Abstract
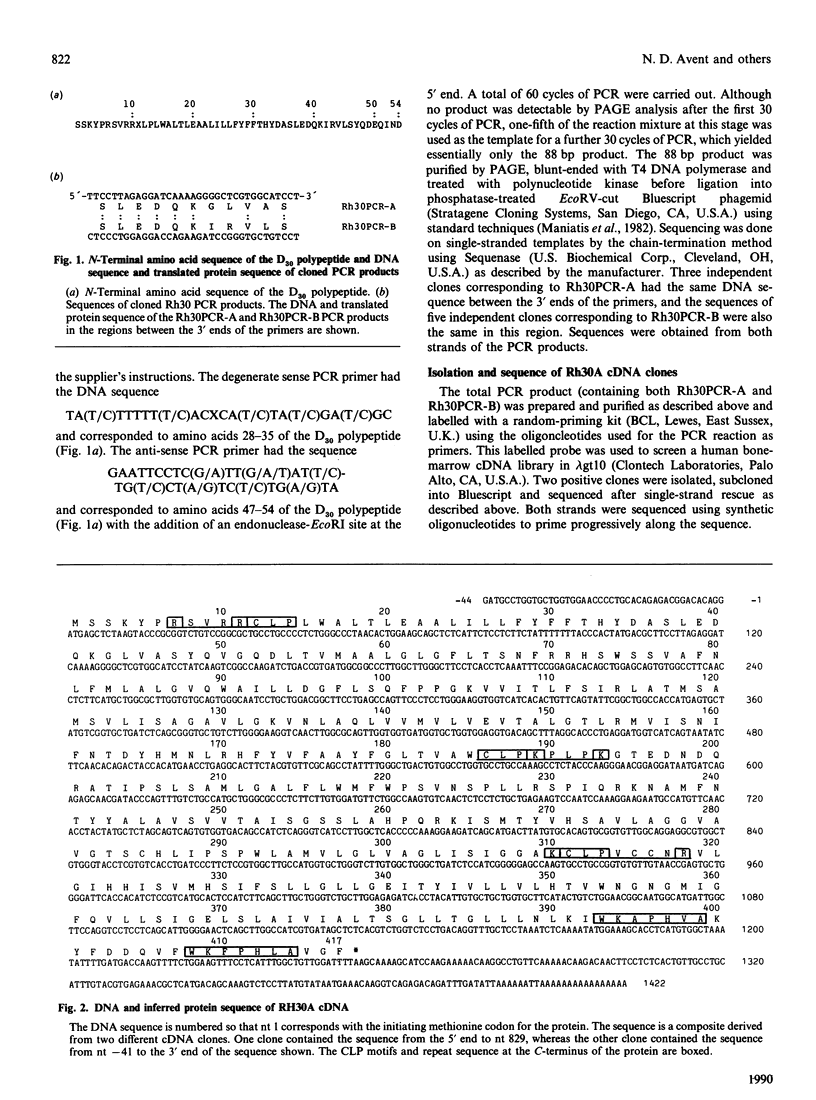
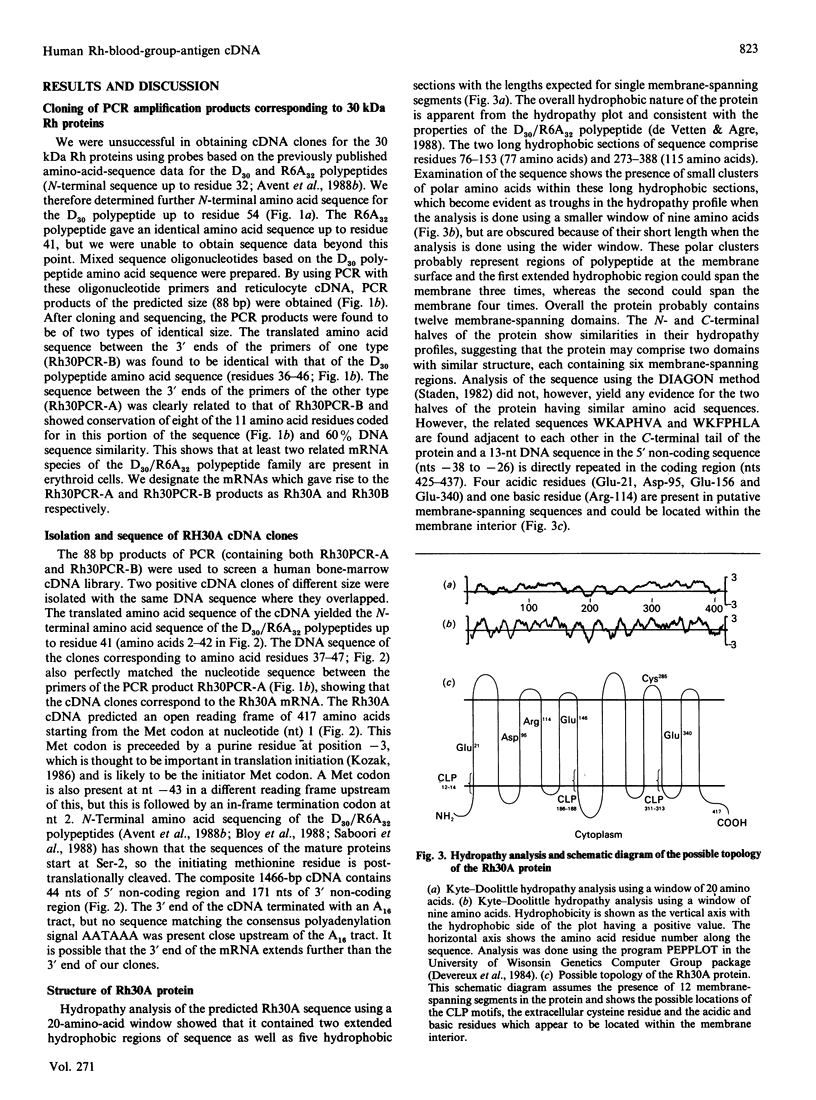
The Rh-blood-group antigens (often described as Rhesus antigens) are associated with erythrocyte membrane proteins of approx. 30 kDa. We have determined the N-terminal 54 amino acid residues of the 30 kDa Rh D polypeptide (D30 polypeptide). We used primers based on these sequence data and the polymerase chain reaction (PCR) on human reticulocyte cDNA and genomic DNA to clone two types of PCR product of identical size. The two PCR products had related translated amino acid sequences between the 3' ends of the primers, one of which was identical with that found for the D30 polypeptide. We designate the two related mRNA species which gave rise to the PCR products as Rh30A and Rh30B, the latter corresponding to the D30 polypeptide. We have isolated cDNA clones for the Rh30A protein which encode a hydrophobic membrane protein of 417 amino acids. The Rh30A protein has the same N-terminal 41 amino acids as the D30 polypeptide, but beyond this point the sequence differs, but is clearly related. The Rh30A protein probably corresponds to the R6A32 polypeptide, another member of the Rh 30 kDa family of proteins, which may carry the C/c and/or E/e antigens. Hydropathy analysis suggests that the Rh30A protein has up to 12 transmembrane domains. Three of these domains are bordered by a novel cysteine-containing motif, which might signal substitutions at these cysteine residues. Information which supplements this paper (amino-acid-sequence-analysis histograms) is reported in Supplementary Publication SUP 50160 (4 pages), which has been deposited at the British Library Document Supply Centre, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1990) 265, 5.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. E., Schachter D. Impermeant maleimides. Oriented probes of erythrocyte membrane proteins. J Biol Chem. 1976 Nov 25;251(22):7176–7183. [PubMed] [Google Scholar]
- Avent N. D., Ridgwell K., Mawby W. J., Tanner M. J., Anstee D. J., Kumpel B. Protein-sequence studies on Rh-related polypeptides suggest the presence of at least two groups of proteins which associate in the human red-cell membrane. Biochem J. 1988 Dec 15;256(3):1043–1046. doi: 10.1042/bj2561043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avent N., Judson P. A., Parsons S. F., Mallinson G., Anstee D. J., Tanner M. J., Evans P. R., Hodges E., Maciver A. G., Holmes C. Monoclonal antibodies that recognize different membrane proteins that are deficient in Rhnull human erythrocytes. One group of antibodies reacts with a variety of cells and tissues whereas the other group is erythroid-specific. Biochem J. 1988 Apr 15;251(2):499–505. doi: 10.1042/bj2510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D., Bloy C., Hermand P., Cartron J. P., Saboori A. M., Smith B. L., Agre P. Two-dimensional iodopeptide mapping demonstrates that erythrocyte Rh D, c, and E polypeptides are structurally homologous but nonidentical. Blood. 1988 Oct;72(4):1424–1427. [PubMed] [Google Scholar]
- Bloy C., Blanchard D., Dahr W., Beyreuther K., Salmon C., Cartron J. P. Determination of the N-terminal sequence of human red cell Rh(D) polypeptide and demonstration that the Rh(D), (c), and (E) antigens are carried by distinct polypeptide chains. Blood. 1988 Aug;72(2):661–666. [PubMed] [Google Scholar]
- Chérif-Zahar B., Bloy C., Le Van Kim C., Blanchard D., Bailly P., Hermand P., Salmon C., Cartron J. P., Colin Y. Molecular cloning and protein structure of a human blood group Rh polypeptide. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6243–6247. doi: 10.1073/pnas.87.16.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J., Schroit A. J. Transbilayer movement of phosphatidylserine in nonhuman erythrocytes: evidence that the aminophospholipid transporter is a ubiquitous membrane protein. Biochemistry. 1989 Dec 12;28(25):9680–9685. doi: 10.1021/bi00451a021. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E. Positively charged residues are important determinants of membrane protein topology. Trends Biochem Sci. 1990 Jul;15(7):253–257. doi: 10.1016/0968-0004(90)90047-f. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN F. A. STUDIES ON THE RH (D) ANTIGEN. Vox Sang. 1965 Jan-Feb;10:32–53. doi: 10.1111/j.1423-0410.1965.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular characterization of the human red cell Rho(D) antigen. EMBO J. 1983;2(2):223–227. doi: 10.1002/j.1460-2075.1983.tb01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular identification of the human Rho (D) antigen. FEBS Lett. 1982 Apr 5;140(1):93–97. doi: 10.1016/0014-5793(82)80528-0. [DOI] [PubMed] [Google Scholar]
- Green F. A. Erythrocyte membrane sulfhydryl groups and Rh antigen activity. Immunochemistry. 1967 Jul;4(4):247–257. doi: 10.1016/0019-2791(67)90186-3. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers F., van Linde-Sibenius-Trip M., Roelofsen B., Tanner M. J., Anstee D. J., Op den Kamp J. A. Rhnull human erythrocytes have an abnormal membrane phospholipid organization. Biochem J. 1984 Aug 1;221(3):931–934. doi: 10.1042/bj2210931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Green C. The identification of specific Rhesus-polypeptide-blood-group-ABH-active-glycoprotein complexes in the human red-cell membrane. Biochem J. 1987 Jun 15;244(3):735–741. doi: 10.1042/bj2440735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Ridgwell K., Roberts S. J., Tanner M. J., Anstee D. J. Absence of two membrane proteins containing extracellular thiol groups in Rhnull human erythrocytes. Biochem J. 1983 Jul 1;213(1):267–269. doi: 10.1042/bj2130267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboori A. M., Smith B. L., Agre P. Polymorphism in the Mr 32,000 Rh protein purified from Rh(D)-positive and -negative erythrocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4042–4045. doi: 10.1073/pnas.85.11.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M. Fatty acids covalently bound to erythrocyte proteins undergo a differential turnover in vivo. J Biol Chem. 1988 Sep 25;263(27):13615–13622. [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vetten M. P., Agre P. The Rh polypeptide is a major fatty acid-acylated erythrocyte membrane protein. J Biol Chem. 1988 Dec 5;263(34):18193–18196. [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]


