6;158(1-2):177–178. doi: 10.1016/0014-2999(88)90271-3. [DOI] [PubMed] [Google Scholar]
- Hu J. R., Von Harsdorf R., Lang R. E. Endothelin has potent inotropic effects in rat atria. Eur J Pharmacol. 1988 Dec 13;158(3):275–278. doi: 10.1016/0014-2999(88)90079-9. [DOI] [PubMed] [Google Scholar]
- Hughes P. J., Drummond A. H. Formation of inositol phosphate isomers in GH3 pituitary tumour cells stimulated with thyrotropin-releasing hormone. Acute effects of lithium ions. Biochem J. 1987 Dec 1;248(2):463–470. doi: 10.1042/bj2480463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Hansen C. A., Williamson J. R. Inositol 1,3,4,5-tetrakisphosphate increases the duration of the inositol 1,4,5-trisphosphate-mediated Ca2+ transient. FEBS Lett. 1987 Jul 13;219(1):125–129. doi: 10.1016/0014-5793(87)81203-6. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Pertussis toxin blocks angiotensin II-induced calcium influx but not inositol trisphosphate production in adrenal glomerulosa cell. FEBS Lett. 1986 Aug 18;204(2):347–351. doi: 10.1016/0014-5793(86)80841-9. [DOI] [PubMed] [Google Scholar]
- Lips D. L., Majerus P. W., Gorga F. R., Young A. T., Benjamin T. L. Phosphatidylinositol 3-phosphate is present in normal and transformed fibroblasts and is resistant to hydrolysis by bovine brain phospholipase C II. J Biol Chem. 1989 May 25;264(15):8759–8763. [PubMed] [Google Scholar]
- Marie J., Jard S. Angiotensin II inhibits adenylate cyclase from adrenal cortex glomerulosa zone. FEBS Lett. 1983 Aug 8;159(1-2):97–101. doi: 10.1016/0014-5793(83)80424-4. [DOI] [PubMed] [Google Scholar]
- Molina Y Vedia L., Nolan R. D., Lapetina E. G. Subcellular localization of the enzymes that dephosphorylate myo-inositol polyphosphates in human platelets. Biochem J. 1988 Nov 1;255(3):795–800. doi: 10.1042/bj2550795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravec C. S., Reynolds E. E., Stewart R. W., Bond M. Endothelin is a positive inotropic agent in human and rat heart in vitro. Biochem Biophys Res Commun. 1989 Feb 28;159(1):14–18. doi: 10.1016/0006-291x(89)92397-8. [DOI] [PubMed] [Google Scholar]
- Morishita R., Higaki J., Ogihara T. Endothelin stimulates aldosterone biosynthesis by dispersed rabbit adreno-capsular cells. Biochem Biophys Res Commun. 1989 Apr 28;160(2):628–632. doi: 10.1016/0006-291x(89)92479-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Peter-Riesch B., Fathi M., Schlegel W., Wollheim C. B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest. 1988 Apr;81(4):1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijuan V., Litosch I. Norepinephrine stimulates the production of inositol trisphosphate and inositol tetrakisphosphate in rat aorta. Biochem Biophys Res Commun. 1988 Oct 14;156(1):240–245. doi: 10.1016/s0006-291x(88)80831-3. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Capponi A. M., Vallotton M. B. Metabolism of inositol 1,4,5-trisphosphate in permeabilized rat aortic smooth-muscle cells. Dependence on calcium concentration. Biochem J. 1987 Jul 1;245(1):305–307. doi: 10.1042/bj2450305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serunian L. A., Haber M. T., Fukui T., Kim J. W., Rhee S. G., Lowenstein J. M., Cantley L. C. Polyphosphoinositides produced by phosphatidylinositol 3-kinase are poor substrates for phospholipases C from rat liver and bovine brain. J Biol Chem. 1989 Oct 25;264(30):17809–17815. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Traynor-Kaplan A. E., Harris A. L., Thompson B. L., Taylor P., Sklar L. A. An inositol tetrakisphosphate-containing phospholipid in activated neutrophils. Nature. 1988 Jul 28;334(6180):353–356. doi: 10.1038/334353a0. [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan A. E., Thompson B. L., Harris A. L., Taylor P., Omann G. M., Sklar L. A. Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J Biol Chem. 1989 Sep 15;264(26):15668–15673. [PubMed] [Google Scholar]
- Van Renterghem C., Vigne P., Barhanin J., Schmid-Alliana A., Frelin C., Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Wallnöfer A., Weir S., Rüegg U., Cauvin C. The mechanism of action of endothelin-1 as compared with other agonists in vascular smooth muscle. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S23–S45. doi: 10.1097/00005344-198900135-00008. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E. A., Mcleod J. K., Johnston C. I. Vasopressin stimulates phosphatidylinositol turnover and aldosterone synthesis in rat adrenal glomerulosa cells: comparison with angiotensin II. Endocrinology. 1986 Jun;118(6):2432–2436. doi: 10.1210/endo-118-6-2432. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., Smith A. I., Wallace C. A., White L. B. Evidence for a lack of inositol--(1,4,5)trisphosphate kinase activity in norepinephrine-perfused rat hearts. Biochem Biophys Res Commun. 1987 Oct 14;148(1):68–77. doi: 10.1016/0006-291x(87)91077-1. [DOI] [PubMed] [Google Scholar]
- Woodcock E. A., Smith A. I., White L. B. Angiotensin II-stimulated phosphatidylinositol turnover in rat adrenal glomerulosa cells has a complex dependence on calcium. Endocrinology. 1988 Mar;122(3):1053–1059. doi: 10.1210/endo-122-3-1053. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


