Abstract
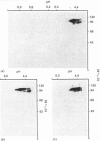
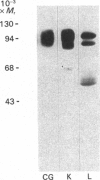
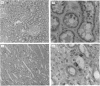
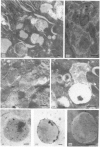
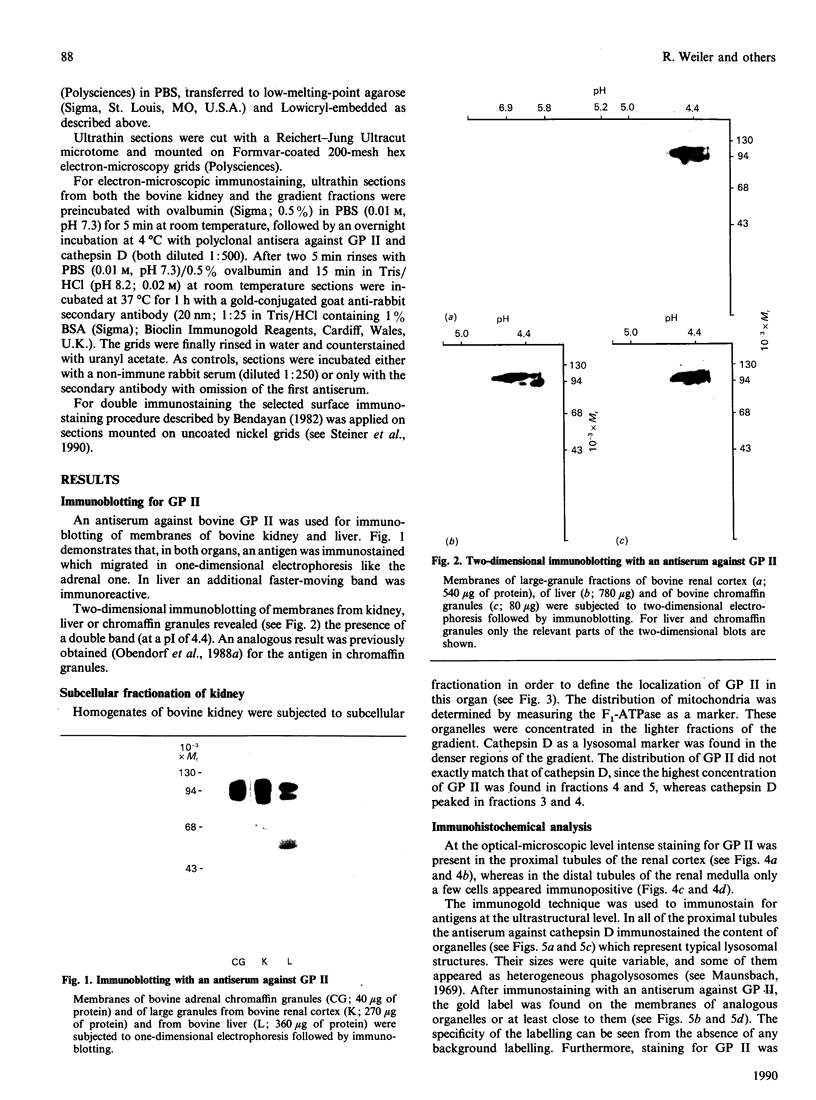
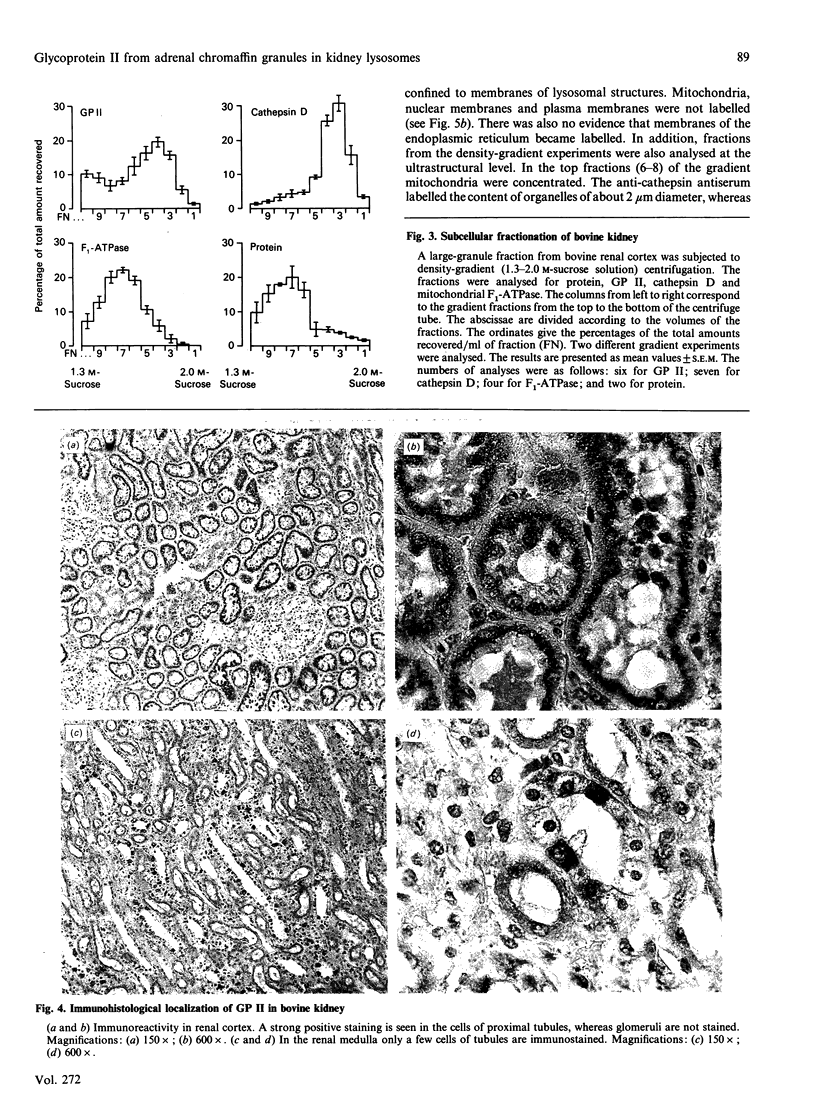
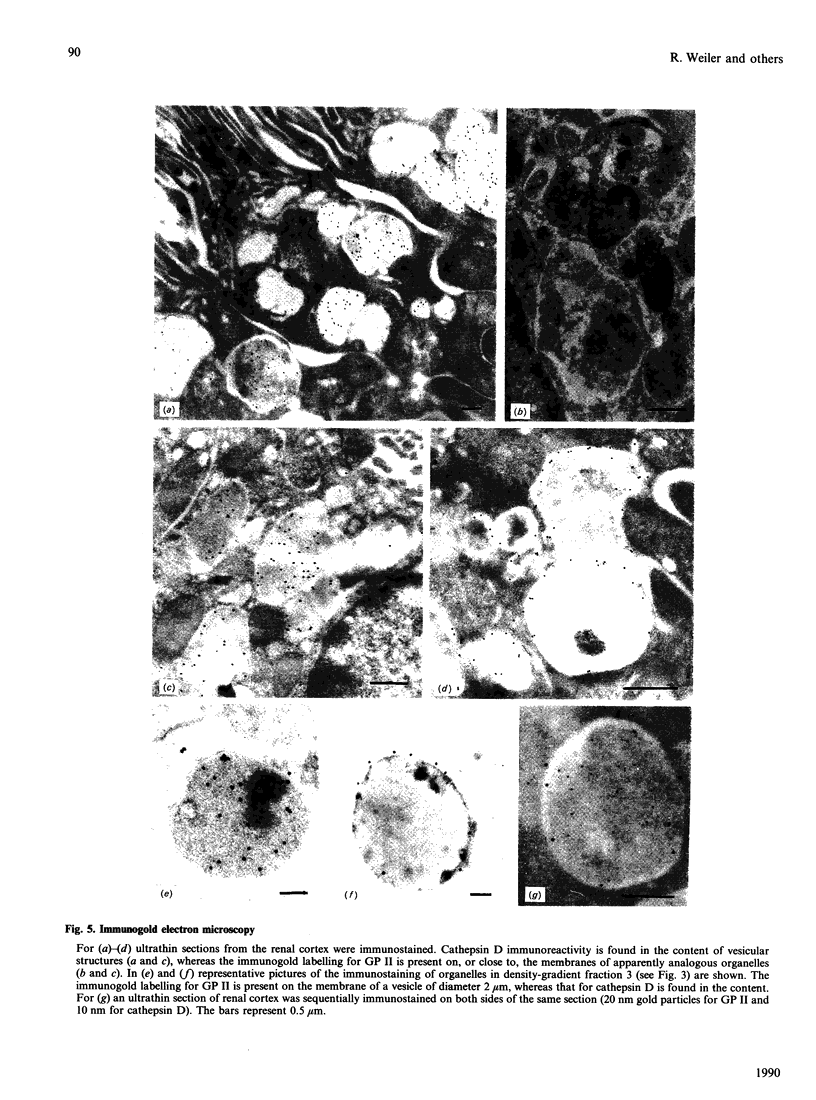
Glycoprotein II (GP II) is a protein found in the membranes of chromaffin granules from adrenal medulla. Immunoblotting (one- and two-dimensional) revealed that this antigen is also present in liver and in kidney. Subcellular fractionation of the latter organ indicated that GP II was present in lysosomes. This was confirmed by immunoelectron microscopy. The antiserum against GP II immunolabelled the membranes of organelles which could be identified as lysosomes by the labelling of their contents with an antiserum against cathepsin D. Thus GP II is an antigen common to secretory vesicles and lysosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apps D. K., Pryde J. G., Phillips J. H. Cytochrome b561 is identical with chromomembrin B, a major polypeptide of chromaffin granule membranes. Neuroscience. 1980;5(12):2279–2287. doi: 10.1016/0306-4522(80)90143-8. [DOI] [PubMed] [Google Scholar]
- Bendayan M. Double immunocytochemical labeling applying the protein A-gold technique. J Histochem Cytochem. 1982 Jan;30(1):81–85. doi: 10.1177/30.1.6172469. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Structure of human lysosomal membrane glycoprotein 1. Assignment of disulfide bonds and visualization of its domain arrangement. J Biol Chem. 1989 Dec 5;264(34):20526–20531. [PubMed] [Google Scholar]
- Chen J. W., Murphy T. L., Willingham M. C., Pastan I., August J. T. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985 Jul;101(1):85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Frischenschlager I. Immunological characterization of secretory proteins of chromaffin granules: chromogranins A, chromogranins B, and enkephalin-containing peptides. J Neurochem. 1985 Jun;44(6):1854–1861. doi: 10.1111/j.1471-4159.1985.tb07179.x. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Zangerle R., Frischenschlager I., Weber A., Winkler H. Isolation and immunological characterization of a glycoprotein from adrenal chromaffin granules. J Neurochem. 1984 Apr;42(4):1008–1016. doi: 10.1111/j.1471-4159.1984.tb12704.x. [DOI] [PubMed] [Google Scholar]
- Gavine F. S., Pryde J. G., Deane D. L., Apps D. K. Glycoproteins of the chromaffin granule membrane: separation by two-dimensional electrophoresis and identification by lectin binding. J Neurochem. 1984 Nov;43(5):1243–1252. doi: 10.1111/j.1471-4159.1984.tb05379.x. [DOI] [PubMed] [Google Scholar]
- Grimaldi K. A., Hutton J. C., Siddle K. Production and characterization of monoclonal antibodies to insulin secretory granule membranes. Biochem J. 1987 Jul 15;245(2):557–566. doi: 10.1042/bj2450557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber E., König P., Schuler G., Aberer W., Plattner H., Winkler H. Characterization and topography of the glycoproteins of adrenal chromaffin granules. J Neurochem. 1979 Jan;32(1):35–47. doi: 10.1111/j.1471-4159.1979.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H., Lochs H. Membrane proteins of chromaffin granules, dopamine -hydroxylase, a major constituent. Biochem J. 1972 Aug;129(1):187–195. doi: 10.1042/bj1290187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Wiederanders B., Brömme D., Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989 Dec 1;264(2):467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laslop A., Fischer-Colbrie R., Hook V., Obendorf D., Winkler H. Identification of two glycoproteins of chromaffin granules as the carboxypeptidase H. Neurosci Lett. 1986 Dec 23;72(3):300–304. doi: 10.1016/0304-3940(86)90530-6. [DOI] [PubMed] [Google Scholar]
- Mane S. M., Marzella L., Bainton D. F., Holt V. K., Cha Y., Hildreth J. E., August J. T. Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys. 1989 Jan;268(1):360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. Isolation and purification of acid phosphatase-containing autofluorescent granules from homogenates of rat kidney cortex. J Ultrastruct Res. 1966 Sep;16(1):13–34. doi: 10.1016/s0022-5320(66)80020-5. [DOI] [PubMed] [Google Scholar]
- Nelson N., Taiz L. The evolution of H+-ATPases. Trends Biochem Sci. 1989 Mar;14(3):113–116. doi: 10.1016/0968-0004(89)90134-5. [DOI] [PubMed] [Google Scholar]
- Obendorf D., Schwarzenbrunner U., Fischer-Colbrie R., Laslop A., Winkler H. Immunological characterization of a membrane glycoprotein of chromaffin granules: its presence in endocrine and exocrine tissues. Neuroscience. 1988 Apr;25(1):343–351. doi: 10.1016/0306-4522(88)90030-9. [DOI] [PubMed] [Google Scholar]
- Obendorf D., Schwarzenbrunner U., Fischer-Colbrie R., Laslop A., Winkler H. In adrenal medulla synaptophysin (protein p38) is present in chromaffin granules and in a special vesicle population. J Neurochem. 1988 Nov;51(5):1573–1580. doi: 10.1111/j.1471-4159.1988.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Rinne A., Järvinen M., Kirschke H., Wiederanders B., Hopsu-Havu V. K. Demonstration of cathepsins H and L in rat tissues. Biomed Biochim Acta. 1986;45(11-12):1465–1476. [PubMed] [Google Scholar]
- Rowley D. Phagocytosis and immunity. The central role of phagocytosis in immune reactions. Experientia. 1966 Jan 15;22(1):1–5. [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. The localization of lysosomal enzymes in chromaffin tissue. J Physiol. 1966 Mar;183(1):179–188. doi: 10.1113/jphysiol.1966.sp007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H. J., Weiler R., Ludescher C., Schmid K. W., Winkler H. Chromogranins A and B are co-localized with atrial natriuretic peptides in secretory granules of rat heart. J Histochem Cytochem. 1990 Jun;38(6):845–850. doi: 10.1177/38.6.2139887. [DOI] [PubMed] [Google Scholar]
- Winkler H., Apps D. K., Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986 Jun;18(2):261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]