Abstract
Multiple sclerosis is an inflammatory demyelinating disease and represents a global health concern. Ocrelizumab, a humanized IgG monoclonal antibody, selectively targets CD20 on B cells and CD20-expressing T cells. This study aimed to compare the efficacy and safety of the biosimilar ocrelizumab candidate (Xacrel) to the originator product (Ocrevus) in Relapsing Multiple Sclerosis (RMS) patients. In this randomized trial, patients received either Xacrel or Ocrevus for 96 weeks. The primary endpoint was the equivalency of the medications in reducing the annualized relapse rate (ARR) at week 48. The secondary endpoints included time to the onset of disability progression confirmed at 12 and 24 weeks, the proportion of relapse-free patients, magnetic resonance imaging (MRI) evaluations, safety assessments, and immunogenicity over 96 weeks. A total of 170 patients were randomized (1:1 ratio). In the per protocol analysis, the upper and lower limits of 95% two-sided confidence intervals of difference between treatments in the 48-week ARR rate were in the predefined margin of − 0.2 to 0.2 (− 0.002; 95% CI − 0.080 to 0.075). The two products were also comparable in terms of other efficacy parameters, safety, and immunogenicity. The results confirmed that Xacrel is equivalent to Ocrevus in terms of 48-week ARR in RMS patients, with no considerable difference in other efficacy parameters and the safety profile during the 96 weeks. The trial was registered in Iranian registry of clinical trials (IRCT) on 10/06/2019 with the registration number of IRCT20150303021315N13 and in Clinicaltrials.gov on 19/07/2021 with the registration code of NCT04966338.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75745-y.
Subject terms: Multiple sclerosis, Neurology, Randomized controlled trials
Introduction
Multiple sclerosis (MS) is an autoimmune and inflammatory demyelinating disease affecting around three million people worldwide with a female preponderance1,2. The disease is characterized by the attack of immune cells on myelin in the central nervous system, leading to acute exacerbations and progressive disability3. MS lesions can be formed in different regions, such as the optic nerves, brainstem, cerebellum, periventricular, and spinal cord. The cerebral grey matter may also be affected4. Beside cognitive impairment, it can also cause fatigue, which is a frequent and disabling symptom5. In terms of activity level and progression, MS is classified into relapsing-remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS)6. The available management approaches focus on recovering from acute attacks, alleviating symptoms, and reducing biological activity through disease-modifying therapies (DMTs)3.
Initiating the treatment of MS with a more aggressive approach by the use of high-efficacy DMTs in the patients with highly active disease can have a positive impact on delaying the progression of the disease and many newly diagnosed patients are receiving high-efficacy DMTs at initial diagnosis7.
The effects of the immune response in the pathogenesis of MS are crucial. Peripheral proinflammatory B cells in relapsing disease mechanisms and meningeal collections of B cells in CNS-compartmentalized disease, as well as the T cells, play an important role in MS pathology. Hence, the B cell-targeting medications can have effective therapeutic results. CD20-targeted therapies including rituximab, ocrelizumab, and ofatumumab have shown acceptable efficacy and safety profile in MS8,9.
Ocrelizumab -a humanized IgG monoclonal antibody- selectively targets CD20 on immature and mature B cells and CD20-expressing T cells that play an important role in producing autoantibodies, presenting antigens, regulating cytokines, and aggregating ectopic lymphoid follicles10,11. Previous studies of ocrelizumab, including two phase III clinical trials (OPERA I and OPERA II) on relapsing MS (RMS) patients, have demonstrated the efficacy of ocrelizumab in reducing annualized relapse rate (ARR) and the number of new gadolinium-enhancing and new/enlarging T2 hyperintense lesions on MRI10,12. Ocrelizumab was first approved by the US Food and Drug Administration (FDA) in March 2017 for the treatment of both relapsing and primary progressive forms of MS and is the first approved medication for PPMS10.
Biosimilars are biological medications very similar to their licensed originator biologics with no clinically meaningful differences in safety and efficacy in the same treating indications as the originator. Biosimilars can be beneficial by increasing the patients’ access to proper medication while reducing the healthcare costs13. Neurological disorders, such as MS, are a leading cause of overall worldwide disease burden requiring effective prevention, treatment, and rehabilitation strategies14. According to the increasing incidence of MS worldwide, the development of therapeutic strategies and providing access to available treatment options are imperative. Biosimilars are effective and safe medications comparable to the reference product, that are available at lower cost and with better access, especially in lower-income countries. In this study, we aimed to assess the equivalency of a biosimilar ocrelizumab candidate (Xacrel, CinnaGen, Iran) compared to the reference product (Ocrevus, Roche, Switzerland) in terms of reducing ARR in RMS patients at 48 weeks of treatment.
Methods
Study design and intervention
This was a randomized, two-armed, double-blind, phase III clinical trial performed from August 2019 to October 2021 in 15 centers in Iran. Patients were randomly assigned (1:1) to receive 300 mg of either Xacrel or Ocrevus at an interval of 2 weeks for the first two doses, followed by 600 mg every 24 weeks for 96 weeks.
To reduce the infusion reactions, all patients received 100 mg intravenous methylprednisolone (or the equivalent dose of the other corticosteroids) 30 min prior to infusion. An oral or intravenous antihistamine (i.e., chlorpheniramine) and an antipyretic medication (i.e., acetaminophen) were also administered 30–60 min before infusion.
Participants
Adult patients diagnosed with MS (according to the McDonald 2010 criteria) were enrolled in the study if they were 18–55 years of age and had a history of at least two documented relapses in the last two years or one documented relapse in the last year before the screening visit. The Expanded Disability Status Scale (EDSS) score had to be between 0 and 5.5 for inclusion. The exclusion criteria were as follows: the diagnosis of PPMS; disease duration of more than 10 years in patients with EDSS ≤ 2 at the screening visit; previous anti B-cell targeted therapy (including rituximab, ocrelizumab, atacicept, belimumab, and ofatumumab); the presence of known neurological disorders that mimic the MS symptoms (including neuromyelitis optica, untreated vitamin B12 deficiency, neurosarcoidosis, and cerebrovascular disorders); the presence of any limitation for MRI conduction (including claustrophobia, body weight > 140 kg, etc.); pregnancy and lactation; suffering from any chronic disease requiring corticosteroids (oral or parenteral) or immunosuppressive therapy during the study; history or a case of primary or secondary immunodeficiency; inaccessibility of peripheral vessels; history of hypersensitivity reactions or anaphylaxis to monoclonal antibodies; the presence of any serious or uncontrolled medical disorders that prevent the patient from being enrolled in the study (including heart failure class III or IV according to New York Heart Association (NYHA) criteria); the presence of any clinical symptoms of active bacterial, viral, fungal, mycobacterial or other infections; suffering from any infection requiring hospitalization or treatment with parenteral antibiotics within four weeks before the baseline visit or oral antibiotics within two weeks before the baseline visit; history of chronic or recurrent infections (including HIV, hepatitis B, and hepatitis C); history of progressive multifocal leukoencephalopathy (PML); history of malignancies (including solid tumor and hematologic malignancies except for basal cell carcinoma); drug or alcohol abuse within 24 weeks before the screening visit; history of coagulation disorders; receiving any live vaccines within 6 weeks before the screening visit; being under treatment with any other investigational medications within 24 weeks before the screening visit; contraindications or intolerance to oral and parenteral corticosteroids; being under treatment with dalfampridine (unless the patient was receiving a constant dose within 30 days before the screening visit and throughout the study); receiving systemic corticosteroids within 4 weeks before the screening visit (for patients who received parenteral corticosteroids for MS attacks, the duration could be extended to 8 weeks); previous treatment with alemtuzumab, anti-CD4 therapies, cladribine, mitoxantrone, daclizumab, teriflunomide and laquinimod; history of whole-body radiotherapy or hematopoietic stem cell transplantation; treatment with cyclophosphamide, azathioprine, mycophenolate mofetil, cyclosporine, methotrexate, or natalizumab within 24 months before the screening visit (patients receiving natalizumab for less than 1 year could be enrolled); treatment with fingolimod or dimethyl fumarate within 4 weeks before the screening visit (patients receiving these medications more than 4 weeks prior to screening could be enrolled provided that their lymphocyte count was more than the lower limit of normal (LLN)); receiving intravenous immunoglobulin (IVIg) within 12 weeks before the baseline visit; a positive beta-hCG result at the screening visit; and laboratory abnormalities (including CD4 + levels < 300/mcL, AST/SGOT or ALT/SGPT ≥ 2 times of the upper limit of normal (ULN), platelet count < 100,000/mcL, serum IgG levels of 18% below the LLN, serum IgM levels of 8% below the LLN, and absolute neutrophil count (ANC) < 1500/mcL).
Written informed consent was provided by all the participants. The study was carried out in accordance with the Declaration of Helsinki and was approved by the ethics committees of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1398.164) and Shahid Beheshti University of Medical Sciences (IR.SBMU.REC.1398.024). The trial was registered in Iranian registry of clinical trials (IRCT) on 10/06/2019 with the registration number of IRCT20150303021315N13 and in Clinicaltrials.gov on 19/07/2021 with the registration code of NCT04966338.
Randomization and blinding
Eligible patients were assigned to each treatment arm using a dynamic randomization algorithm (minimization) designed to achieve an overall balance between groups. Randomization was stratified according to the baseline EDSS score (≤ 4 vs. > 4). The participants, the medication administrators, and the outcome assessors were all blinded to the allocation.
Outcomes
The study duration was 96 weeks. The primary outcome of the study was to evaluate the equivalency of Xacrel to Ocrevus in reducing the annualized relapse rate (ARR) at week 48 in patients with RMS. Relapse was defined as new or worsening MS-related neurological symptoms (at least 0.5 scores increase in EDSS, 2 points increase in one of the appropriate FSS, or 1 point increase in at least two of the appropriate FSS) that persisted for more than 24 h, proceeded after at least 30 days of stable or improving neurological conditions, and were not attributed to other clinical conditions (e.g., infection, fever, injury, drug-related adverse reactions). Episodic spasms, sexual dysfunction, fatigue, mood changes, urinary incontinence, and urinary retention were not considered a relapse.
The secondary efficacy outcomes included the time to the onset of disability progression confirmed at 12 and 24 weeks over 96 weeks. Disability progression was defined as an increase in the EDSS score by at least 1.5 steps if the baseline EDSS was 0, at least 1 step if the baseline EDSS was 0 > and ≤ 5.5, or at least 0.5 steps if the baseline EDSS was > 5.5. Other secondary outcomes were the proportion of relapse-free patients at 96 weeks, the total number of gadolinium-enhancing lesions, and new or enlarged T2 hyperintense lesions identified on brain MRI at weeks 24, 48, and 96. The change in the volume of T2 lesions identified on brain MRI from baseline to week 96 was also assessed.
Safety outcomes included the incidence of adverse events (AE) every 12 weeks and infusion reactions every 24 weeks for 96 weeks. All the reported events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 15. In addition, the causality relation was assessed based on World Health Organization (WHO) criteria. All AEs were classified based on the Medical Dictionary for Regulatory Activities (MedDRA) terms as the preferred term (PT) and system organ class (SOC). Serious adverse events (SAEs) were documented according to ICH guidelines (E2B(R2)); SAE was defined as any AE that “results in death, is life-threatening, requires in-patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, results in congenital anomaly or birth defect.” Infusion-related reactions were defined as symptoms including pruritus, rash, urticaria, erythema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, throat irritation, oropharyngeal pain, dyspnoea, pharyngeal or laryngeal oedema, nausea and tachycardia during or within 24 h after the infusion.
Adverse events of special interest (AESIs), included infusion-related reactions, infections and infestations (including herpes infections, urinary tract infections, and respiratory tract infections), and neoplasm benign, malignant, and unspecified (including cysts and polyps).
The immunogenicity assessments were also conducted at baseline as well as weeks 24, 48, and 96. The enzyme-linked immunosorbent assay (ELISA) was used for anti-drug antibodies (ADAs) measurements.
All the assessments and interventions complied with the valid international guidelines and Good Clinical Practice (GCP) standards. The laboratory methods were validated and implemented by trained staff using reliable kits and in compliance with international and national standards.
Statistical analysis
A sample size of 76 patients in each group was assumed to provide at least 80% power to detect equivalency when assessed by the risk difference between groups for ARR with a predefined margin of ± 0.20 and a significance level of 0.05. ARR in the reference group (Ocrevus) was reported as 0.16 8. The total sample size of 170 patients was calculated considering the drop-out rate of 10% 16.
The primary outcome of ARR at 48 weeks was compared between the two groups using a Poisson regression model, adjusted for baseline EDSS score, and the LS-Means was used to estimate the rate difference between the two groups. Xacrel efficacy was judged equivalent to Ocrevus if the lower and the upper limits of the two-sided 95% confidence intervals (95% CIs) of difference in the ARR between the two study groups (Xacrel and Ocrevus) were within the accepted equivalence margin (− 0.2, 0.2). The primary outcome analysis was performed using the per protocol set (PPS) and the intention to treat (ITT) population.
PPS was defined as the patients who completed the study without any major protocol deviation. The ITT population was defined as all randomized patients receiving at least one dose of the study treatment. Data for patients were analyzed based on the assigned study arm. All secondary efficacy analyses were based on the ITT principle.
To evaluate the time to the onset of confirmed disability progression (CDP) for 12 and 24 weeks, the overall hazard ratio and its corresponding 95% CI were estimated using a stratified Cox regression model with the same stratification factors used in the stratified log-rank test. In addition, the cumulative probability of disease progression was estimated using the Kaplan–Meier methodology. Finally, the percentages of relapse-free patients between the treatment groups at week 96 were compared using the chi-squared test.
The total number of new and/or enlarging T2 hyperintense lesions identified on the brain MRI at weeks 24, 48, and 96 were compared between study arms using a negative binomial model adjusted for baseline EDSS score and baseline T2 lesions number. The percent change in the volume of T2 lesions on brain MRI from baseline to week 96 was compared between the two groups using t-test. The exploratory endpoint of ARR at 96 weeks was compared between two treatment groups using a Poisson regression model, adjusted for baseline EDSS score. The proportion of patients experiencing CD19 levels of less than 1% at least once (%) between treatment groups was compared using the chi-squared test.
A missing value for the number of T2 hyperintense lesions at a particular week was imputed by the average number of new and/or enlarging lesions on available scans obtained during the 96 weeks of study, and the missing values in the volume of T2 lesions were imputed using last-observation-carried forward (LOCF) methodology. In addition, the missing values were imputed for patients in any study arm if they had a baseline and at least one post-baseline MRI assessment.
Safety assessments were performed on all randomly assigned patients who received at least one dose of the medication. Safety outcomes were reported based on the incidence rate of AEs and SAEs.
All the statistical analyses were performed using STATA (version 14, StataCorp LP, USA) and R 4.0.3 17.
Results
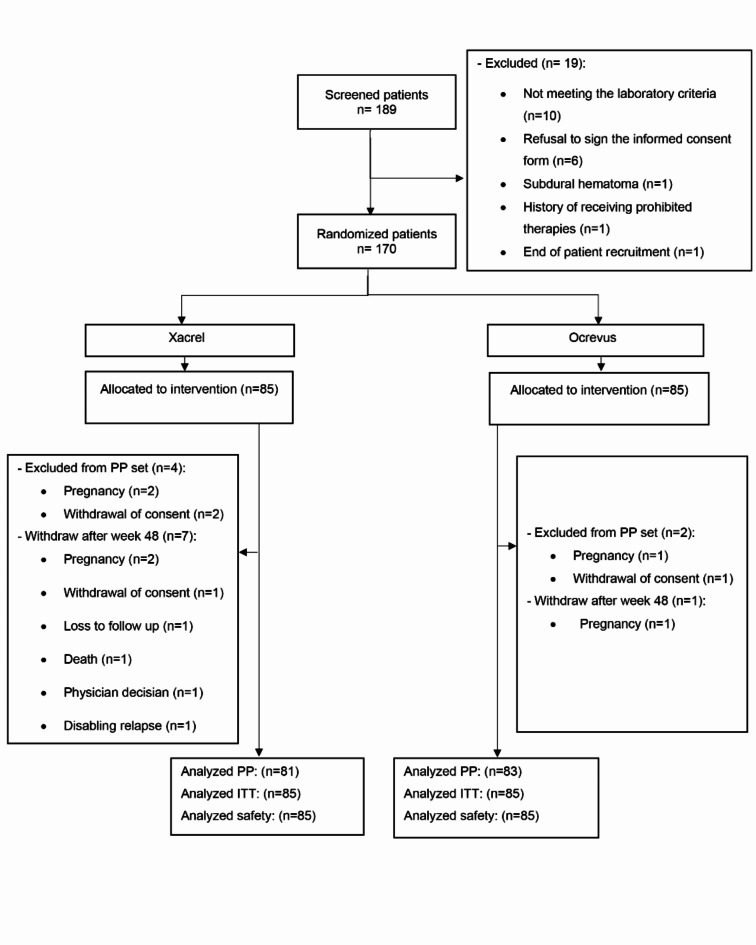
Overall, 189 patients were screened, of which 19 patients were excluded, and 170 patients fulfilled the study eligibility criteria. Eighty-five patients were randomly assigned to each study group, receiving Xacrel or Ocrevus (Fig. 1).
Fig. 1.
Patient flow diagram. ITT intention to treat, PP per protocol.
Demographics and baseline characteristics of the patients are shown in Table 1.
Table 1.
Demographics and disease characteristics of the patients at baseline (intention to treat population).
| Characteristic* | Xacrel (N = 85) | Ocrevus (N = 85) |
|---|---|---|
| Age, year | 33.14 ± 7.22 | 31.12 ± 7.27 |
| Female sex, no. (%) | 64 (75.29) | 67 (78.82) |
| No. of relapses in previous 12 month | 1.16 ± 0.43 | 1.13 ± 0.37 |
| Previous disease-modifying therapy, no./total no. (%)§ | 56 (65.88) | 63 (74.12) |
| No medication | 27 (31.76) | 21 (24.71) |
| Interferons | 25 (29.41) | 33 (38.82) |
| Fingolimod | 15 (17.65) | 13 (15.29) |
| Dimethyl fumarate | 10 (11.76) | 11 (12.94) |
| Glatiramer acetate | 8 (9.41) | 7 (8.24) |
| Mean EDSS score | 2.35 ± 1.01 | 2.18 ± 1.13 |
| Baseline values related to MRI assessment** | ||
| No. of gadolinium-enhancing lesions on T1-weighted MRI, no./total no. (%) | ||
| 0 | 60/77 (77.92) | 56/76 (73.70) |
| 1 | 9/77 (11.70) | 13/76 (17.11) |
| 2 | 3/77 (3.90) | 3/76 (3.95) |
| 3 | 2/77 (2.60) | 3/76 (3.95) |
| ≥ 4 | 3/77 (3.90) | 1/76 (1.32) |
| No. of lesions on T2-weighted MRI | 27.32 ± 20.32 | 27.33 ± 20.90 |
| Volume of lesions on T2-weighted MRI (cm3) | 11.81 ± 12.92 | 9.37 ± 10.10 |
EDSS expanded disability status scale, MRI magnetic resonance imaging.
*Plus-minus values are means ± SD.
**Information related to MRI assessment was not available for 17 patients (8 patients in the Xacrel and 9 patients in the Ocrevus).
§Data on previous treatment were collected only one year before the screening. Patients could be counted in several categories.
Efficacy outcome measures
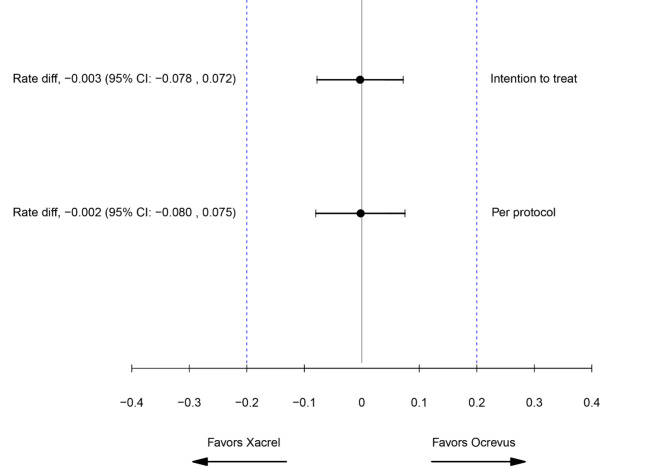
The results of the clinical and MRI findings are summarized in Table 2. ARR at 48 weeks was evaluated in 164 patients in the PP set (81 patients in the Xacrel group and 83 patients in the Ocrevus group). The result was 0.067 in the Xacrel group (95% CI 0.029–0.155) versus 0.070 in the Ocrevus group (95% CI 0.031–0.158). No significant difference was noticed in the rate difference between the two groups (P = 0.95; 95% CI − 0.080 to 0.075). In the ITT set, 48-week ARR was evaluated in 170 patients (85 patients in each study group). The result was 0.065 in the Xacrel group (95% CI 0.028 to 0.149) and 0.068 in the Ocrevus group (95% CI 0.030 to 0.153). There was no statistically significant difference in the rate difference between the two study groups (P = 0.95; 95% CI − 0.078 to 0.072).
Table 2.
Clinical and MRI endpoints during the trial.
| Endpoint | Xacrel (N = 85) | Ocrevus (N = 85) | P-Value |
|---|---|---|---|
| Primary endpoint | |||
| Annualized relapse rate at 48 weeks (PP)** | 0.067 (0.029 to 0.155) | 0.070 (0.031 to 0.158) | |
| Rate difference (95% CI) | 0.002 (− 0.080 to 0.075) | 0.95 | |
| Annualized relapse rate at 48 weeks (ITT) | 0.065 (0.028 to 0.149) | 0.068 (0.030 to 0.153) | |
| Rate difference (95% CI) | − 0.003 (− 0.078 to 0.072) | 0.95 | |
| Secondary clinical endpoints | |||
| Disability progression confirmed at 12 weeks¥ | |||
| Patients with event (%) | 6 (7.06) | 5 (5.90) | |
| Hazard ratio (95% CI) | 1.25 (0.38 to 4.10) | 0.71 | |
| Disability progression confirmed at 24 weeks¥ | |||
| Patients with event (%) | 6 (7.06) | 2 (2.35) | |
| Hazard ratio (95% CI) | 3.15 (0.64 to 15.60) | 0.16 | |
| Proportion of relapse-free patients by Week 96 (%) | 76 (89.41) | 74 (87.10) | 0.63 |
| Secondary MRI endpoints | |||
| Total no. of new or newly enlarged hyperintense lesions on T2-weighted MRI by week 96§ | |||
| Mean no. of lesions per scan (95% CI) | 0.16 (0.08 to 0.33) | 0.08 (0.03 to 0.20) | |
| Rate ratio (95% CI) | 1.98 (0.70 to 5.73) | 0.21 | |
| T2-volume change from baseline to week 96 | − 0.60 (5.94) | − 0.70 (2.60) | 0.88 |
| Exploratory endpoints | |||
| Annualized relapse rate at 96 weeks | 0.112 (0.041 to 0.184) | 0.124 (0.049 to 0.198) | |
| Rate difference (95% CI) | − 0.011 (− 0.113 to 0.089) | 0.82 | |
| Proportion of patients experiencing CD19 levels of less than 1% at least once (%) | 55 (64.71) | 54 (63.53) | 0.87 |
CI confidence Interval, ITT intention to treat, MRI magnetic resonance imaging, PP per protocol.
*All rate ratios, hazard ratios, and rate difference values are for the Xacrel versus the Ocrevus.
**Per protocol set (81 patients in the Xacrel group and 83 patients in the Ocrevus group).
¥Disability progression that was confirmed at 12 or 24 weeks was defined as an increase from the baseline EDSS score of at least 1.5 score if the baseline value was zero; at least 1 score increase in EDSS if 0 < baseline EDSS ≤ 5.5 and at least 0.5 score increase in EDSS if the baseline EDSS > 5.5, that was sustained for at least 12 or 24 weeks.
§The total number of lesions was calculated as the sum of the individual number of lesions at weeks 24, 48, and 96, divided by the total number of MRI scans of the brain.
In the PP set, the primary endpoint of the study was met, as the upper and lower limits of 95% two-sided confidence intervals of differences in the 48-week ARR were all in the predefined margin of − 0.2 to 0.2 in Poisson regression model (Fig. 2). Similarly, in the ITT set, the efficacy of Xacrel in the 48-week ARR was equivalent to Ocrevus (Table 2).
Fig. 2.
Forest plot for the primary endpoint (annualized relapse rate). CI confidence interval.
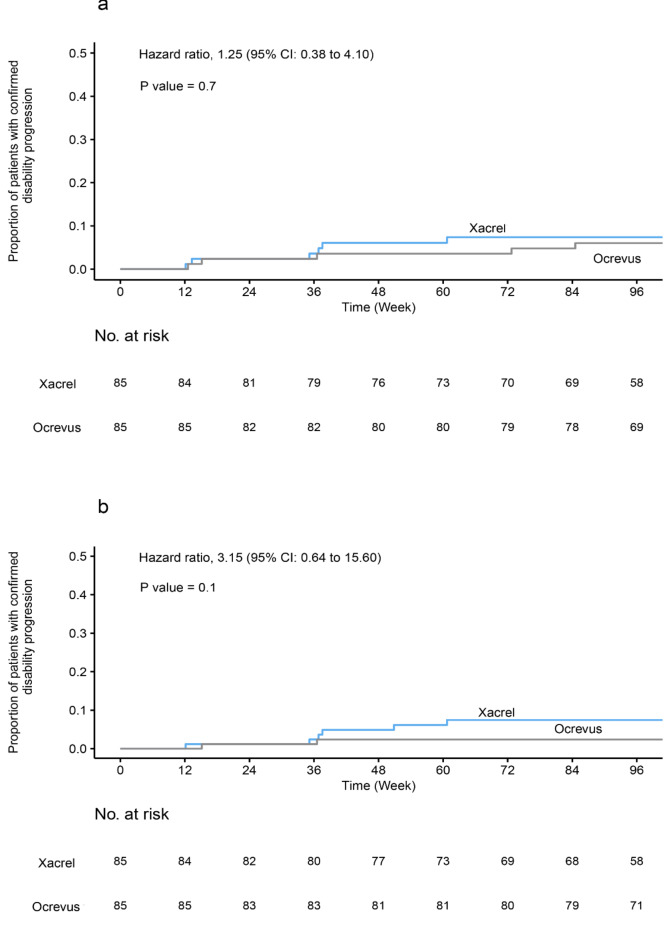
Over the 96-week trial period, the rate of 12 weeks of confirmed disability progression was 7.06% in the Xacrel group, as compared with 5.90% in the Ocrevus group (P = 0.71; 95% CI 0.38 to 4.10) (Fig. 3a). The percentage of patients with confirmed disability progression at 24 weeks was 7.06% in the Xacrel group versus 2.35% in the Ocrevus group (P = 0.16; 95% CI 0.64 to 15.60) (Fig. 3b).
Fig. 3.
Kaplan-Meier plots for proportion of patients with confirmed disability progression. (a) Confirmed disability progression at 12 weeks. (b) Confirmed disability progression at 24 weeks. The reported p-values were calculated using the Log-Rank test. CI confidence Interval.
Within 96 weeks of the study duration, 89.41% of patients in the Xacrel group were relapse-free, compared to 87.10% in the Ocrevus group (P = 0.63).
No new gadolinium-enhancing lesions were observed within the 96-week study period among the two groups.
The total mean number of new or enlarging T2 hyperintense lesions per MRI scan was 0.16 (95% CI 0.08 to 0.33) with the Xacrel group versus 0.08 (95% CI 0.03 to 0.20) with the Ocrevus group. No statistically significant difference was noticed between the two groups (P = 0.21).
T2 lesions’ volume was also reduced over time in the two groups. The absolute change was − 0.60 in the Xacrel group compared to − 0.70 in the Ocrevus group (P = 0.88).
Safety outcome measures
Based on the study results, in both groups, 83/85 patients (97.65%) experienced at least one AE (Table 3). The most common SOC in both groups was general disorders and administration site conditions. The most common PT was infusion-related reactions, respectively.
Table 3.
Adverse events (safety population).
| Variable | Xacrel (N = 85) |
Ocrevus (N = 85) |
|---|---|---|
| Number of patients (%) | ||
| Any adverse event | 83 (97.65) | 83 (97.65) |
| Infusion-related reaction | 51 (60) | 54 (63.53) |
| Infections and infestations | ||
| Herpes zoster | 1 (1.2) | 1 (1.2) |
| Oral herpes | 1 (1.2) | 3 (3.5) |
| Genital herpes | 0 (0) | 1 (1.2) |
| Urinary tract infection | 4 (4.7) | 1 (1.2) |
| Corona virus infection | 21 (24.7) | 25 (29.4) |
| Neoplasms benign, malignant and unspecified | 1 (1.2) | 1 (1.2) |
| Any serious adverse event | 8 (9.41) | 12 (14.12) |
| Serious infections and infestations | 5 (5.90) | 8 (9.41) |
In the Xacrel group, 51/85 patients (60%) and in the Ocrevus group, 54/85 patients (63.53%) experienced at least one infusion-related reaction (Table 3).
Throughout the study, 24 serious adverse events (SAEs) were reported in 20 patients; in the Xacrel group, six SAEs leading to hospitalization or prolongation of hospitalization, one life-threatening SAE, and one death resulting from coronavirus infection were reported. In the Ocrevus group, 15 SAEs leading to hospitalization or prolongation of hospitalization and one life-threatening SAE were reported. Five SAEs were considered at least possibly related to the treatment in both groups. There were no meaningful differences between the number of patients experiencing SAE in both groups (8/85 patients (9.41%) in the Xacrel group vs. 12/85 patients (14.12%) in the Ocrevus group). (Table 3)
Serious infections and infestations reported in the Xacrel group were pharyngitis (in one patient), urinary tract infection (in one), and coronavirus infection (in three). Serious infections and infestations reported in the Ocrevus group were appendicitis (in two patients) and coronavirus infection (in six).
The whole trial subjects were assessed for immunogenicity. Among all the samples, there was no positive sample for anti-ocrelizumab antibodies at all time points, and all patients’ samples were negative.
Post hoc analysis
We also evaluated ARR within 96 weeks of the study period. The results showed no statistically significant difference among the two groups. (0.122 in the Xacrel group vs. 0.124 in the Ocrevus group; 95% CI − 0.113 to 0.089; P = 0.82). Moreover, to compare the efficacy of the two medications in reducing the levels of CD19, the proportion of patients who experienced CD19 levels of less than 1% at least once throughout the study was assessed. In the Xacrel group, the result was 55/85 patients (64.71%) versus 54/85 patients (63.53%) in the Ocrevus group (P = 0.87). Moreover, the trend of CD19 changes is shown in Fig. S1 in the supplementary information.
Discussion
The results of this study showed that Xacrel is equivalent to Ocrevus in reducing the ARR at 48 weeks in patients with RMS. In addition, study results also provided evidence for comparability of Xacrel and Ocrevus in terms of other efficacy parameters and safety.
According to the sensitivity analysis, including the analysis in the ITT set, no statistically significant difference was found between the two groups. Therefore, the robustness of results can be concluded, and patients who were withdrawn from the study or had protocol deviations could not influence the reported results. It is noteworthy that patients’ demographics and other baseline characteristics were almost the same between the two groups. Hence, the effects of potential confounding factors were minimal.
OPERA studies reported an ARR of 0.14 for 48 weeks12. Moreover, in the open-label extension (OLE) of OPERA studies, ARR was reported to be 0.098 by the end of the first year, which is more than our results (0.067 in Xacrel group versus 0.070 in Ocrevus group)18. It is noteworthy that the reported results in the OPERA OLE study were only for patients who switched from interferon beta-1a to ocrelizumab at OLE baseline. The reason for this greater rate might be the difference in patients’ disease severity at the time of study entrance according to baseline MRI lesions. In a nationwide observational Danish population-based cohort study with prospectively enrolled cases and a median follow-up of 1.3 years, the mean ARR was 0.09 in the RRMS group during the whole ocrelizumab treatment period19. In another real-world, multicenter, retrospective, observational study on the Spanish population with a median follow-up of 10 months performed by Fernandez-Diaz et al. in the year 2020, ARR was reported to be 0.10 20. The reported results in these studies were compatible with our study results. Additionally, a multicenter observational study with a median follow-up of 204 days reported an ARR of 0.17 21. We speculate that differences in follow-up durations could explain the difference between the reported and our study results. Another main point to consider is that all these studies were in a real-world setting, which is much different from our design.
Along with the 48-week ARR, there were no significant differences between the two groups regarding the 96-week ARR results. The results were in line with the study, which led to FDA approval of ocrelizumab12. In a non-randomized controlled trial on patients with a suboptimal response after ≥ 6 months on another DMT, 96-week adjusted ARR based on baseline EDSS (< 2.5 vs. ≥ 2.5) and the number of previous DMTs (= 1 vs. > 1), was 0.046 22. In a real-world, retrospective, single-center observational cohort study in Qatar with a median follow-up period of 19 months, ARR was 0.21 23.
In our study, 12- and 24-week CDP were also evaluated, and there were no statistically significant differences between the two groups in terms of these parameters. The clinical efficacy data in our study were generally in line with those reported in the OPERA studies and the few real-world studies conducted so far12,19,21,22.
In general, 20 patients experienced relapses within 96 weeks of the study. Nine patients were in the Xacrel group, and the other 11 patients were in the Ocrevus group. The proportion of relapse-free patients did not differ between the two groups, and the results were also in line with OPERA and some other studies12,20,22,23.
In addition, comparable efficacy between Xacrel and Ocrevus was confirmed by evaluating patients’ MRI activity. There was no statistically significant difference between the two study groups in the mean number of the new/enlarging T2 hyperintense lesions per scan. In OPERA trials, new or enlarging T2 lesions per MRI scan were reported to be 0.32 and 0.33. Considering baseline T2 lesions values in OPERA studies, the difference in results can be justified. Patients enrolled in OPERA studies had more T2 lesions at the time of study entrance12. T2 lesions’ volume change was another efficacy parameter evaluated in this study. It was shown that T2 lesions’ volume change was similar between the two groups. In addition, our results were in line with a non-randomized controlled trial which reported overall T2 lesions’ volume change to be − 0.56 cm3 from baseline to week 9622. This study showed no new gadolinium-enhancing lesions throughout the study period among the two study groups.
CD19 is considered to be a sensitive -but not specific- pharmacodynamic marker for evaluating anti-CD20 therapies’ efficacy12. Therefore, decreased CD19 levels following B-cell depleting treatments could be regarded as an effective medication in terms of pharmacodynamics. In addition to all analyses explained above, in this study, the proportion of patients who experienced CD19 levels of less than 1% throughout the study period at least once was almost the same between the two groups. Evaluating the trend of CD19 levels during the study period also showed that Xacrel and Ocrevus are comparable regarding the pharmacodynamic characteristics.
Based on the trial findings, Xacrel and Ocrevus were generally comparable in terms of safety parameters. The overall incidence of AEs and SAEs were comparable between the two arms.
This study had some limitations, including the influence of the COVID-19 pandemic on patients’ compliance that might have affected their attendance at their scheduled visits. In addition, even though there was a central physician for evaluating MRI reports, MRI instruments variability between different study centers could possibly affect the results.
Conclusion
The results of this study confirmed that Xacrel is equivalent to the originator Ocrevus in terms of 48-week ARR in RMS patients. In addition, the results of other efficacy parameters, including 12- and 24-week CDP, MRI lesions, and safety, were also comparable among the two groups. Therefore, Xacrel can be considered a biosimilar to Ocrevus.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The medical writing team are gratefully acknowledged by the authors.
Author contributions
M.S., R.A., V.S., F.A., N.M., S.N., S.M.B., B.S., A.N.M., M.N., H.G.L., S.E.M., N.B., H.A., A.N., M.G., N.R., E.A., and A.S. participated in the study design and coordination, as well as revising the manuscript. H.K. was the head of the medical department and S.A. was the manager of the clinical research unit of Orchid Pharmed Company. They supervised the clinical trial conduction, contributed to the writing of the manuscript draft, and analyzed the data. M.S. conducted the study in accordance with the accepted protocol, drafted the manuscript, and decided to submit the manuscript for publication. All authors read and approved the final manuscript and are accountable for all aspects of the work.
Funding
This trial was funded by CinnaGen Company. The sponsor participated in the conducting of study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
M.S., V.S., S.M.B., B.S., A.N.M., M.N., and S.E.M. have received educational, research grants, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, Bayer, Roche, Novartis, CinnaGen, Osveh, Zistdaru, Zahravi, Nanoalvand, and Genzyme. R.A. has received support for scientific meetings and honorarium for advisory board from CinnaGen, Merck, Roche, Zistdaru, Nanoalvand, Zahravi, Cobel Daru, and Actoverco. F.A. has received educational, research grants, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, Bayer, Roche, Novartis, CinnaGen, Osveh, Zistdaru, Zahravi, and Nanoalvand. N.M. has received educational, research grants, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, Roche, Novartis, CinnaGen, Osveh, Zistdaru, and Nanoalvand. S.N. has received educational, research grants, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, Roche, CinnaGen, Zistdaru, and Nanoalvand. H.G.L. has received educational, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, Bayer, Roche, CinnaGen, Zistdaru, Zahravi, Nanoalvand, and Genzyme. N.B. has received travel supports to attend scientific meetings from Biogen, Merck, Bayer, Novartis, CinnaGen, Zistdaru, and Nanoalvand. H.A. has received educational, research grants, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, Bayer, Novartis, CinnaGen, Zistdaru, Zahravi, and Nanoalvand. A.N. has received speaker fee to attend scientific meetings from Merck, CinnaGen, Zistdaru, and Nanoalvand. N.R. has received educational, research grants, lecture honorarium, travel supports to attend scientific meetings from Biogen, Merck, CinnaGen, Osveh, Zahravi, and Nanoalvand. E.A. has received payment from CinnaGen for the interpretation of MRI scans. M.G. has declared no competing interests. A.S. is a member of CinnaGen medical biotechnology research center, which collaborates with universities and researchers all over the world with regards to research and development of medications and health issues. H.K. is the head of the medical department and S.A. is the manager of the clinical research unit of Orchid Pharmed Company; the CinnaGen Company partner in conducting clinical trials.
Ethical approval
This study was performed in line with the principles of the 1964 Declaration of Helsinki and its later amendments, and Good Clinical Practice guidelines. The study was approved by the ethics committees of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1398.164) and Shahid Beheshti University of Medical Sciences (IR.SBMU.REC.1398.024). These ethical approvals covered all the necessary approvals for 15 study centers. Written informed consent was obtained from each individual participant involved in the study. Consent for publication was not applicable in this study.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walton, C. et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler.26, 1816–1821. 10.1177/1352458520970841 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirmosayyeb, O., Shaygannejad, V., Bagherieh, S., Hosseinabadi, A. M. & Ghajarzadeh, M. Prevalence of multiple sclerosis (MS) in Iran: a systematic review and meta-analysis. Neurol. Sci.43, 233–241. 10.1007/s10072-021-05750-w (2022). [DOI] [PubMed] [Google Scholar]
- 3.Hauser, S. L. & Cree, B. A. C. Treatment of multiple sclerosis: a review. Am. J. Med.133, 1380–1390e1382. 10.1016/j.amjmed.2020.05.049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford, H. Clinical presentation and diagnosis of multiple sclerosis. Clin. Med. (Lond). 20, 380–383. 10.7861/clinmed.2020-0292 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vucic, S., Burke, D. & Kiernan, M. C. Fatigue in multiple sclerosis: mechanisms and management. Clin. Neurophysiol.121, 809–817. 10.1016/j.clinph.2009.12.013 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Ciotti, J. R. & Cross, A. H. Disease-Modifying treatment in progressive multiple sclerosis. Curr. Treat. Options Neurol.2010.1007/s11940-018-0496-3 (2018). [DOI] [PubMed]
- 7.Simpson, A., Mowry, E. M. & Newsome, S. D. Early aggressive treatment approaches for multiple sclerosis. Curr. Treat. Options Neurol.23, 19. 10.1007/s11940-021-00677-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Amico, E. et al. Placing CD20-targeted B cell depletion in multiple sclerosis therapeutic scenario: Present and future perspectives. Autoimmun. Rev.18, 665–672. 10.1016/j.autrev.2019.05.003 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Michel, L. et al. B cells in the multiple sclerosis Central Nervous System: trafficking and contribution to CNS-Compartmentalized inflammation. Front. Immunol.6, 636. 10.3389/fimmu.2015.00636 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulero, P., Midaglia, L. & Montalban, X. Ocrelizumab: a new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 11, 1756286418773025. 10.1177/1756286418773025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinoda, K. et al. Differential effects of anti-CD20 therapy on CD4 and CD8 T cells and implication of CD20-expressing CD8 T cells in MS disease activity. Proc. Natl. Acad. Sci. U S A. 120, e2207291120. 10.1073/pnas.2207291120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauser, S. L. et al. Ocrelizumab versus Interferon Beta-1a in relapsing multiple sclerosis. N Engl. J. Med.376, 221–234. 10.1056/NEJMoa1601277 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Greenberg, B. & Giovannoni, G. A place for biosimilars in the changing multiple sclerosis treatment landscape. Mult Scler. Relat. Disord. 77, 104841. 10.1016/j.msard.2023.104841 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Global Regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the global burden of Disease Study 2021. Lancet Neurol.23, 344–381. 10.1016/s1474-4422(24)00038-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freites-Martinez, A., Santana, N., Arias-Santiago, S. & Viera, A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of Anticancer therapies. Actas Dermosifiliogr (Engl Ed). 112, 90–92. 10.1016/j.ad.2019.05.009 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Blackwelder, W. C. Equivalence Trials. In Encyclopedia of Biostatistics, vol. 21367–1372 (Wiley, 1998).
- 17.Team, R. C. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org/ (2020).
- 18.Hauser, S. L. et al. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology. 95, e1854–e1867. 10.1212/wnl.0000000000010376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pontieri, L. et al. Ocrelizumab treatment in multiple sclerosis: a Danish population-based cohort study. Eur. J. Neurol.29, 496–504. 10.1111/ene.15142 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Diaz, E. et al. Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann. Clin. Transl Neurol.8, 385–394. 10.1002/acn3.51282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellwardt, E. et al. Ocrelizumab initiation in patients with MS: a multicenter observational study. Neurol. Neuroimmunol. Neuroinflamm. 7. 10.1212/nxi.0000000000000719 (2020). [DOI] [PMC free article] [PubMed]
- 22.Weinstock-Guttman, B. et al. Ocrelizumab treatment for relapsing-remitting multiple sclerosis after a suboptimal response to previous disease-modifying therapy: a nonrandomized controlled trial. Mult Scler.28, 790–800. 10.1177/13524585211035740 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Cañibano, B. et al. Real-world experience of ocrelizumab in multiple sclerosis in an arab population. J. Drug Assess.10, 106–113. 10.1080/21556660.2021.1989193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.