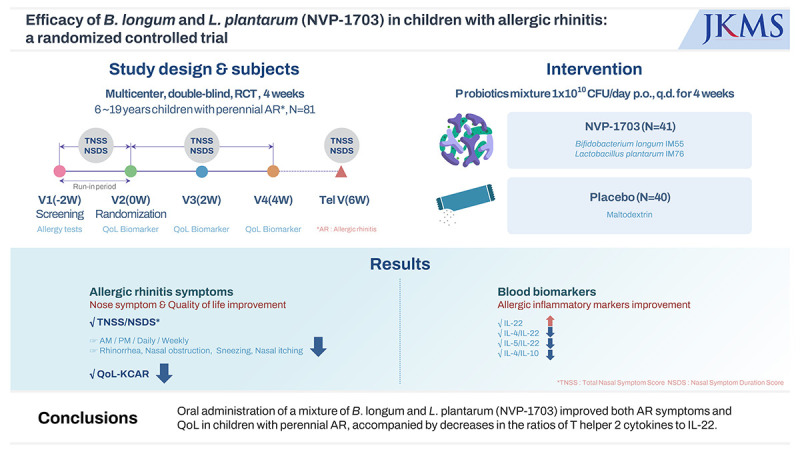
Abstract
Background
There is increasing evidence that probiotics are effective in treating allergic rhinitis (AR), while some controversies remain. This study was performed to evaluate the therapeutic effect and safety of a mixture of Bifidobacterium longum and Lactobacillus plantarum (NVP-1703) in children with AR.
Methods
In a randomized, double-blind, placebo-controlled study, children aged 6 to 19 years with perennial AR were treated with NVP-1703 at a dose of 1 × 1010 CFU/day or placebo once a day for 4 weeks. Total nasal symptom score (TNSS), nasal symptom duration score (NSDS), quality of life (QoL), allergic inflammatory markers, and safety parameters were evaluated.
Results
After 4 weeks of treatment, the TNSS in the NVP-1703 group significantly decreased compared to that in the placebo group (P = 0.011), both in the morning and the evening (P = 0.031 and P = 0.004, respectively). The NSDS also significantly decreased in the NVP-1703 group compared to that in the placebo group (P = 0.018). QoL scores, particularly those related to mouth breathing and itchy nose, showed a significant improvement in the NVP-1703 group compared to the placebo group. The ratios of interleukin (IL)-4/IL-22 and IL-5/IL-22 were significantly reduced in the NVP-1703 group after the treatment compared to the baseline values. No notable adverse events were reported in the NVP-1703 group.
Conclusion
Oral administration of a mixture of B. longum and L. plantarum (NVP-1703) improved both AR symptoms and QoL in children with perennial AR, accompanied by decreases in the ratios of T helper 2 cytokines to IL-22.
Trial Registration
Clinical Research Information Service Identifier: KCT0002661
Keywords: Allergy, Microbiota, Nasal Symptoms, Probiotics, Quality of Life
Graphical Abstract
INTRODUCTION
Allergic rhinitis (AR) is a common chronic disease that affects up to 40% of children1 and significantly reduces quality of life (QoL) in terms of impaired sleep quality, cognitive function, and behavioral problems.2,3 Children with AR frequently rely on symptomatic treatments such as antihistamines, which may cause adverse reactions such as drowsiness and dry mouth. Allergen-specific immunotherapy, which is the only disease-modifying treatment, has limited indications and requires considerable effort, time, and cost. Therefore, microbes such as probiotics have been featured as disease-preventive modalities and are considered candidates as immunomodulating agents to prevent and treat allergic diseases, mainly atopic dermatitis and respiratory allergic diseases.4,5,6
Balanced intestinal microbes modulate AR.7,8 Lactiplantibacillus plantarum derived from Korean kimchi promotes the production of interferon (IFN)-γ and interleukin (IL)-12, decreases airway hyperresponsiveness and leukocyte infiltration in mice, and reduces T helper 2 (Th2) cytokine production in specific mucosal lesions.9 Oral administration of Bifidobacterium breve exerts anti-allergic effects by inhibiting the Th2 response and inducing CD4+/CD25+ regulatory T-cells.10 The effects of probiotics on AR have been mainly studied in Lactobacillus spp. and Bifidobacterium spp., but the study protocols, outcome measures, and results are relatively heterogeneous, and the evidence is limited in children.11,12
Treatment with a mixture of Bifidobacterium longum and Lactobacillus plantarum significantly reduces allergic nasal symptoms, accompanied by a Th2 cytokine reduction in nasal tissues and bronchoalveolar lavage fluid in ovalbumin-induced AR mice, and its clinical therapeutic effect has been shown in adults with AR.13,14 We aimed to investigate the therapeutic effects of probiotic NVP-1703, a mixture of B. longum and L. plantarum, in children with perennial AR. We conducted a 4-week, double-blind, randomized, placebo-controlled trial in children using NVP-1703 and investigated its effect on the total nasal symptom score (TNSS), nasal symptom duration score (NSDS), QoL, and several biomarkers related to the immunomodulatory mechanism of AR.15,16,17
METHODS
Study population
We included children and adolescents aged 6–19 years with perennial AR for more than 2 consecutive years, a mean TNSS ≥ 2 points for 2 nasal symptoms during the run-in period, and positive sensitization in a skin prick test (SPT; Lofarma SpA, Milan, Italy) or serum specific immunoglobulin (Ig)E (Inhalant MAST; LG Chem., Seoul, Korea and ImmunoCAP; Thermo Fisher Scientific, Uppsala, Sweden). Mean wheal size ≥ 3 mm and greater than positive control (histamine) in SPT, serum specific IgE ≥ 0.35 kUA/L in ImmunoCAP, or serum specific IgE ≥ class 1 in MAST was considered positive. The primary criterion for defining perennial AR was the presence of symptoms for more than 2 consecutive years, rather than the specific type of allergens to which the subjects were sensitized. We excluded participants taking medication that might affect AR during the study period; those taking systemic corticosteroids, nasal cromolyn, or antihistamines within 2 weeks from the screening; and those taking probiotic health functional foods or drinking fermented milk (≥ 4 times per week) within 1 week from the screening. During the study period, the use of antihistamines, health supplements for improving allergic rhinitis, immune-related health supplements (e.g., red ginseng, herbal medicine), steroids, nonsteroidal anti-inflammatory drugs, probiotic-containing foods (e.g., fermented milk), probiotic health functional foods, and antibiotics were not allowed.
Study design
Participants visited our center 4 times during the 4-week study period. During the screening visit (Visit 1), we recorded demographic information and medical and surgical histories. Physical examinations, clinical laboratory tests, allergy tests (SPT, Inhalant MAST, or Immuno CAP), and TNSS were also performed.
During the run-in period (between Visits 1 and 2), all participants had their TNSS recorded daily for a minimum of 5 days. After the run-in period, participants who met the inclusion criteria were randomly assigned to one of 2 treatment groups: the probiotic NVP-1703 group or the placebo group. Detailed information regarding sample size determination, randomization, and protocol access is provided in Supplementary Data 1, 2, 3, 4. All participants recorded TNSS and NSDS daily during study periods. At Visits 2, 3, and 4, the QoL Questionnaire in Korean Children with AR (QoL-KCAR) was evaluated; serum immunological biomarkers were analyzed at Visits 2 and 4. TNSS was assessed by phone at week 6.
Probiotics
The study product, NVP-1703, is a mixture of 2 probiotic strains: B. longum IM55 (isolated from the human gut microbiota) and L. plantarum IM76 (isolated from Korean kimchi). NVP-1703 contains a 1 × 1010 CFU live bacterial mixture of 2 g per sachet, and the placebo product contains 2 g of maltodextrin per sachet. Both products have identical appearances, are indistinguishable, and were produced at a Good Manufacturing Practice-certified facility. All participants orally consumed one sachet of the product per day.
Clinical efficacy evaluation
TNSS and NSDS
The primary outcome was the TNSS, defined as the sum of 4 nasal symptoms: rhinorrhea, nasal obstruction, sneezing, and nasal itching. Each symptom was evaluated on a 4-point scale (0, no symptoms; 1, mild symptoms; 2, moderate symptoms; and 3, severe symptoms) for a total of 0–12 points. All the participants recorded their symptom scores in a diary twice daily (morning and evening). The NSDS for the 4 nasal symptoms were similarly recorded. Each duration was assessed on a 4-point scale (0: none, 1: < 30 minutes, 2: between 30 minutes and 2 hours, and 3: > 2 hours). Baseline data were defined as the average daily scores in the morning, evening, or the sum of the morning and evening scores during the run-in period. Changes from the baseline to each week were analyzed.
QoL
The QoL-KCAR was administered to the participants. It consisted of 10 questions, including mouth breathing, itchy nose, rubbing nose, stuffy/blocked nose, rubbing eyes, sneezing, runny nose, blowing nose, taking medicine, and thirst. Each symptom was rated on a five-point scale (0–4 points).
Laboratory tests
Serum immunological biomarkers such as IL-4, IL-5, IL-13, IL-10, and IFN-γ were measured using a high-sensitivity T-cell magnetic bead panel kit for human samples (Millipore Sigma, Burlington, MA, USA) and a Luminex LX200 instrument (Thermo Fisher Scientific) before and 4 weeks after the administration of either product. IL-17 and IL-22 were measured using an immunoassay kit (R&D Systems, Minneapolis, MN, USA). Serum Dermatophagoides pteronyssinus (Dp)- and Dermatophagoides farinae (Df)-specific IgE levels were measured using ImmunoCAP (Thermo Fisher Scientific).
Statistical analysis
All clinical data were analyzed using SAS (version 9.4; SAS Institute, Cary, NC, USA). Baseline characteristics were compared between the 2 groups using the χ2 test or Fisher’s exact test for categorical variables and the 2-sample t-test for continuous variables. An analysis of covariance (ANCOVA), adjusted for baseline, baseline IL-22, and IgE (Dp), was performed to compare changes in TNSS, NSDS, QoL-KCAR, and serum cytokine levels between the groups. A paired t-test was performed for within-group analysis. The TNSS and NSDS were analyzed on a weekly basis, and QoL-KCAR was analyzed for each visit. Serum biomarkers were compared before and after ingestion. If the values were non-normally distributed, the Wilcoxon rank-sum test was used. Statistical significance was set at P < 0.05.
Ethics statement
All participants agreed to take part in the study, and their parents (or legal guardians) signed an informed consent form before the initiation of the study. This study was approved by the Institutional Review Board of Seoul National University Hospital (No. H-1707-117-873) and Ajou University Hospital (No. AJIRB-MED-FOD-17-469). The study was registered with the Clinical Research Information Service, Korea (No. KCT0002661).
RESULTS
Participant characteristics
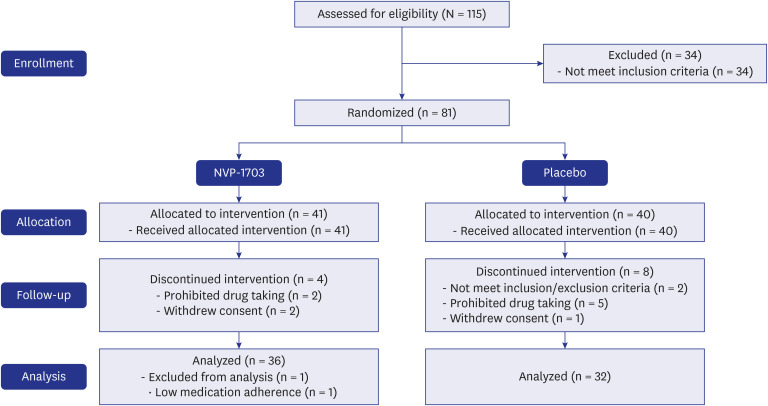
After screening 115 participants, 81 were enrolled in the study. Of these, 41 received NVP-1703 probiotics, and 40 received a placebo. Sixty-eight participants (NVP-1703 group: 36; placebo group: 32) completed the study per the protocol and were analyzed (Fig. 1). There were no significant differences in baseline characteristics, including age, sex, duration of AR, or family history of AR, between the 2 groups (Table 1). There was also no significant difference between groups in response to the allergens as measured by the SPT, immune CAP, and inhalant MAST.
Fig. 1. NVP-1703 clinical trial flowchart.
Table 1. Baseline characteristics of the clinical study participants.
| Characteristics | Placebo (n = 32) | NVP-1703 (n = 36) | P value | |
|---|---|---|---|---|
| Age, yr | 10.44 ± 3.34 | 10.92 ± 3.68 | 0.579a | |
| Sex | 0.117b | |||
| Female | 10 (31.3) | 18 (50.0) | ||
| Male | 22 (68.8) | 18 (50.0) | ||
| BMI, kg/m2 | 19.61 ± 4.05 | 20.52 ± 3.69 | 0.337a | |
| Duration of allergic rhinitis, mon | 72.69 ± 32.30 | 68.08 ± 40.70 | 0.610a | |
| Family history of allergic disease | 0.203c | |||
| Yes | 31 (96.9) | 31 (86.1) | ||
| No | 1 (3.1) | 5 (13.9) | ||
| Sensitization to major inhalant allergens | ||||
| Dermatophagoides pteronyssinus | 22 (68.8) | 26 (72.2) | 0.754b | |
| Dermatophagoides farinae | 24 (75.0) | 27 (75.0) | 1.000b | |
| Alternaria | 5 (15.6) | 2 (5.6) | 0.241c | |
| Aspergillus | 1 (3.1) | 2 (5.6) | 1.000c | |
| Cat fur | 6 (18.8) | 11 (30.6) | 0.262b | |
| Dog hair | 1 (3.1) | 7 (19.4) | 0.058c | |
| Cockroach | 2 (6.3) | 3 (8.3) | 1.000c | |
| Alder | 4 (12.5) | 8 (22.2) | 0.353c | |
| Ragweed | 1 (3.1) | 5 (13.9) | 0.203c | |
| Birch | 6 (18.8) | 7 (19.4) | 0.942b | |
| Japanese hop | 4 (12.5) | 3 (8.3) | 0.699c | |
| Mugwort | 5 (15.6) | 8 (22.2) | 0.490b | |
| Oak | 6 (18.8) | 7 (19.4) | 0.942b | |
Values are expressed as mean ± standard deviation or number (%).
BMI = body mass index.
P value using aindependent t-test, bχ2 test, and cFisher’s exact test between 2 groups.
TNSS and NSDS
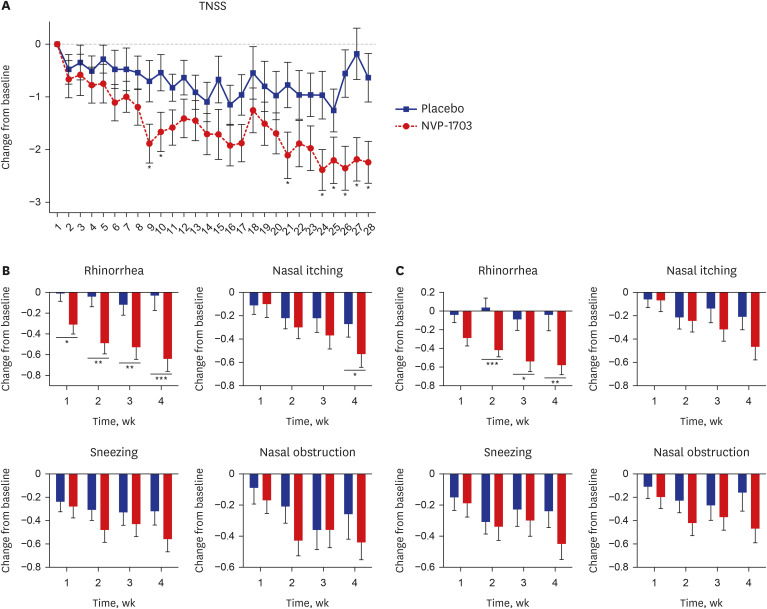
After 4 weeks of treatment, the TNSS in the NVP-1703 group in the morning (AM) significantly decreased compared to those in the placebo group (−1.90 ± 2.07 vs −1.02 ± 2.62, P = 0.031, Table 2, Supplementary Fig. 1A). For the evening (PM), TNSS were significantly reduced in the NVP-1703 group after 2 and 4 weeks of treatment than in the placebo group (−1.70 ± 1.72 vs. −0.78 ± 1.49, P = 0.017 and −2.17 ± 2.14 vs. −0.88 ± 2.22, P = 0.004, respectively; Supplementary Fig. 1A). The sum of the morning and evening (AM + PM) TNSS was significantly improved after 2 and 4 weeks of treatment in the NVP-1703 group (−2.99 ± 3.46 vs. −1.45 ± 3.11, P = 0.043 and −4.07 ± 4.05 vs. −1.90 ± 4.64, P = 0.011, respectively; Supplementary Fig. 1B). Likewise, the NSDS significantly decreased after 4 weeks of treatment in the NVP-1703 group compared to the placebo group in the morning and evening (−1.94 ± 1.97 vs. −0.60 ± 2.29, P = 0.023 and −1.97 ± 2.05 vs. −0.65 ± 2.18, P = 0.019, respectively; Supplementary Fig. 2). The sum of the morning and evening duration scores was significantly reduced in the NVP-1703 group at 4 weeks (−3.91 ± 3.91 vs. −1.25 ± 4.35, P = 0.018; Table 2). The improvement in nasal itching and sneezing duration score in the evening NSDS was maintained for 2 weeks after product administration in the NVP-1703 group compared with the placebo group (Supplementary Fig. 3). The improvement in the daily evening TNSS was significantly greater in the NVP-1703 group than in the placebo group from day 9 to 4 weeks (Fig. 2A). Among the TNSS subscales, the rhinorrhea symptom score significantly decreased in the NVP-1703 group every week after probiotic intake compared with that in the placebo group. The changes in the nasal itching score in the NVP-1703 group were significantly greater than those in the placebo group at 4 weeks (Fig. 2B). The rhinorrhea duration score also significantly improved in the NVP-1703 group at 2, 3, and 4 weeks compared to that in the placebo group (Fig. 2C).
Table 2. Changes in TNSS and NSDS during the study period.
| Variables | Placebo (n = 32) | NVP-1703 (n = 36) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 2 | Week 4 | Week 6 (after 2 weeks) | Baseline | Week 2 | Week 4 | Week 6 (after 2 weeks) | ||
| TNSS | |||||||||
| AM | 5.59 ± 2.07 | 4.92 ± 1.91 | 4.64 ± 2.52 | 5.55 ± 2.11 | 4.27 ± 1.74 | 3.65 ± 1.69 | |||
| Change from baseline | −0.67 ± 2.01 | −1.02 ± 2.62 | −1.28 ± 1.87 | −1.90 ± 2.07 | |||||
| P valueb | 0.941a | 0.141 | 0.031* | ||||||
| PM | 5.49 ± 1.95 | 4.72 ± 2.16 | 4.70 ± 2.55 | 5.13 ± 3.05 | 5.58 ± 2.27 | 3.88 ± 1.71 | 3.41 ± 1.76 | 4.25 ± 2.42 | |
| Change from baseline | −0.78 ± 1.49 | −0.88 ± 2.22 | −0.37 ± 2.82 | −1.70 ± 1.72 | −2.17 ± 2.14 | −1.33 ± 2.78 | |||
| P valueb | 0.849a | 0.017* | 0.004** | 0.088 | |||||
| AM + PM | 11.08 ± 3.83 | 9.63 ± 3.83 | 9.34 ± 5.02 | 11.13 ± 4.25 | 8.15 ± 3.28 | 7.07 ± 3.32 | |||
| Change from baseline | −1.45 ± 3.11 | −1.90 ± 4.64 | −2.99 ± 3.46 | −4.07 ± 4.05 | |||||
| P valueb | 0.959a | 0.043* | 0.011* | ||||||
| NSDS | |||||||||
| AM | 5.24 ± 2.13 | 4.85 ± 2.14 | 4.72 ± 2.53 | 5.72 ± 2.07 | 4.51 ± 2.15 | 3.79 ± 1.97 | |||
| Change from baseline | −0.39 ± 1.67 | −0.60 ± 2.29 | −1.21 ± 1.65 | −1.94 ± 1.97 | |||||
| P valueb | 0.346a | 0.149 | 0.023* | ||||||
| PM | 5.36 ± 2.10 | 4.63 ± 2.39 | 4.80 ± 2.58 | 5.03 ± 2.96 | 5.60 ± 2.21 | 4.18 ± 2.04 | 3.63 ± 2.21 | 4.17 ± 2.17 | |
| Change from baseline | −0.73 ± 1.49 | −0.65 ± 2.18 | −0.33 ± 2.75 | −1.42 ± 1.49 | −1.97 ± 2.05 | −1.43 ± 2.10 | |||
| P valueb | 0.644a | 0.129 | 0.019* | 0.055 | |||||
| AM + PM | 10.59 ± 4.14 | 9.48 ± 4.31 | 9.52 ± 5.06 | 11.32 ± 4.20 | 8.69 ± 4.12 | 7.41 ± 4.09 | |||
| Change from baseline | −1.12 ± 2.87 | −1.25 ± 4.35 | −2.63 ± 3.02 | −3.91 ± 3.91 | |||||
| P valueb | 0.475a | 0.110 | 0.018* | ||||||
Data are expressed as mean ± standard deviation for TNSS and NSDS.
TNSS = total nasal symptom score, NSDS = nasal symptom duration score, AM = morning, PM = evening, IL = interleukin, Ig = immunoglobulin.
aP value for a 2-sample t-test or Wilcoxon rank-sum test between 2 groups. bP value using analysis of covariance adjusted for baseline, the baseline of IL-22, and IgE Dermatophagoides pteronyssinus between 2 groups.
*P < 0.05, **P < 0.01.
Fig. 2. Effect of NVP-1703 on AR symptoms and duration. Effect of NVP-1703 on (A) daily TNSS, (B) weekly AR symptom scores, and (C) AR symptom duration scores. Scores are shown as mean change from the baseline for the TNSS and subscale score of TNSS or NSDS during the study period. Data are expressed as mean ± standard error of the mean.
TNSS = total nasal symptom score, AR = allergic rhinitis, NSDS = nasal symptom duration score.
*P< 0.05, **P< 0.01, ***P< 0.001.
QoL-KCAR
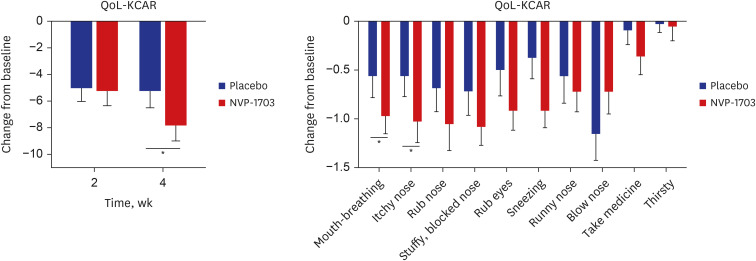
The QoL-KCAR score showed a significant decrease in the NVP-1703 group compared to the placebo group at 4 weeks after treatment (P = 0.047). Among the subscales, mouth breathing and itchy nose scores were significantly reduced in the NVP-1703 group compared to the placebo group at 4 weeks (P < 0.05). We also observed a trend toward improvement in other subscales in the NVP-1703 group compared with the placebo group (Fig. 3).
Fig. 3. Effects of NVP-1703 on QoL-KCAR. Data are expressed as mean ± standard error of the mean.
QoL-KCAR = Quality of Life Questionnaire in Korean Children with Allergic Rhinitis.
*P < 0.05.
Serum biomarkers
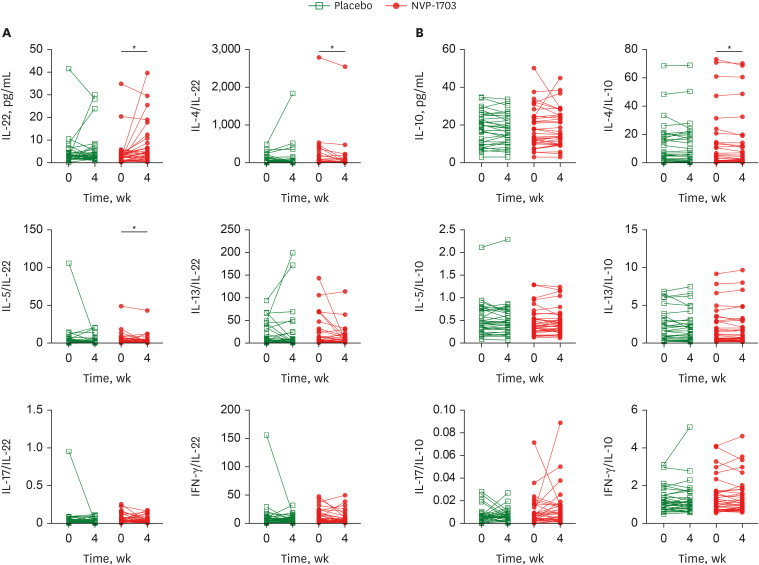
Serum IL-22 concentration significantly increased in the NVP-1703 group but not in the placebo group (P = 0.0293; Fig. 4A). However, no significant differences were observed between the 2 groups (Supplementary Table 1). The ratios of Th2 cytokines, including those of IL-4/IL-22 and IL-5/IL-22, were significantly reduced in the NVP-1703 group (Supplementary Table 2). For the anti-inflammatory cytokine IL-10, the IL-4/IL-10 ratio significantly decreased in the NVP-1703 group, but there was no significant difference between the 2 groups (Fig. 4B). There were no significant differences in serum Dp-specific IgE, Df-specific IgE, eosinophil count, and eotaxin levels between the 2 groups (Supplementary Table 3).
Fig. 4. Effects of NVP-1703 on blood levels of IL-22, cytokines/IL-22, IL-10, and cytokines/IL-10. Effect of NVP-1703 on blood levels of IL-22, IL-10, and associated cytokine ratios. (A) Effect of NVP-1703 on blood levels of IL-22 and associated cytokine ratios, (B) Effect of NVP-1703 on blood levels of IL-10 and associated cytokine ratios.
IL = interleukin, IFN = interferon.
*P < 0.05.
Safety and compliance
At Visits 3 and 4, there were 4 cases of adverse events in the NVP-1703 group (7.32%; anal incontinence, constipation, and urticaria in 3 patients) and 7 cases in the control group (10.00%; conjunctivitis allergy, abdominal pain, diarrhea, rhinitis allergy, and urticaria in 7 patients). One participant in the placebo group dropped out owing to adverse reactions, and all adverse reactions were completely cured. In addition, there were no significant differences between the groups in the clinical laboratory tests conducted at Visits 2 and 4. In the compliance evaluation conducted at Visits 3 and 4, the mean compliance of the NVP-1703 group was 96.14 ± 6.23%, and that of the placebo group was 95.38 ± 8.18%, with no significant difference between groups.
DISCUSSION
We conducted a double-blind, randomized, placebo-controlled trial to evaluate the therapeutic effects of NVP-1703 in children aged 6–19 years. The change in TNSS and NSDS significantly decreased in the NVP-1703 group compared to the placebo group at week 4; therefore, the primary outcome was fulfilled. Furthermore, QoL-KCAR improved in the NVP-1703 group compared to the placebo group at week 4. In terms of immunological biomarkers, serum IL-22 significantly increased, and the IL-4/IL-10, IL-4/IL-22, and IL-5/IL-22 ratios significantly decreased in the NVP-1703 group, although there were no significant differences compared to the placebo group.
Probiotics contribute to the regulation of allergic inflammation through various mechanisms and exert preventive and/or therapeutic effects on respiratory allergic diseases, including AR.5,10,12,18 In most studies, the primary outcome is the improvement in clinical symptoms from baseline to the final visit. However, whereas most studies reporting improvement of AR symptoms with probiotics mention total symptom scores, we tried to analyze the changes in each component of the TNSS, specifically rhinorrhea, nasal obstruction, sneezing, and nasal itching. We showed that NVP-1703 was particularly effective in improving rhinorrhea and nasal itching symptoms. One potential reason for the improvement in histamine-related symptoms is the mechanism of probiotic strains, which modulate the immune system by balancing Th1/Th2 and reducing histamine release from mast cells. This may have led to more noticeable improvements in these symptoms. Additionally, a longer period of probiotic administration might be necessary to see significant improvements in nasal congestion, which warrants further research exploring the optimal strain, dose, and duration of probiotic treatment in pediatric AR. Compared with several studies on the effect of probiotics in pediatric AR with a 12-week administration period, we showed that symptoms significantly improved after a relatively short treatment period of 4 weeks.19,20,21 This effect of NVP-1703 was similarly reported in adult AR patients,14 showing a fairly rapid clinical response to this probiotic mixture. The improvement in evening symptoms was observed at 2 weeks, while morning symptom improvement was noted at 4 weeks in this study. Rhinitis symptoms are often more severe in the evening due to factors like parasympathetic activity, cortisol levels, and reduced airflow related to body position. This could explain why evening symptoms improved earlier. To further elucidate the mechanisms behind the difference in the timing of diurnal symptom improvement, additional research on various factors contributing to symptom exacerbation may be necessary.
Several individual immunologic markers such as eosinophil, IL-4, IL-5, IFN-γ, IL-10, IL-13, and transforming growth factor (TGF)-β have been proposed as mechanisms related to the therapeutic effect of probiotics on AR.20,22,23 Along with AR symptom improvement, reduced levels of Th2 cytokines and increased levels of IL-10 and IFN-γ are generally observed, with insufficient results for TGF-β. In our study, serum IL-22 levels were significantly increased in the NVP-1703 group but not in the placebo group. IL-22 belongs to the IL-10 family, originally named the IL-10-related T-cell-derived inducible factor, and is a homeostatic cytokine that preserves the integrity of the skin, airways, and gastrointestinal system. Dysregulation of IL-22 may play a role in various immune-mediated inflammatory diseases, including psoriasis and inflammatory bowel diseases,24,25 but the role of IL-22 in allergic diseases remains unclear. The tissue-protective role of IL-22 in the early stages of asthma and atopic eczema has been reported26,27; however, it exhibits both pro- and anti-inflammatory properties.28 Tang et al.29 reported that AR severity correlates with IL-22 level, which is, to some extent, contradictory to the results of our study. However, the latest research is lacking; therefore, further clarification is needed regarding the mechanistic role of IL-22 in AR. The reduction in the IL-4/IL-10 ratio in the NVP-1703 group is consistent with other previous studies.14,20,30
Oral administration of NVP-1703 for 4 weeks significantly improved the QoL scores compared to our placebo group. Probiotic treatment improves QoL in adults with AR31,32; however, there are relatively few studies involving QoL in children and adolescents with AR. Miraglia Del Giudice et al.21 reported that a Bifidobacterium mixture significantly improved QoL in children with pollen-induced AR and intermittent asthma and, in a study investigating the effect of Lactobacillus johnsonii, QoL improved in both the study and placebo groups, with no significant difference between groups.20
This study has several limitations. The intestinal microbial environment is influenced by various factors, such as dietary patterns, in addition to probiotic administration, and it was not possible to control completely these factors in this study targeting children and adolescents. Efforts have been made to minimize these effects by limiting the intake of supplements other than the study product, fermented milk, and typical lactic acid-containing foods. In addition, medications such as antihistamines and systemic steroids were strictly restricted to evaluate objectively the effect of the study product.
In conclusion, NVP-1703, a mixture of B. longum and L. plantarum, significantly improved AR symptoms and QoL in children with perennial AR in a 4-week, double-blind, randomized, placebo-controlled trial. Improvement in clinical symptoms was related to increased IL-22 levels and decreased IL-4/IL-10 and IL-4/IL-22 ratios. No significant differences in the various safety parameters were found between the 2 groups.
Footnotes
Funding: This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) & funded by the Korean government (MSIT) (NRF-2017M3A9G2049280).
Disclosure: Jang SW, Han SW, and Kang SH are employed by NVP Healthcare Co., Ltd. The remaining authors report no conflicts of interest in this work.
Data Availability Statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
- Conceptualization: Han SW, Suh DI, Lee S.
- Data curation: Jeong K, Jeon SA, Seo HJ.
- Formal analysis: Jeong K, Jang SW, Kang SH.
- Funding acquisition: Suh DI, Lee S.
- Investigation: Jeong K, Jeon SA, Seo HJ, Suh DI, Lee S.
- Methodology: Jang SW, Han SW, Suh DI, Lee S.
- Resources: Jeon SA, Seo HJ.
- Supervision: Suh DI, Lee S.
- Validation: Suh DI, Lee S.
- Visualization: Jang SW, Kang SH.
- Writing - original draft: Jeong K, Jang SW.
- Writing - review & editing: Jeong K, Jang SW, Han SW, Suh DI, Lee S.
SUPPLEMENTARY MATERIALS
Sample size determination
Randomization
Losses and exclusions after randomization
Protocol access and the periods of recruitment and follow-up
Effects of NVP-1703 on blood levels of pro-inflammatory and anti-inflammatory cytokines
Effects of NVP-1703 on blood levels of the ratio of pro-inflammatory to anti-inflammatory cytokines
Effects of NVP-1703 on blood levels of immune cells and antibodies associated with allergies
Effect of NVP-1703 on morning and evening AR symptoms. Effect of NVP-1703 on (A) TNSS, (B) subscale of TNSS (Rhinorrhea).
Effect of NVP-1703 on morning and evening AR symptom duration. Effect of NVP-1703 on (A) NSDS, (B) subscale of NSDS (Rhinorrhea).
Effect of NVP-1703 on AR symptoms and duration subscales in the evening. Effect of NVP-1703 on (A) TNSS and subscale of TNSS (B) NSDS and subscale of NSDS in the evening.
References
- 1.Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016;36(2):235–248. doi: 10.1016/j.iac.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.D’Elia C, Gozal D, Bruni O, Goudouris E, Meira E Cruz M. Allergic rhinitis and sleep disorders in children - coexistence and reciprocal interactions. J Pediatr (Rio J) 2022;98(5):444–454. doi: 10.1016/j.jped.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki A, Burks CA, Bhattacharyya N. Cognitive and quality of life-related burdens of illness in pediatric allergic airway disease. Otolaryngol Head Neck Surg. 2020;162(4):566–571. doi: 10.1177/0194599820908202. [DOI] [PubMed] [Google Scholar]
- 4.Rusu E, Enache G, Cursaru R, Alexescu A, Radu R, Onila O, et al. Prebiotics and probiotics in atopic dermatitis. Exp Ther Med. 2019;18(2):926–931. doi: 10.3892/etm.2019.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West CE, Jenmalm MC, Kozyrskyj AL, Prescott SL. Probiotics for treatment and primary prevention of allergic diseases and asthma: looking back and moving forward. Expert Rev Clin Immunol. 2016;12(6):625–639. doi: 10.1586/1744666X.2016.1147955. [DOI] [PubMed] [Google Scholar]
- 6.Jeong K, Kim M, Jeon SA, Kim YH, Lee S. A randomized trial of Lactobacillus rhamnosus IDCC 3201 tyndallizate (RHT3201) for treating atopic dermatitis. Pediatr Allergy Immunol. 2020;31(7):783–792. doi: 10.1111/pai.13269. [DOI] [PubMed] [Google Scholar]
- 7.Farahmandi K, Mohr AE, McFarland LV. Effects of probiotics on allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. Am J Rhinol Allergy. 2022;36(4):440–450. doi: 10.1177/19458924211073550. [DOI] [PubMed] [Google Scholar]
- 8.Ried K, Travica N, Paye Y, Sali A. Effects of a probiotic formulation on Seasonal allergic rhinitis in adults-a randomized double-blind placebo-controlled trial: the probiotics for hay fever trial. Front Nutr. 2022;9:887978. doi: 10.3389/fnut.2022.887978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Bae J, Choi CY, Choi SP, Yun HS, Chun T. Oral administration of Lactiplantibacillus plantarum NR16 isolated from Kimchi ameliorates murine allergic rhinitis. Lett Appl Microbiol. 2022;75(1):152–160. doi: 10.1111/lam.13716. [DOI] [PubMed] [Google Scholar]
- 10.Ren J, Zhao Y, Huang S, Lv D, Yang F, Lou L, et al. Immunomodulatory effect of Bifidobacterium breve on experimental allergic rhinitis in BALB/c mice. Exp Ther Med. 2018;16(5):3996–4004. doi: 10.3892/etm.2018.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, Billoo AG, Iqbal K. Efficacy of probiotic in perennial allergic rhinitis under five year children: a randomized controlled trial. Pak J Med Sci. 2019;35(6):1538–1543. doi: 10.12669/pjms.35.6.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anania C, Di Marino VP, Olivero F, De Canditiis D, Brindisi G, Iannilli F, et al. Treatment with a probiotic mixture containing Bifidobacterium animalis subsp. Lactis BB12 and Enterococcus faecium L3 for the prevention of allergic rhinitis symptoms in children: a randomized controlled trial. Nutrients. 2021;13(4):1315. doi: 10.3390/nu13041315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim WG, Kang GD, Kim HI, Han MJ, Kim DH. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef Microbes. 2019;10(1):55–67. doi: 10.3920/BM2017.0146. [DOI] [PubMed] [Google Scholar]
- 14.Kang MG, Han SW, Kang HR, Hong SJ, Kim DH, Choi JH. Probiotic NVP-1703 alleviates allergic rhinitis by inducing IL-10 expression: a four-week clinical trial. Nutrients. 2020;12(5):1427. doi: 10.3390/nu12051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer EO, Kunjibettu S, Hall N, Wingertzahn MA, Murcia C, Berger W, et al. Efficacy and safety of ciclesonide, 200 microg once daily, for the treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2007;98(2):175–181. doi: 10.1016/s1081-1206(10)60693-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Wei P, Chen B, Li X, Luo X, Chen X, et al. Intranasal fluticasone furoate in pediatric allergic rhinitis: randomized controlled study. Pediatr Res. 2021;89(7):1832–1839. doi: 10.1038/s41390-020-01180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JW, Kang HR, Ji GE, Park MS, Song WJ, Kim MH, et al. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011;3(2):103–110. doi: 10.4168/aair.2011.3.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin WY, Fu LS, Lin HK, Shen CY, Chen YJ. Evaluation of the effect of Lactobacillus paracasei (HF.A00232) in children (6-13 years old) with perennial allergic rhinitis: a 12-week, double-blind, randomized, placebo-controlled study. Pediatr Neonatol. 2014;55(3):181–188. doi: 10.1016/j.pedneo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. 2007;62(2):215–220. doi: 10.1203/PDR.0b013e3180a76d94. [DOI] [PubMed] [Google Scholar]
- 20.Lue KH, Sun HL, Lu KH, Ku MS, Sheu JN, Chan CH, et al. A trial of adding Lactobacillus johnsonii EM1 to levocetirizine for treatment of perennial allergic rhinitis in children aged 7-12 years. Int J Pediatr Otorhinolaryngol. 2012;76(7):994–1001. doi: 10.1016/j.ijporl.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Miraglia Del Giudice M, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. 2017;43(1):25. doi: 10.1186/s13052-017-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivory K, Chambers SJ, Pin C, Prieto E, Arqués JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy. 2008;38(8):1282–1289. doi: 10.1111/j.1365-2222.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh A, Hacini-Rachinel F, Gosoniu ML, Bourdeau T, Holvoet S, Doucet-Ladeveze R, et al. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: an exploratory, randomized, placebo-controlled clinical trial. Eur J Clin Nutr. 2013;67(2):161–167. doi: 10.1038/ejcn.2012.197. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87(5):523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 25.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagome K, Imamura M, Kawahata K, Harada H, Okunishi K, Matsumoto T, et al. High expression of IL-22 suppresses antigen-induced immune responses and eosinophilic airway inflammation via an IL-10-associated mechanism. J Immunol. 2011;187(10):5077–5089. doi: 10.4049/jimmunol.1001560. [DOI] [PubMed] [Google Scholar]
- 27.Pennino D, Bhavsar PK, Effner R, Avitabile S, Venn P, Quaranta M, et al. IL-22 suppresses IFN-γ-mediated lung inflammation in asthmatic patients. J Allergy Clin Immunol. 2013;131(2):562–570. doi: 10.1016/j.jaci.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JR, Nishioka M, Chakir J, Risse PA, Almaghlouth I, Bazarbashi AN, et al. IL-22 contributes to TGF-β1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res. 2013;14(1):118. doi: 10.1186/1465-9921-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, Xiao P, Luo X, Bai J, Xia W, Chen W, et al. Increased IL-22 level in allergic rhinitis significantly correlates with clinical severity. Am J Rhinol Allergy. 2014;28(6):197–201. doi: 10.2500/ajra.2014.28.4088. [DOI] [PubMed] [Google Scholar]
- 30.Torre E, Sola D, Caramaschi A, Mignone F, Bona E, Fallarini S. A pilot study on clinical scores, immune cell modulation, and microbiota composition in allergic patients with rhinitis and asthma treated with a probiotic preparation. Int Arch Allergy Immunol. 2022;183(2):186–200. doi: 10.1159/000518952. [DOI] [PubMed] [Google Scholar]
- 31.Costa DJ, Marteau P, Amouyal M, Poulsen LK, Hamelmann E, Cazaubiel M, et al. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN Study) Eur J Clin Nutr. 2014;68(5):602–607. doi: 10.1038/ejcn.2014.13. [DOI] [PubMed] [Google Scholar]
- 32.Yonekura S, Okamoto Y, Okawa T, Hisamitsu M, Chazono H, Kobayashi K, et al. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009;30(4):397–405. doi: 10.2500/aap.2009.30.3256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample size determination
Randomization
Losses and exclusions after randomization
Protocol access and the periods of recruitment and follow-up
Effects of NVP-1703 on blood levels of pro-inflammatory and anti-inflammatory cytokines
Effects of NVP-1703 on blood levels of the ratio of pro-inflammatory to anti-inflammatory cytokines
Effects of NVP-1703 on blood levels of immune cells and antibodies associated with allergies
Effect of NVP-1703 on morning and evening AR symptoms. Effect of NVP-1703 on (A) TNSS, (B) subscale of TNSS (Rhinorrhea).
Effect of NVP-1703 on morning and evening AR symptom duration. Effect of NVP-1703 on (A) NSDS, (B) subscale of NSDS (Rhinorrhea).
Effect of NVP-1703 on AR symptoms and duration subscales in the evening. Effect of NVP-1703 on (A) TNSS and subscale of TNSS (B) NSDS and subscale of NSDS in the evening.