Abstract
Water pollution is a significant issue resulting from past long-term actions. The remediation projects carried out under law constraints for industrial plants, which have been the major contributors to environmental and water pollution, are currently providing a significant amount of data about contaminated soil, surface waters, and groundwater. Most of such plants worldwide are in coastal zones. Based on a significant amount of chemical and environmental data for a coastal contaminated site subject to variable groundwater salinization, this study aimed to understand the mobility of some trace elements because of coastal zone dynamics. Data concerned 688 groundwater samples, including As, Hg, Cd, Crtot, Cu, Ni, Pb, V, Se, Zn, pH, electrical conductivity, chlorides, total organic carbon and organic contaminants as quantitative variables, enhanced by additional qualitative variables such as groundwater salinity, season, water level, precipitation, and industrial activity type to make the dataset as representative as possible of the site under investigation. The study used robust multivariate statistical analyses to analyse the complex dataset and explain the relevant factors influencing contaminant behaviour under different environmental conditions. The Multivariate Statistical Analysis distinguished three clusters of trace elements with diverse reactivity to changes in groundwater salinization. The first includes Se, Cu, Crtot, V, and Ni, showing the highest correlation with electrical conductivity and chlorides because of their high affinity to form chloride or organic chloride complexes and for ion competition. Zn and Pb cluster in the second group: they are less reactive to groundwater salinization and more influenced by cation and anion competition and organic matter. The mobility of Hg and As (third cluster) significantly correlates with Fe and Mn, underlining the dominant role of reductive dissolution of trace elements-bearing minerals (Fe/Mn/Al-oxy-hydroxides) and metal-organic complexes. The correlation between the clustering of variables in groundwater and soils shows the influence of sediment structure, mineral composition, and physical and chemical soil conditions on the distribution in soils of trace elements and their transport to groundwater. The study proposes a valuable approach for assessing the effects of salinization in contaminated coastal aquifers. It supports planning multi-purpose characterization and monitoring campaigns of contaminated coastal sites and provides guidance on the correct associated remediation projects.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75974-1.
Keywords: Trace elements mobility, Contaminated industrial site, Coastal zone, Groundwater salinization, Univariate analysis, Multivariate statistical analysis
Subject terms: Environmental sciences, Environmental chemistry, Environmental impact
Introduction
Apart from the evident damage to the atmosphere, soil, surface waters, marine waters, sediments, and biota, industrial plants located near coastal areas determine not as so obvious deterioration to the environment when they modify the natural geological structures and the interconnected hydrodynamics with engineering works, infrastructures, and back-fillings. The environmental components are generally interconnected by groundwater (acting like an in-between reservoir) and complex geochemical relations, which may change so much to alter the whole system’s functioning completely. Industrial activities may cancel, divert, or pollute the natural discharge of groundwater. The groundwater input to the oceans (Submarine Groundwater Discharge, SGWD)1–4 through the “subterranean estuary”5 is often overlooked, if not disregarded, compared to surface water, even in studies of natural environments, thus forgetting a significant component of the coastal balance.
The high vulnerability of coastal aquifers to seawater intrusion highlights the importance of hydrochemical analysis and careful groundwater management to prevent further degradation6. GIS-based assessments could provide essential data for improving groundwater quality and ensuring its suitability for use7. However, studies on mass transport and chemical cycling in subterranean estuaries are difficult and uncommon8–12. The SGWD input varies in space and time and has three main components4: freshwater, seawater subject to recycling on tidal to seasonal timescales13, and saline groundwater. Multiple factors control SGWD chemical composition. Climate affects the chemistry of infiltrating water, while the geological framework, controlling the lithology and permeability of the unsaturated and saturated zone and the flow system scale, condition the hydrochemical processes (dissolution, precipitation, base exchange, and microbially-driven diagenesis14–17), the residence time and the extent of hydrogeochemical evolution. Groundwater circulation and oscillating flow due to wave setup and tides may account for up to 96% of the SGWD and induce fluctuations in the rates of chemical transfer over short periods18.
Investigations within industrialized environments are even more limited in considering all the interacting components, given that they generally focus on the quality of narrow areas. However, SGWD remains crucial in transporting mass flux from coastal zones to marine sediments and seawater on whatever environment.
Aquifer sediments and soils are the primary reservoirs of pollutants transported by SGWD. The impact of stored contaminants might only become noticeable when changing environmental conditions cause the soil’s ability to hold them to exceed. Over time, soils and sediments naturally have a capacity to handle the buildup of contaminants up to a saturation point, after which they can shift from being a storage for harmful chemicals to a source of them. During this period, the obvious effects of pollution on the environment may not be readily visible or predictable. When the effects of post-saturation and subsequent movement of contaminants finally become apparent, they typically occur long after the activities that initially caused the issue. Because of this delayed environmental response and the unforeseeable effects, polluted soils and sediments have been likened to “chemical time bombs”19.
Among pollutants, trace elements (TEs) merit more attention because some of them are of toxicologic significance. In this work, we will use the term “trace elements” in the general context. However, whenever opportune, we will distinguish heavy metals, metalloids, and non-metals according to Ali and Khan20. For this categorization Hg, Cd, Crtot, Cu, Ni, Pb, V, and Zn are “heavy metals”, As is a metalloid, Be is an alkaline earth metal and Se is a non-metal. For the literature review, we will use the terms used by authors.
TEs show a diverse mobility within groundwater and sediments. The form of such elements in soils and aquifers depends on the type of sediment or soil and the chemical and physical conditions21 like the pH, the redox potential, concentration of dissolved organic matter, the competitiveness of other ions for exchange sites, groundwater ionic strength, textural characteristics of soils and mineral sources presence. Under low redox potential, relatively neutral pH, and low salinity of waters, sediments act as “sinks” for many TEs.
However, sediments can become a source of heavy metals following a redox potential and groundwater salinity increase19,22, and a pH decrease. In a coastal environment under salinization, heavy metals may be released into or removed from groundwater depending on their phase (solid, dissolved or colloid)23. The control over heavy metal solutes occurs through chemical processes such as dissolution, oxidation and reduction, acidification, absorption and desorption, Fe/Mn/Al-hydroxide coprecipitation, where such factors also depend on the residence time, groundwater depth and flow24,25. In general, increasing salinity increases the availability of inorganic ligands for formation of complex with cations26, decreases activity coefficients due to increase in ionic strength27,28 and cation exchange29,30. The cation exchange effect results from the competition of salt-derived cations with positively charged minor and trace elements for sorption sites on the solid phase30. In addition, high levels of salinity can influence the microbial processes regulating trace element mobilization. Groundwater salinity increase may enhance heavy metal mobility because complexing agents form soluble metal complexes31,32. The salinity increase may involve negative (Pb, Hg), and positive correlations (As) with Cl and with the major cations Na+, K+, Ca2+, and Mg2+33,34. Hg seems to overcome the subterranean barrier35, which is a barrier for As, which is trapped in sediments36, and for Fe, which precipitates as iron oxide and accumulates concurrently with phosphorous12,13. Cd, Co, Cr, Pb and Zn concentrations in SGWD are higher (of one order of magnitude) than in seawater, while Cu and Ni show lower concentrations than in seawater37. Mn shows two orders of magnitude compared to seawater. However, dissolved Co, Cd, Pb and Zn decrease with increased salt content. All TEs do not show conservative behaviour during mixing due to the action of different and often overlapping factors. Redox conditions and co-precipitation with manganese oxides drive the cycling of elements in the discharge areas. Under reduction conditions, high levels of sulphate salts can enhance the activity of sulphate-reducing bacteria in sediments. Along with SO42− reduction to HS–, the reduction of Fe oxide mineral occurs, resulting in the leaching of some TE and their partitioning between sediment and groundwater38–40.
Understanding the transfer of TEs to the sea with SGWD and their fluxes is still a subject of research. The matter requires further efforts considering the complexity of TEs and especially heavy metals’ behaviour and the land-ocean interface. Recognizing such processes in industrialised environments is even more tricky, as heavy pollution and human pressures add their effects to those of the natural framework. Contaminated sites on coastal zones can show TEs mobilisation not only under the impact of natural seawater intrusion: groundwater salinization may also derive from industrial effluents or interconnections of aquifers because of over-pumping from deep wells, or because remediation actions such as hydraulic barriers cause groundwater salinization. All salinization factors significantly alter sediments and groundwater’s physical, chemical, and biological properties. From the technical point of view, remediation works under groundwater salinization might promote the transfer of TEs to the marine environment rather than their removal.
Guidelines or directives for evaluating contaminated sites do not consider the connections between groundwater pollution and the natural or non-natural increase of salt content during their assessment and for remediation design, so much so that laws do not even require the analysis of chlorides in coastal contaminated sites characterisation.
Research on transfer of TEs of toxicological significance from contaminated coastal sites to the sea can rely only on data generated under legal constraints, which generally only covers the terrestrial part of industrial settlements. This research deals with the contamination released in soils and groundwater in a parcel of Taranto’s coastal area (Puglia, Southern Italy). The study area is in a broader Site of National Interest (SIN), which includes numerous industrial plants (refineries and steelworks) liable for severe environmental problems affecting all environmental matrices (soil, air, water and marine sediments)41. The qualitative degradation of groundwater in the SIN area depends not only on the presence of industrial centres but also on human activities in the area (military, port, shipbuilding, etc.) and the incorrect use of fertilisers for agricultural use42–44. The study refers to a complex dataset comprising multiple monitoring surveys on soil and groundwater from a superficial aquifer affected by groundwater salinization (GWS) from different sources and processes for four years. Monitoring refers to a hydraulic barrier’s functioning period aimed at remediation by the “pump and treat” method. The monitoring period includes season variability, thus comprising a range of combinations of natural and human influential factors. Hydrocarbons and TEs contaminate the study area in different focal points. They generated plumes, which are disconnected from the origin today, giving a current picture of contamination due to the overlap in space and time of contaminants of different natures released at various times with different doses. The transport of pollutants in the aquifer is multi-component within a complex geochemical system because of GWS. Such salinization does not come directly from seawater intrusion because the aquifer is at a higher elevation than sea level. Still, activities related to the industrial plant and the remediation works likely triggered it.
The study neither aims at characterizing the site nor to verify remediation efficiency of the contaminated site. We deal with monitoring data from groundwater and soils to recognize TEs’ behaviour in a complex coastal environment with human impact. The significant amount of monitoring data enabled their effective elaboration by the chemometric approach, able to deal with heterogeneous datasets (of hydrological, hydrogeological, and geochemical data), which typically require different expert judgements41,43,45–47. The approach comprises the application of univariate and unsupervised multivariate statistical techniques (Principal Component Analysis, PCA, Correlation Analysis, CA, and Hierarchical Cluster Analysis, HCA).
The present study’s primary goal and novelty is to demonstrate the benefit of an interdisciplinary and comprehensive approach for monitoring a complex coastal industrial environment. This comprehensive approach involves combining water and soil quality surveys and measures of coastal dynamics with advanced statistical analysis. It can offer insights into the behaviour of contaminants in groundwater that would not be easily recognizable through the analysis of smaller datasets and classic data processing.
Specifically, the study aims to:
-
(i)
Verify the effectiveness of combining different chemometric techniques to understand complex contamination patterns in a coastal area, addressing the need to integrate advanced statistical methods to analyse the interactions between salinization and contaminant mobility.
-
(ii)
Identify the key factors and interactions that control the behaviour of contaminants in polluted coastal aquifers affected by salinization.
-
(iii)
Update investigation protocols for the characterization and monitoring of coastal contaminated sites, providing a practical tool for policymakers and environmental managers.
Materials and methods
Geographic and hydrogeological setting of the Taranto area
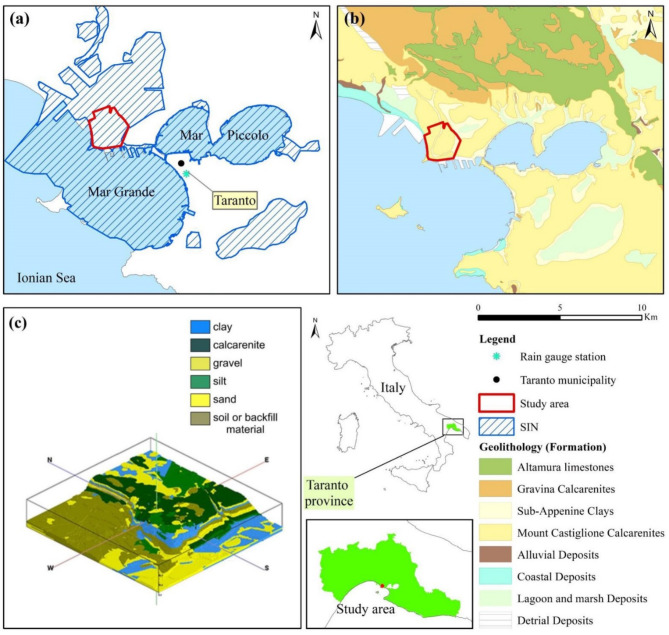
The study site locates in the industrial area of Taranto town (Puglia, Southern Italy, Fig. 1a) belonging to the Taranto Province. The Ionian Sea borders the Taranto Province to the S, where the elliptical seas of the Mar Piccolo and the Mar Grande develop. To the N and NW, the elevation rapidly decreases from the highlands to the coastal plain, and the territory (average elevation of 168 m above mean sea level, AMSL) shows deep karstic canyons (gravine). The province area is generally hilly and flat, arched around the Taranto town. The Taranto area is geologically characterised by a formation (Fm) succession, from ancient to recent (Fig. 1b), of: Mesozoic limestones (“Altamura limestone” Fm), which outcrop to the east and north at higher altitudes; Plio-Pleistocene calcarenites (“Gravina Calcarenite” Fm) consisting of clasts derived from the degradation of Cretaceous limestones as well as fossiliferous fragments, and marly clays (Sub-Apennine Clays Fm), outcropping along the incisions and the coast of the Mar Piccolo and impermeable, preventing the contact with the sea of the basement; Medium and Upper Pleistocene transgressive calcarenite deposits (the “Mount Castiglione Calcarenites”), mainly coarse and compact and showing a variable hydraulic conductivity because of alternating compact and macro fossiliferous layers; “Alluvial Deposits” of Holocene, comprising polygenic pebbles, sometimes cemented, with sandy-clayey intercalations and volcanic products, ancient lacustrine and terraced, and current alluviums; “Coastal Deposits” (Holocene) consisting of modest thickness of sands, sometimes coarse and cross-stratified and including the present-day and recent coastal dunes present along the coastline; Upper Pleistocene to Holocene “Lagoon and marsh deposits” of modest thickness showing from low to very low hydraulic conductivity; Holocene “Detrital Deposits”48,49.
Fig. 1.
(a) Geographic framework of the study area with indication of the SIN “Taranto” borders as provided by the Italian Ministry of Ecological Transition (MiTE); (b) geo-lithological map; (c) hydro-stratigraphic model of the study site.
Upstream of the physical-geographical sector of the study site, the hydrography is karstic in type, with ephemeral water courses. Perennial courses develop on short lengths only as tide channels of the coastal karstic springs. The climate is Mediterranean: rainfall is modest and concentrated mainly in winter. In the study area, the climate is classified as Csa (Hot-summer Mediterranean climate)50 according to Köppen and Geiger, with a mean temperature of 17.4 °C and 450 to 575 mm of average yearly precipitation. Holocene and Upper Pleistocene deposits’ modest thicknesses constitute multi-level superficial aquifers whenever clays are at their base. They discharge through small surface (contact) springs inland. Where the superficial aquifers are close to the coast, the elevation of the clay layers is generally lower than sea level: they discharge into the sea and suffer seawater intrusion. The limestone aquifer in the area is in continuity in the entire southern sector of the Murge. Limestone is permeable due to fracturing and karstification. Groundwater circulates under pressure under the impermeable clay cover along the coasts, locally discharging through coastal and submarine springs.
Geology and hydrogeology of the study site
One hundred sixty-five stratigraphic records out of the 1100 available constituted the base for the modelling of the study site. The hydro-stratigraphic model develops between a minimum altitude of -0.8 m AMSL and a maximum altitude of 20 m AMSL (Fig. 1c). The model includes the general lithotypes of the larger scale description. The lithotypes incorporate more lithological terms according to their hydraulic properties. They are soil (and backfill ground), sand, silt, gravel, calcarenite, and clay. The term clay includes the less permeable lithologies, such as silt, silty clays, and clays. The succession of such lithotypes is irregular. Sometimes, one or more are absent; in others, a single lithotype may occur at diverse elevations in alternation with other lithotypes. The only lithotypes nearly always present are the soil, also comprising anthropic litho-structure deposits with iron and steel industry wastes (the first layer in the stratigraphic succession) and the clays (the basal layer). The first layer shows significant heterogeneity, both vertical and areal. In the substratum, layers of clays are heteropically interspersed with layers of sand, calcarenite and silt, thus constituting a complex hydro-stratigraphic succession.
Groundwater circulates in a modest aquifer comprising sandy silts and silty sands, and in some areas, also carbonate soils. An extensive basal impermeable level, mainly comprising silt and silty clays and intercepted between 1.0 and 7.5 m from the ground level, hydraulically limits the aquifer. The superficial aquifer, therefore, has a maximum thickness of around 7 m. The superficial aquifer shows medium-low permeability between 10−2 and 10−4 cm/s, and water table elevation varies between 2 and 18.5 m AMSL. Some boreholes demonstrate the persistence of the basal level up to the maximum depth reached (15 m), thus excluding possible interconnections of the superficial aquifer with the limestone aquifer at depth. A clay level (sub-Apennine clays) under the basal one is found mainly below mean sea level, likely allowing communication between the subsoil under the superficial aquifer and the sea.
Environmental setting
The Taranto area underwent intense industrialisation since the second half of the XIX century. The study area is on the west coast of the town, within a coastal SIN (Site of National Interest) perimeter. It extends for 275 hectares and is approximately 60 km wide and 35 km long (Fig. 1a). It hosts an oil refinery. At its borders, there has been, for a long time, one of the largest cement and concrete plants in southern Italy, and to the NW, it still operates the largest steel plant in Europe. Two municipal wastewater treatment plants and a fish farm are to the W of the area. Approximately 250 m from it, there are (currently inactive or active) oil deposits.
A collector of liquid effluents from upstream industries has been discharging on the coast since 1979. For many decades, the coast of the SIN has been subject to the reclamation of maritime state-owned areas for building industrial facilities.
The coastal morpho-structure has been completely disrupted, particularly in the Taranto port areas. Reclamation works have led to a significant redefinition of the coastline bends. Two successive fillings of inlets along the S coast of the study site have caused a modification of the main morpho-structural protrusion into the sea. Anthropic litho-structures hide, in this area, recent and current sandy deposits. Additionally, a recent sheet pile boundary has been built along the coast to the W of the study site to collect contaminated marine sediments. Another sheet pile boundary today separates from seawater an industrial landfill of blast furnace slags with other complex back-fillings developed along the western coast through land reclamation. The development of industrial and harbour settlements and infrastructures has also obliterated the distal part of most of the hydrographic networks around the study site. Supplementary Fig. S1a shows the study area’s detailed land use map dated 2011 (https://webapps.sit.puglia.it/freewebapps/UDS2011/index.html).
Several chemical characterisation surveys have documented severe contamination by hydrocarbons and TEs in various focal points of the industrial area, likely caused by industrial losses, material storage or uncontrolled inputs of polluted fluids.
Data collection
The monitoring surveys on soils and groundwater covered the period from the end of 2002 to the end of 2005 and involved monitoring wells properly designed for site characterization and assessing the effectiveness of a pump-and-treat system for remediation. The concurrence timing of soil and groundwater surveys ensures consistent and comparable data, enabling the study to accurately observe the interactions between soil and groundwater, especially considering the groundwater salinization51. This strategy improves the understanding of TE movement under different environmental conditions and increases the reliability of the multivariate statistical analysis by reducing the dataset’s dimensionality.
The location of the soil sampling points, and groundwater monitoring wells depended on the presence or absence of underground structures.
Two thousand eighty-nine (2089) soil samples were collected from 945 drillings (Fig. 2a) during ten (10) sampling surveys (December 2002, May 2003, June-December 2004, and January-February 2005). They refer to variable depths from 1 to 8 m below ground. The mesh for soil sampling was 50 × 50 m, according to the sampling strategy of EPA 600/R-92/128.
Fig. 2.
Study area with (a) location of soil sampling points and hydraulic barriers, (b) monitoring wells and activity area typologies, and (c) water level map related to December 2005.
The groundwater monitoring network includes 132 monitoring wells distributed in the study site (Fig. 2b and c). One hundred twenty-eight (128) concern the shallow aquifer (PT, PE, PZ, PO wells; Table 1) while four (4) reach the carbonate Mesozoic aquifer (PP wells). Seven hundred eighty (780) groundwater samples (178 in 2003, 299 in 2004 and 303 in 2005) come from nine (9) groundwater sampling surveys (May and October 2003; April, October, and December 2004; February, May, August, and December 2005).
Table 1.
Identity (ID) (supplementary Fig. S1b) and location of groundwater monitoring wells within the areas of Fig. 2b.
| Aquifer | Monitoring well ID | Location | Number of wells |
|---|---|---|---|
| Shallow | PT | Hydraulic barriers | 18 |
| Shallow | PE | Western external area | 20 |
| Shallow | PZ | Southern external area | 6 |
| Shallow | PO | Internal area | 84 |
| Deep | PP | Internal area | 4 |
| Total | 132 |
The monitoring net for water level measurements included at the end of 2005 one hundred twenty (120) wells selected among the 132 monitoring wells for groundwater monitoring. Manual groundwater level measurements were performed using a hand-held groundwater level meter during nine surveys using an increasing number of monitoring wells because of availability (depending on the progress of drillings), however variable in the time due to their conditions (sometimes dry or obstructed) of wells. The wells interested by surveys were 95 in October 2003; 53, 99, 93, 93 and 112 in January, April, June, September, and December 2004; 116, 115, and 115 in August, October, and December 2005. During the monitoring period, the water levels varied from maximum values of 18.5 to 0 m AMSL. Figure 2c shows the water level map for December 2005. Groundwater flows towards the coast to the W, SW, and S directions. However, the water level maps of other dates do not show significant changes in the water level pattern.
The collection of the monthly precipitation data from 2002 to 2005 (Taranto rain gauge station, Fig. 1a, Supplementary Table S1) aimed at defining the precipitation regime (labelled as wet, dry, or semi-dry, Fig. 3) during each groundwater or soil sampling survey. Such a label based on the trend characterizing the month before each sampling date of the cumulative precipitation, calculated over 15-day intervals (Fig. 3). The years 2000 to 2003 show a lower precipitation pattern compared to average annual precipitation of the study area (472 mm over the period 1921–2005).
Fig. 3.
Cumulative precipitation over 15-day intervals (blue line); dry, semi-dry, and wet time ranges mark groundwater and soil sampling survey dates.
The Italian Ministerial Decree 471 of 25 October 199952 regulates the soil quality. Soils granulometric features refer to the finest grain size (d < 2 mm), sandy grain size (soils with 2 mm < d < 2 cm and the gravel grain size fraction with d > 2 cm. The parameters considered for defining soil quality are CEC (cation exchange capacity), pH, and TOC (Total Organic Carbon). Chemical analyses concerned As, Be, Cd, Crtot, Hg, Ni, Pb, Cu, Se, V, Zn as well as the Light molecular hydrocarbon (C < 12), semi volatile hydrocarbons (12 < C < 25, and C > 25) also including the total of Polycyclic aromatic hydrocarbons (PAHs). All analyses were carried out by certified agencies according to highly standardized protocols (Supplementary Table S2).
Groundwater chemical analyses include ten TEs (As, Hg, Cd, Crtot, Cu, Ni, Pb, V, Se, Zn), pH, chlorides, electrical conductivity (EC), and TOC. Among the analyzed 52 organic compounds determined (PCB, PAH, Pesticides, fluorinated compounds) we selected MTBE (methyl tert-butyl ether) as representative of anthropogenic organic compounds. Analytical methods are in Supplementary Table S3.
Methods
The study employs two statistical technique groups. Univariate analysis considers the entire dataset comprising 782 data (including outliers), to demonstrate the influence of land use by depicting the spatial distribution of pollutants and pinpointing the hot spots near the industrial loading area. Multivariate analysis is then employed to reduce the dataset dimensionality to fewer factors. After discarding all outliers, a refined dataset of 688 samples with data closer to normality allowed to draw inferences about the relationship between variables and observations. Three different unsupervised multivariate techniques (PCA, CA and HCA) were then applied to the data. This approach enables recognising the most significant, often concealed, relationships between contaminants and the factors controlling their behaviour in groundwater. Univariate Analysis is performed with the Origin Program (version 16.05) and Statistica v. 13, while SIMCA 17 Program (17, MKS Umetrics AB, Sweden) is used for the Multivariate Statistical Analysis (MVSA).
To enhance the performance of MVSA, besides considering the quantitative variables, groundwater samples were also categorized according to qualitative variables (Table 2): year of monitoring, salt content (based on chloride concentration, herein referred to as salinity), water level at the sampling point, season, precipitation regime, and activity type. The grain-size and hydrocarbon content are two additional categories for soil samples.
Table 2.
Categorization of groundwater and soil samples according to qualitative variables.
| Classification variable (groundwater) | Class label | Class range | Explanation |
|---|---|---|---|
| Year of monitoring | 2003 | 1st Jan − 31 Dec. 2003 | |
| 2004 | 1st Jan − 31 Dec. 2004 | ||
| 2005 | 1st Jan − 31 Dec. 2005 | ||
| Salt content (salinity) | Fresh | 0 < Cl (mg/L) < 500 | Modified by Stuyfzand and Stuurman53 |
| Brackish | 501 < Cl (mg/L) < 5,000 | ||
| Saline | Cl (mg/L) > 5,000 | ||
| Water level | Low | 0–3.5 m AMSL | Location of samples in low water level area (see Fig. 2c) |
| Medium | 3.51–11 m AMSL | Location of samples in medium water level area (see Fig. 2c) | |
| High | 11.1–18.50 m AMSL | Location of samples in high water level area (see Fig. 2c) | |
| Season | A | 1st Sept. − 30 Nov. | Autumn |
| W | 1st Dec. − 28/29 Feb. | Winter | |
| Sp | 1st March − 31 May | Spring | |
| S | 1st June − 31 August | Summer | |
| Precipitation regime | Dry | see Fig. 3 | Based on a null increase of the 15-day cumulative precipitation one month before the sampling date |
| Semi-Dry (SD) | see Fig. 3 | Based on a modest increase of the 15-day cumulative precipitation one month before the sampling date | |
| Wet | see Fig. 3 | Based on a significant increase of the 15-day cumulative precipitation one month before the sampling date | |
| Activity type | Area 1 | See Fig. 2b | External Refinery area - The area between the refinery limits and the limit of the study area |
| Area 2 | See Fig. 2b | Storage area - Includes former oil deposits and storage tanks | |
| Area 3 | See Fig. 2b | Industrial plant area - Includes LPG, petrol, paraffin, diesel, fuel, and bitumen production plants | |
| Area 4 | See Fig. 2b | External area - To the N and NW of refinery limits | |
| Area 5 | See Fig. 2b | Loading area - Includes product loading shelters by tanker trucks | |
| Classification variable (soils) | Class label | Class Range | Explanation |
| Grain size | Finest | diameter < 2 mm | |
| Medium | 2 mm < diameter < 2 cm | ||
| Coarse | diameter > 2 cm | ||
| Hydrocarbons content | C < 12 | Low molecular weight hydrocarbons | |
| 12 < C < 25 | Semi-volatile hydrocarbons | ||
| C > 25 | High molecular weight hydrocarbons |
Before the unsupervised analysis, all data were mean-centred and log-transformed to avoid the influence of outliers on the final classification and correlations assessment. We used half of the detection limit where the concentrations resulted below it. Before the statistical analysis, all variables were scaled to unit-variance (UV). Due to the high number of outliners in our dataset, we exploited the non-parametric techniques that are more robust when there are extremes in the distribution of the variables to be correlated54–56.
The quality of statistical models was evaluated based on R2 (goodness-of-fit), which expresses the fraction of the Sum of Squares (SS) explained by the model, and Q2 (goodness-of-prediction in cross-validation), which represents the fraction of the total variation of X or Y that can be predicted by a component, as estimated by cross-validation.
Results
Univariate analysis of groundwater and soil
Groundwater quality
The EC of groundwater samples varies from 728 to 48,600 mS/cm. Some values notably overcoming this range have been discarded because of likely affected by errors.
Considering that seawater shows EC values around 56,000–58,000 mS/cm in the Mediterranean area, the EC range may derive from GWS by seawater intrusion. However, the hydrogeological condition of the superficial aquifer does not allow communication with the sea. Unluckily, the groundwater chemical analyses do not include parameters (such as the major and minor ions) helpful in supporting other explanations. The only available parameter is chloride, whose concentration between 29 and 23,208 mg/L coherently increases as the EC increases. The geological setting may justify seawater as a source only considering that exploitation by deep wells can make salinization emerge because of the interconnection between the shallow aquifer and the subsoil under the clay level which is in communication with the sea. In the concerned site, GWS may anyhow come from sources other than seawater, such as cooling or desalination fluids or treated contaminated waters released on the ground or in the barriers, which all derive from coastal groundwater and may produce the same effects of mixing with seawater.
The value of pH varies from 6.51 to 8.84: it is normal or slightly alkaline in almost all samples but tends to be acidic in some areas. The Total Organic Carbon (TOC) ranges from 1.3 to 284 mg/L. For human consumption TOC is generally set as less than 2 mg/L; stagnant waters show values from 5 to 10 mg/L, while a TOC > 50 indicates a pollution level that makes drinking water unsafe. Since TOC concentrations in the study site exceed almost times 10 mg/L the maximum permissible TOC concentration for human consumption (according to the Communication of the Ministry of Health57) this means that oxidizable organic and inorganic pollutants have contaminated groundwater.
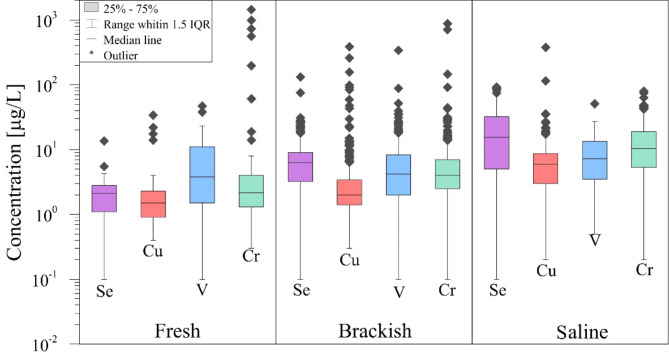
Figure 4 shows the box and whisker plots of trace element concentrations in groundwater. Almost all TEs show outlying values. Table 3 shows the concentration range (1.5 IQR), the average and the details the areas (Fig. 2b) where the wells (Supplementary Fig. S1b) with the highest outliers of the TE’s concentrations are located. The legal limits set by the Italian Legislative Decree No. 152/0658 for groundwater in industrial areas allow evaluating the exceedance of outlier values.
Fig. 4.
Box and whisker plot of TEs in groundwater. The concentrations are reported in logarithmic scale due to the high values of outliers.
Table 3.
Concentration range and average concentrations of trace elements in groundwater samples, outlier values and their position.
| Trace elements (TEs) | Legal limit (µg/L)59 | Concentration range 1.5 IQR | Average conc. (µg/L) | Highest outlying values (µg/L) | Wells showing the highest outlying values (ID details in Supplementary Fig. S1b) | Location of wells with highest outlying values with reference to Fig. 2b |
|---|---|---|---|---|---|---|
|
Arsenic (As) |
10 | 4.2–19.3 | 51.76 | 1,963 2,405 2,444 |
P052, P561 P226 |
Industrial plant and storage areas |
|
Copper (Cu) |
1000 | 1.60–6.80 | 7.74 |
132 379 400 |
PZ006 P054 P129 |
External, Industrial plant and storage areas |
|
Iron (Fe) |
200 | 0.5–156 | 713 | 33,700 | P565 | Storage area |
|
Lead (Pb) |
10 | 0.00-0.80 | 1.73 |
83.7, 136 110 |
P247, 1-PT1 P227 |
Loading and storage areas |
| Manganese (Mn) | 50 | 32.7–578 | 477 | 8,927 | P227 | Storage area |
|
Mercury (Hg) |
1 | 0.00-0.25 | 0.175 | 1.33, 1.4, 2.09 | 4-PT1, P052 | Industrial plant areas |
|
Nichel (Ni) |
20 | 1.9- 9.0 | 15.07 |
1,258 1,780 |
PZ002 P177 |
External and storage areas |
|
Selenium (Se) |
10 | 2.1–15.9 | 14.80 |
103, 132 105, 108 |
PE018, PZ002 3-PT1, PE006 |
External and loading areas |
| Total Chromium (Cr tot) | 50 | 2.8-12.98 | 20.59 |
875 1,446 1446 |
PZ002 P250 |
External and external refinery areas |
|
Vanadium (V) |
2.9–11.20 | 8.64 | 341 | PZ002 | External area | |
|
Zinc (Zn) |
3000 | 3.8–23.7 | 25.68 |
2,067 2,211 |
1-PT1 PP4 |
Loading and storage areas |
Soil/Sediment quality
The values of pH in soil samples ranges from neutral (7.24) to alkaline, with a significantly highest value around 11.5 in the Industrial plant, near the Storage Area, and in the External areas. The Cation Exchange Capacity (CEC) ranges from 5.5 to 56.1 meq/100 g. The organic matter content, expressed as TOC, ranges from 0.01 to 3.82% (Fig. 5a). The ternary diagram (Fig. 5b) shows that the soils and sediments collected in the study site are primarily of the finest texture (d < 2 mm).
Fig. 5.
(a) Box and whisker plots of water content (Wc), CEC and TOC in soil samples; (b) grain size of soil samples.
Figure 6 shows the box plots of concentrations of the TEs in the soil samples. Be and Hg are available only for samples sites collected in 2002 and 2003. All elements show outliers within the 1.5 IQR (Table 4). However, when comparing the concentrations with the legal concentration limits58, Table 4), only As, Cu and Pb show exceeding concentrations. The concentrations of Be, Hg, Cd, Ni, Se, V and Zn are below the legal limits. The areas of highest concern with the highest number of outliers were those near the storage area and near the refinery.
Fig. 6.
Box and whisker plot of trace element’s concentrations in the soil samples. The concentrations are reported in logarithmic scale due to the high values of outliers.
Table 4.
Average concentrations of TEs in soil samples, outlier values and their position.
| Trace elements (TEs) | Legal limit for Industrial Site Areas (mg/Kg d.s*) 58 | Concentration range 1.5 IQR (mg/Kg) | Average conc. (mg/Kg d.s*) | Highest outlying values (mg/Kg d.s.*) |
|---|---|---|---|---|
|
Arsenic (As) |
50 | 4.12–10.77 | 8.35 | 138 |
|
Cadmium (Cd) |
15 | 0.06–0.16 | 0.19 | – |
|
Copper (Cu) |
600 | 2.80–9.80 | 9.29 |
467 871 904 |
|
Lead (Pb) |
1000 | 2.50–5.80 | 8.50 | 4493 |
|
Mercury (Hg) |
5 | 0.002–0.015 | 0.014 | – |
|
Nichel (Ni) |
500 | 5.30–37.10 | 19.65 | – |
|
Selenium (Se) |
15 | 0.40–3.55 | 2.46 | – |
|
Vanadium (V) |
250 | 10.80–28.10 | 19.84 | – |
|
Zinc (Zn) |
1500 | 8.92–41.10 | 27.85 | – |
| C < 12 | 250 | 59.41 | – | |
| C12 - C25 | – | 0.20–3.50 | 51.15 | – |
| C > 25 | – | 46.48 | – | |
| C > 12 | 750 | 0-2.80 | 69.64 | – |
Supplementary Fig. S2 shows the trace element’s box plots sorted per year of monitoring. Among all TEs, As and Cu show the less pronounced differences in the time (Supplementary Fig. S3a and S3c) and space (Supplementary Fig. S3b and S3d).
The box-whisker plots of soil and groundwater show that the areas with the highest concentrations of TEs are the storage area and the industrial plant areas. These areas correspond to where some organic compounds of anthropogenic origin, like C < 12, C > 25, MTBE, xylene, benzene, and toluene, show the highest concentrations, which also suggests that organic pollution is of hot-spot origin (Supplementary Fig. S4 and S5). The samples in the other areas show organic pollutant concentrations below the legal limits.
Because hot-spot pollution may disguise the effect of soils and GWS on heavy metal dynamics, before the multivariate analyses we pre-treated the dataset removing all extreme outliers.
Multivariate analysis of groundwater and soil
Groundwater quality investigation
The multivariate analysis aimed to reduce the original dataset’s huge dimensionality without losing essential information by constructing new variables, called principal components, PC (or latent components), that account for the most significant variance of the original system, projected into a new hyperspace (i.e., statistical model). Each PC explains a portion of the existing variance among the data; for a given PC, each observation has a score indicating its contribution to the PC, while each original variable has a loading indicating its weight on that PC.
The first analysis was the unsupervised Principal Component Analysis (PCA) to get a preliminary overview of groundwater data and to find hidden correlations between different chemical variables and observations. The dataset for PCA consists of 688 observations and 16 quantitative variables related to the concentration of TEs (As, Crtot, Cu, Hg, Ni, Pb, Se, V, Zn), Fe and Mn, pH, EC, Cl– concentration, TOC, and MTBE. The statistical analysis does not include Cd (because of the high number of missing data, more than 72%) and the Cr(VI) concentrations (mainly under the detection limit). The PCA also considers the six qualitative variables of Table 2. We considered a PCA model with four principal components (PCs) accounting for a cumulative variance of 60% (Fig. 7). Despite excluding spike concentrations and variables with negligible influence on the PCs, the cumulative variance explained by the first four PCs remained almost unchanged, likely because of too many concurrent and multi-source effects, each marginally influencing the pattern of contamination. The PCA analysis was combined with HCA, performed on the four selected PCs and the overall data.
Fig. 7.
(a and b) Score plot PC1/PC2 and PC1/PC3 projections sorted according to the salinity range; (c and d) Loading (PC1/PC2 and PC1/PC3) projections; e) Biplot (score and loading plots) PC1/PC3, sorted according to salinity range; (f) Dendrogram of HCA analysis based on PCs Model.
The PC1/PC2 score plot projections (Fig. 7a and b), sorted according to salinity (Table 2), show a distinct sample grouping. On the hyperspace, a highly negative value of PC1 characterises the fresh samples, while on the opposite hyperspace, a highly positive value of PC1 marks the saline samples. Values of PC1 less negative than fresh samples characterise the brackish sample group. The analysis of the loading plots (variables) of the PC1/PC2 projection (Fig. 7c and d) identifies three clusters. The first one, associated with the saline samples, shows the most positive values of PC1 (Fig. 7c): this cluster contains five TEs (Se, Cu, Crtot, V, and Ni), whose concentration is significantly controlled by EC and chloride concentration, which show a significant correlation. A second cluster, associated with both saline and brackish samples, with less positive PC1 loadings than saline samples and positive PC2 loadings (Fig. 7d), includes Zn and Pb. This cluster seems less influenced by chloride concentration and EC: it falls in the same hyperspace of TOC, distinguished by positive PC2 values. Negative PC1 and extremely positive PC2 values characterise a third cluster, which comprises As and Hg, associated with Fe and Mn. This cluster, representing the main pattern of the brackish groundwater, shows significant correlations with TOC and MTBE: it represents the main pattern of the brackish groundwater. The pH, characterised by negative values of PC1 and PC2, is associated only with the fresh samples, suggesting that it is the main factor controlling their pattern. The pH controls, albeit to a lesser extent, also the concentrations Fe and Mn that fall in the same hyperspace characterised by negative PC1 value.
The biplot projection of PC1/PC3 (Fig. 7e) and the 3D projections highlight a better association between the samples sorted according to salinity (Supplementary Fig. S6a) and the pattern of variables (Supplementary Fig. S6b).
The HCA dendrogram, with linkage distance less than 0.5, confirms the clustering of PCA (Fig. 7f): EC and Cl− control the first cluster, TOC, and pH the second cluster, and Fe-Mn and MTBE the third cluster.
Samples sorted according to low and medium water levels correspond to samples sorted according to saline and brackish salinity in the PC1/PC2 and PC1/PC3 biplots (Supplementary Fig. S7).
Discussion
Findings from PCA and HCA
The PCA and HCA analyses show a different clustering of TEs, which can be explained by the relative dominance of the main processes controlling their mobilization under GWS, namely the complexation with chlorides, the competition with calcium and other cations, such as Mg2+ and Na+, for sorption sites, the complexation with oxyanions (PO43−, NO3− SO42−, but also BO33− or B(OH)4−, HCO3−and CO32−), the reductive dissolution of metal–bearing Fe/Mn/Al-oxy-hydroxide, and the microbial processes that regulate trace element mobilization different redox conditions. The general high content of Fe (> 100 µg/L) (Table 3) and the redox state of groundwater samples (checked according to the classification of McMahon and Chapelle59) indicate a marked anoxic condition of groundwater in the study site.
Considering that the first cluster shows high correlation with EC and chlorides, pH ranges from 6.51 in “fresh” samples to 8.84 in “saline” samples, and the anoxic conditions, we could assume that Cu, Cr, Se, and V of the first cluster, because of their high affinity for halide complexes, form soluble chloride complexes. The box plots of Fig. 8 prove that the mean and median concentrations of Se, Cu, Crtot, and V increase with the increase of groundwater salinity. Literature confirms the high mobility of these TEs in groundwater with increasing salinity60–77.
Fig. 8.
Box plots of Se, Cu, V, and Crtot according to the different salinity range.
Jia et al.60 described the main factors influencing the mobility of Cu in salinized aquifers and stated that the primary process controlling its mobility is its tendency to form soluble complexes with Cl− (as well as other salinity-promoting soluble species) such as [CuOH]+, [CuCl]+, [Cu2(OH)2]2+. A second process governing the Cu mobility is the ion competition. The findings of the same authors also allow excluding the role of the dissolution-precipitation process in determining Cu concentrations in groundwater in the study site because of the undersaturated conditions (i.e., at low Cu concentrations). Cary et al.61 analyzed the factors influencing the mobilization of Se in groundwater. Selenium can be found in groundwater in different soluble (containing Se+4 and Se+6) and insoluble species (containing Se+2 and Se0). The authors explained that the mobility of the main soluble species SeO32− and SeO42− can increase with increasing pH62. Besides seawater intrusion, the increase in Se concentration in groundwater is also attributed to the use of fertilizer/irrigation as well as a side-contaminant of oil refinery63, thus suggesting a coupling of natural and anthropogenic factors. Because of their influence on the adsorption/desorption of Se species from soils, Chen et al.64 assert that some ions (like Fe, Ca2+) commonly present in groundwater promote favorable conditions for Se enrichment in it. Balistrieri and Chao65, concerning the role of phosphate in the stability of selenite oxyanions, estimate that 50% of selenite oxyanions can be replaced by phosphate ions (PO43−) in groundwater when seawater intrusion occurs. The latter anions inhibit the adsorption of selenite even when it is adsorbed on the rock (soil) surface66. Wright et al.67 analyzed the Vanadium mobility in groundwater interested by salinization. Because of its similar structure of Fe3+ it may form complexes with Cl− and SO42−, which could enhance the solubility of Va and its correlation with chlorine content in groundwater.
Eliopoulos et al.68 documented salinity as the driving force for chromium in groundwater. Trivalent and hexavalent chromium are the most abundant species in groundwater. Cr(III) has low solubility while Cr(VI) has high solubility and can easily move through and mix with groundwater. The reducing environment in groundwater and the formation of soluble chloride complexes can explain the association of Cr with Cl. In addition, according to Papazotos et al.69, the mobility of Crtot species may also be related to organic matter, which forms stable Cr(III) complexes with small organic molecules, increasing their mobility and maintaining the solubility of Cr(III) even near neutral pH.
Ni, in the first cluster, usually does not show significant affinity for halide complexes. As for the association of Ni with Cl−, Tian et al.70 suggest that in high Cl− concentration regimes Ni form tetrahedral complexes such as [NiCl3(H2O)]−. Moreover, stable organic ligands formed with increasing salinity, likely because of the high amount of organic matter (which is a source of organic ligands that can act as a sink for the metal) control the Ni concentrations71. The high concentrations (in some cases over 248 mg/L) of TOC in the groundwater of the study site, are consistent with such hypothesis.
The behaviour of Pb and Zn grouped in the second cluster, is different when compared to that of the TEs comprised in the first cluster. We assume that the coupling of different processes explains such difference and that chloride complexes do not play a predominant role. The main process regulating the mobility of Pb and Zn is the competition with Ca2+30. The most important process for Zn mobility is the competition for sorption sites, followed by Zn-sulphate association and chloride complexation, while the next process in order of importance is the competition with Mg2+.
Hg was expected to be within the first cluster due to its high ability to form soluble chloride complexes. An increase of chloride content supports the formation of stable Hg complexes such as HgCl3−, HgCl2−, HgCl42− and HgBrCl2−, leading to increased dissolution of mercury solid phases32. Though, in our case, Hg seems less influenced by the chloride content, likely because of its lower solubility and reactivity with chloride ions compared to the TEs of the first cluster. This effect highly depends on the pH, the solution redox state, and on the organic matter content, which can form stable complexes with mercury and reduce its availability to react with chloride ions. On the other side, the presence of other ions such as sulphates and carbonates can also affect the solubility and mobility of mercury in groundwater, further reducing the influence of chloride content28,30. A possible mechanism for attenuating Hg mobility could also relate to the sorption of Hg(II) onto iron oxides at pH > 5.572. In view of the positive association of Hg(II) with iron (Fe) in iron hydroxide-rich soils, as is our case, sorption of Hg onto Fe hydroxides may likely be another mechanism that differentiates the mobility of Hg in comparison to the TEs of the first cluster73.
Our findings shed light on a significant gap in the literature, as the influence of salinized groundwater on As cycling has been largely overlooked. Moreover, the relative contribution of redox and salinization conditions on As enrichment has remained unclear. We focus on As(III), the main species containing As in reducing groundwater, which is weakly adsorbed by iron oxide, particularly under neutral pH and slightly alkaline conditions. We propose that the association with TOC is the cause of the desorption of As. The reductive dissolution of As–bearing iron oxides emerges as the critical process for As release in groundwater, surpassing the impact of salinization74,75. In addition, Liu et al.76 found that Cl− and SO42−, the main anions of saline water, have little influence on the leaching of As. Our analysis’s results align with these findings. Upon closer examination of the data, we discovered a significant correlation between As and organic matter. This correlation could potentially explain the higher concentration of As in fresh and brackish samples compared to salinized ones, as reported by Giambastiani et al.77.
Findings from CA (correlation analysis)
We investigated the pairwise correlation between TEs and salinity indicators (EC and Cl–). Supplementary Table S4 shows the non-parametric Spearman correlation coefficients.
The Correlation Analysis (CA) confirmed a strong correlation between EC and Cl– (R2 = 0.95) and a low or no significant correlation coefficients (R2 < 0.1) of Cl− with elements of the second cluster (Zn, Pb, Hg) as well as with those of the third cluster (As, Mn, Fe). In addition, a positive correlation of As with Mn and Fe was apparent, supporting the hypothesis of the importance of the reductive dissolution of iron oxides and Mn hydroxides as a principal driving force for As release in coastal groundwater36,74,75. The CA also reveals positive correlations between both EC and Cl– and TEs of the first cluster pair and between the same TEs such as Crtot vs. Cu (R2 = 0.64), or Crtot vs. V (R2 = 0.53), Cu vs. Ni (R2 = 0.57) and Se vs. Cu (R2 = 0.55) supporting the hypothesis of some common fate for these metals in groundwaters26.
However, the correlation values between Cl – and TEs of the first cluster (R2 = 0.56, R2 = 0.53, R2 = 0.50, R2 = 0.29, and R2 = 0.27 for Crtot, Cu, Se, V, and Ni, respectively) are unexpectedly low. A closer look at the pairwise correlations in the scatter plots reveals new factors controlling the distribution of TEs in saline groundwater.
To corroborate the PCA and HCA results for groundwater, Supplementary Fig. S8 shows the spatial distribution map (performed by Ordinary Kriging) of chloride concentration compared to Se and Cu (TEs representatives of the first cluster) and As and Hg (TEs of the second cluster) maps. These maps are based on monitoring data from August 2005, which corresponds to a dry period (refer to Fig. 3), and were selected because of the highest number of monitored wells.
A visual qualitative comparison of the maps reveals that highs and lows of chloride concentrations correspond to highs and lows of Se and Cu at the same locations, providing support for the good Cl-Se and Cl-Cu correlations indicated by Supplementary Table S4 and the HCA results, which place Se and Cu in the same cluster as chlorides. Conversely, the spatial distributions of As and Hg differ from that of chloride, thus confirming the weak correlations between Cl and As and between Cl and Hg, and the HCA results that place As and Hg in a different group from the previous one.
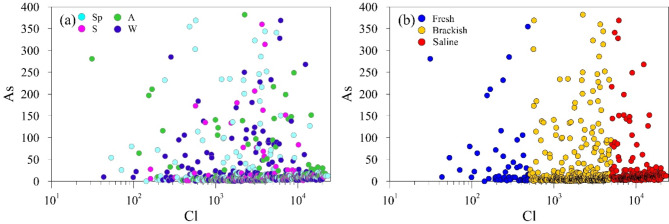
Figure 9 shows the correlation between Cl and Se, Cu, and Cr. The binary plot between Se and Cl (Fig. 9a) shows some sub-clusters of samples depending on the season. In autumn (A samples), the increase in Se concentration with Cl increase is much more pronounced than in spring (Sp samples), summer (S) or winter (W). Furthermore, the winter samples distinguish in two groups: with increase of salinity, a group shows an increase of Se concentration and another one shows unchanged Se concentrations. Such a seasonal splitting justifies the positive, albeit not highly significant, correlation of the TEs of the first cluster with Cl (Supplementary Table S4). Seasonality has a significant effect on the redox processes of groundwater because seasonal variations influence the redox species and redox potentials due to factors such as groundwater recharge. The response of redox species and the indigenous microbial community to seasonal groundwater level fluctuations also play a crucial role in altering redox reactions78. The splitting of seasonal sub-clusters suggests that other factors besides seasonality influence the Se mobility. Figure 9b shows, indeed, the increase of the Se concentration with increasing salinity.
Fig. 9.
Correlation plots between Se, Cu, and Crtot and Cl concentrations with samples sorted according to seasons (a, c, and e, respectively) and salinity (b, d, and f, respectively).
Figure 9c and e show the less significant correlation over the different seasons between Cl and Cu and Crtot, respectively. Our assumption is that the factors that control the mobility of the two elements are different compared with Se and that these factors predominate over the effect of seasonality. Figure 9d and f show the increase of Cu and Crtot from the fresh to the saline range.
Contrary to the trend of the first cluster of TEs, Arsenic shows high concentrations in fresh samples, which moderately increase in brackish samples but decrease in saline waters (Fig. 10). The dynamics of salinization, because of its undoubted influence on the physicochemical processes controlling the mobilization of TEs, is the likely cause of the high variability of the As concentration within the fresh and brackish sample clusters. Considering that Arsenic is more soluble in freshwater than in saline water, after passing a certain salinity threshold, it is supposed that Arsenic tends to precipitate, reducing its concentration in groundwater, as demonstrated by Greene et al.79.
Fig. 10.
Correlation between As and Cl with samples sorted according to (a) season and (b) salinity.
The correlation plots with samples sorted according to the different precipitation regimes did not provide any evident clustering of samples (Supplementary Fig. S9), suggesting further investigation to understand the role of rainfall coupled with changes in temperature, soil redox, or aquifer recharge. Unluckily, monitoring surveys did not include such parameters.
Findings from Soil quality investigation
The MVSA also concerned the soil dataset. We used unit variance scaling and logarithmic transformation to obtain normal distributions of metal concentrations. No data on EC are available for soils. Besides the concentration of the TEs investigated in groundwater, the dataset for multivariate analysis of soil includes the CEC, pH, and moisture content (Wc). We considered the same qualitative variables used for groundwater with two additions: samples are also sorted according to granulometric features and, to represent organic contamination (Table 2), we considered hydrocarbons content classes.
Hg and Be were not measured in all monitoring years and therefore were considered inadequate for MVSA. In addition, Cr(VI) was excluded by the MVSA because always below the detection limits.
Se shows concentrations below the detection limit in 90% of the soil samples. Two views may explain this case: (i) GWS increased the erosion of rocks containing selenites and selenides, leaching Se in groundwater and depleting Se soil content below the detection limits; (ii) Se in groundwater comes from hot spot pollution (as metal smelting, mining, coal-fired power plants and agricultural drainage80) and not from soil.
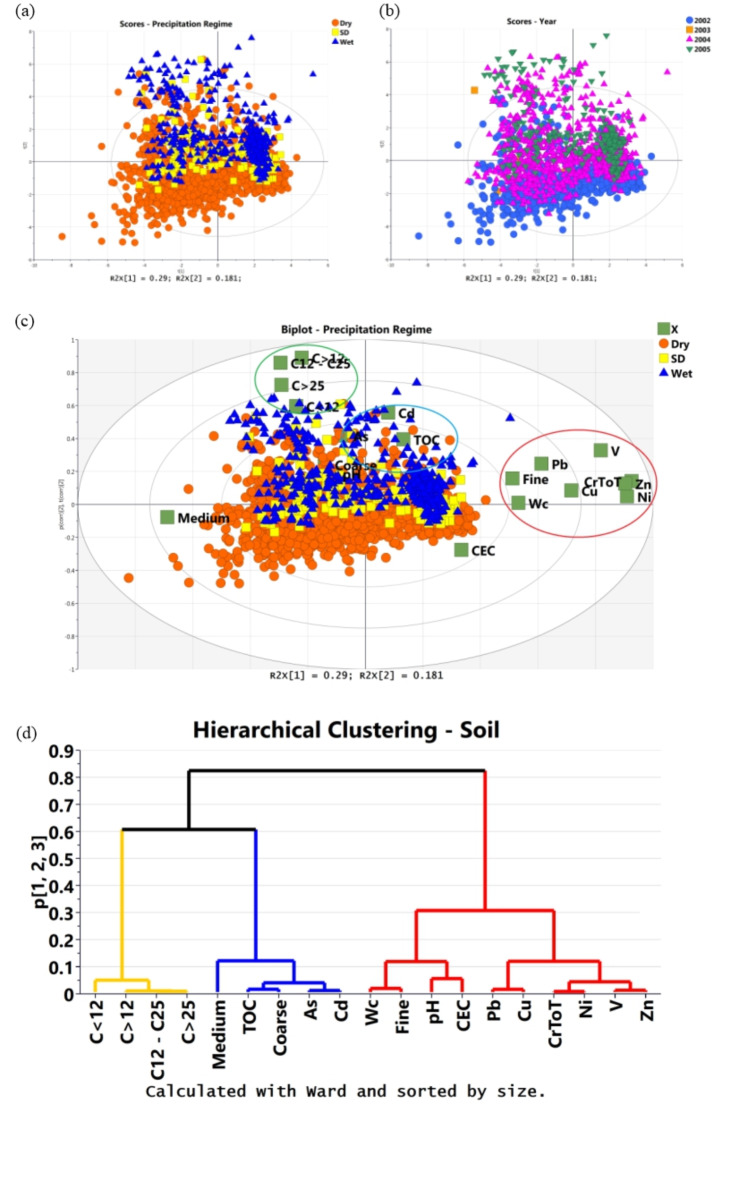
Excluding Se, Hg, Cr(VI) and Be, we performed PCA on the soil dataset obtaining a PCA model with four PCs accounting for 64% of the total variance. Soil samples sorted according to season or water level did not show any evident clustering (Supplementary Fig. S10), while a slight clustering appears when they are sorted according to precipitation regime and different years (Fig. 11a, b).
Fig. 11.
Score Plot PC1/PC2 projections sorted according to (a) the year and (b) the precipitation regime; (c) biplot [score (t) and loading (p)] for PC1/PC2 projection of samples according to precipitation regime; (d) dendrograms according to Ward method calculated on the PCA model.
The biplot (samples and variables) graphs with samples sorted according to the precipitation regime (Fig. 11c) show the clustering of “Wet” and “Semi Dry (SD)” samples alongside the positive PC2 value compared with the “Dry” ones displayed on the negative PC2 space. The “Wet” and “SD” samples associate with two variable-clusters: (i) organic contaminants (C < 12, C > 25, C > 12) associated with As, Cd and pH and with groups including Crtot, Ni, V, Cu, and (ii) Pb associated with Finest sediment and moisture content (Wc).
The Hierarchical Cluster dendrogram (Fig. 11d) distinguishes, indeed, three patterns/clusters of variables, very similar to the variable clusters detected in groundwater samples: a first cluster (characterised by significantly positive PC1 values) includes Crtot, Pb, Cu, Ni and Zn, which appear to be controlled by the finest grain size (d < 2 mm) and moisture content (Wc); a second cluster (characterised by positive PC2 values and less positive PC1 values) which includes As and Cd and appears to be controlled by pH and, to a less extent, by TOC; a third cluster (characterised by extreme positive PC2 values and negative PC1 values) includes all organic contaminants (hydrocarbons).
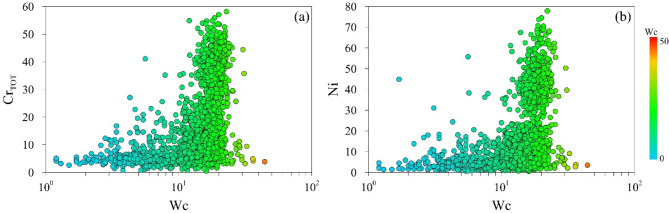
The association of the elements of the first cluster with moisture content Wc, CEC and finest grain size support the hypothesis of the substantial influence of the sediment structure, its mineralogical composition, and the physical-chemical condition on the mobility of these TEs and their migration pathway from soil-water and groundwater. Changes in Wc could influence the CEC of the soils and consequently impact TEs releasing phenomena in the aquatic environment81,82. Fang et al.83 attribute the decreases in CEC to extreme precipitation and consequently soil carbon loss, soil desertification, and soil acidification. Considering that CEC is referred to the clay minerals and organic matter surfaces on which TEs cations are retained, its changes due to the precipitation regime might influence their interaction, impacting the ability of TEs to exchange cations or form complexes with organic molecules when their hydration shells are displaced. The Correlation Analysis supports such findings. The Wc correlates positively with the TEs of the first cluster. Figure 12a shows as an example Crtot vs. Wc (R2 = 0.57), while. Figure 12b shows Ni vs. Wc (R2 = 0.56).
Fig. 12.
Binary plot of (a) Crtot vs. moisture content, and (b) Ni vs. moisture content. Data are sorted according to Wc.
pH, CEC, and TOC contents change during years (Fig. 13a). The samples collected in 2002 and 2003 show pH values lower than those collected in 2004 and 2005. CEC values display a higher variability in 2002 and 2003 than in 2004 and 2005 (Fig. 13b) where a depleting of CEC is registered. Differences in grain size fractions are also noticed (Fig. 13c): samples lose the finest content from 2002 to 2005. These cases justify the clustering of PCA sorted according to years and introduce potential interrelated factors impacting the TEs pathway from soil to water. The positive pairwise correlation between TEs of the first group (Crtot vs. Cu, Ni vs. Zn, Zn vs. Cr) (Supplementary Table S5), and their clustering like that of groundwater samples lead to suppose a similar behaviour of the TEs of the first cluster and potential common sources. The soil analysis also confirms the specific behaviour of arsenic (and cadmium, an element not analysed in groundwater due to its low concentrations) compared to the TEs of the first cluster. HCA states that As is controlled by pH, which, together with CEC, influence the redox state. Consequently, they influence the As leaching from soil to water through reductive dissolution of Fe/Mn/Al oxides, which we consider the main driving force for As release in coastal groundwater.
Fig. 13.
The score of (a) pH, (b) CEC, and (c) finest fractions during years.
Limitations of the study and recommendations for future research
Although this study has produced significant results, it also has some limitations. Firstly, the dataset used in the study was not obtained from a research-oriented setting. Instead, it was gathered as part of activities carried out to comply with legal requirements for characterizing a contaminated site and monitoring remediation efforts. Despite the fact that the study looked into a large number of parameters and measurement points, and the monitoring period and number of samples greatly exceeded legal requirements, additional data would have been beneficial to enhance the interpretation of the findings.
Analyzing major and minor constituents in groundwater, such as the cations Ca2+, Mg2+ and Na+, the oxyanions PO43−, NO3– SO42–, BO33−, HCO3– and CO32−, which are often not covered in legal guidelines for characterizing contaminated sites, could lead to a better understanding of groundwater salinization and its impact on contaminants. Similarly, having a consistent set of parameters for both groundwater and soils could offer a more comprehensive perspective and allow an increase in the predictive power of the statistical models obtained but also in supervised techniques such as PLS and OPLS (not shown in this work). The lack of specific soil measurements has limited our understanding of the movement of trace elements in soils under different salinization conditions. In terms of hydrological and hydrogeological data, it would be beneficial to accompany groundwater levels with temperature measurements, which can easily be carried out using specific in-situ sensors available today.
In addition, although unsupervised multivariate statistical analysis provided a reliable way to categorize TEs (resulting in high goodness-of-fit R2), it may oversimplify interactions in a complex environment where multiple concurrent processes and sources influence outcomes.
It is important to note that the same reported limitations can be viewed as one of the main advantages of the proposed work, which is an ex-ante analysis that paves the way for the definition of a multi-purpose sampling campaign in highly polluted coastal areas. It also directs the selection of a more appropriate set of indicators that accurately represent the evolutionary laws of such fragile, polluted coastal ecosystems.
Conclusions
This work stands out for its interdisciplinary approach to investigating TE mobility in a contaminated coastal site. It particularly emphasises the variable conditions of groundwater salinisation. Unlike conventional research, it addresses ongoing environmental changes influenced by climate and human activity.
The study’s primary findings concern categorisation of trace elements in coastal contaminated sites according to their behaviour under groundwater salinization. Such findings allow (i) to postulate the impact of salinization processes on the transport of TEs from inland towards the sea and (ii) to address the implications for site characterisation procedures and orientation of remediation strategies for contaminated coastal industrial areas. The resulting categorisation is crucial as it links the physical and chemical properties of soils with groundwater dynamics, providing insights into how salinization impacts the transport of TEs from inland areas towards the sea. The advanced multivariate statistical analysis applied to 688 groundwater samples and 2082 soil samples, suggests the influence of concurrent processes and multi-source effects consistent with a complex real-world environment. It effectively categorised the TEs into three clusters displaying a different reactivity to changes in GWS, represented by the changes of EC and chloride variables. The first cluster includes Se, Cu, Crtot, V, and Ni, the second Zn and Pb, and the third As and Hg associated with Fe and Mn. The increase of mobility of four metals of the first cluster (Se, Cu, Crtot, and V) parallel to the enhancement of GWS depends on their high affinity for forming soluble halide complexes. Diversely, Ni-aquo tetrahedral complexes, which predominate at the highest Cl− concentrations, control the Ni mobility. The TEs of the second cluster are less reactive to GWS. Ion (cation or anion) competition and TOC content prevail over chloride complexation in controlling their mobility. The mobility of the TEs of the third cluster correlates with MTBE and Fe and Mn although TOC content impact also their behaviour. The reductive dissolution of Fe/Mn/Al-oxy-hydroxides and the metal-organic complexes dominate the As release in groundwater.
The CA confirmed a moderate correlation between GWS and the mobility of the first cluster TEs and a low correlation with the TEs of the second and third clusters, respectively. The deep analysis of correlation allows the identification of correlated sub-clusters based on the season, which synthesizes the complex variation of variables like precipitation, water level, and salinity, which govern the groundwater redox state.
On the other hand, the PCA model for soils, based on a lower number of trace elements than groundwater, did not show significant clustering with season or water level while distinguishing the role of precipitation regime and year. Finest grain size (d < 2 mm), CEC and moisture content (Wc) control the metals of the first cluster (Crtot, Pb, Cu, Ni, V and Zn), while pH and organic matter content and coarse grain size regulate the mobility of the second cluster’s metals (As and Cd). A third ones includes the organic contaminants, which confirms the identification of hot-spot areas of contamination.
The correspondence of clusters of variables for groundwater and soils demonstrates the primary influence of the sediment structure, mineralogical composition, and physical and chemical conditions of soils on the TE distribution and their transfer to groundwater.
The previous findings indicate that the coastal dynamics at the land-sea interface induce continuous chemical disequilibrium between sediments and ground waters, with significant variations of some trace element concentrations in both reservoirs. This suggests that coastal dynamics can trigger metal release from soil to surface- and ground-waters: the alternation of freshwater flushing and salinization in the aquifers can cause the alternation of attenuation and or mobilization processes. When mobilized by brackish or saline waters, TEs transport to the sea should occur as such salinized waters retreat under flushing pressure.
Considering such a transport pathway, rising sea level involving continuous seawater encroachment may further stimulate sediments, making metals more mobile. Sea level rise could turn zones of aquifers previously occupied by freshwater from sinks into sources of metals, thereby transforming coastal areas into potential sites for environmental re-toxification. Compared to natural coastal sites, those that have been the location of uncontrolled release of industrial waste over the last 70–80 years may show a higher potential for re-toxification by heavy metals. Removing the contaminated marine sediments could be a non-permanent remedy if we do not close the input to the sea of contaminants (and salinization can continue acting and progressing if the various intercommunicating systems are not isolated).
These rational assumptions have implications for coastal zone monitoring, remediation practices, and research. The research allows to demonstrate that characterization and monitoring of the effectiveness of a pump-and-treat system for remediation is inadequate to grasp the system’s dynamics, which nevertheless have repercussions on the remedy designs. The study reveals how pump and treat can be, together with probable short-circuiting of different aquifer levels by deep wells and possible releases of saline liquids deriving from the treatment of salinized water extracted from drainage trenches, the triggering of the salinization leading to an increase of metal mobility. If this were the case, such remediation practice in coastal contaminated sites should be avoided because its progression in time would produce an increasing groundwater salinity, causing the increase of groundwater TE concentrations rather than their progressive reduction. In the case of coastal areas, to safeguard the sea and the environment, it is much better to proceed with physical permanent barriers to prevent communication with the sea. These considerations suggest that there must be a reconciliation even in research because the disciplines that deal with environmental matrices (water, sediment, biota) for the land-sea system, are not interconnected.
In summary, the unique combination of multivariate statistical analysis involving qualitative and quantitative variables in this work provides a comprehensive understanding of coastal environmental dynamics. It clearly demonstrates the behaviour trend of contaminants in groundwater. The results offer valuable insights for environmental management, providing practical recommendations for monitoring and remediation efforts. Additionally, they represent a significant scientific advancement and a useful tool for policymakers. The proposed approach and findings can be used as a starting point for planning multi-purpose sampling campaigns in similar environmental settings to study the impacts of salinization and contaminant mobility. Looking ahead, an integrated and interdisciplinary approach that bridges the gap between different environmental matrices, such as water, sediment, and biota within the land-sea system, could lead to a more holistic understanding of the environmental challenges faced by coastal areas.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
M.D.F developed the research question; M.M. and G.B. developed the methodology; M.D.F. and V.S. collected the data: M.M. and M.R.A. processed the data; M.M., M.D.F., V.S., M.R.A., G.B. and G.R. drafted the manuscript; M.R.A and G.R. edited the text and the figures; MDF reviewed and supervised the work.
Data availability
The data supporting the findings of this study are available upon request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cable, J. E., Burnett, W. C. & Chanton, J. P. Magnitude and variations of groundwater seepage along a Florida Marine shoreline. Biogeochemistry. 38, 189–205. 10.1023/A:1005756528516 (1997). [Google Scholar]
- 2.Moore, W. S. Large groundwater inputs to coastal waters revealed by 226Ra enrichments. Nature. 380, 612–614. 10.1038/380612a0 (1996). [Google Scholar]
- 3.Kim, G. Large submarine groundwater discharge (SGD) from a volcanic island. Geophys. Res. Lett.30, 2098. 10.1029/2003GL018378 (2003). [Google Scholar]
- 4.Burnett, W. C., Bokuniewicz, H., Huettel, M., Moore, W. S. & Taniguchi, M. Groundwater and pore water inputs to the coastal zone. Biogeochemistry. 66, 3–33 (2003). [Google Scholar]
- 5.Moore, W. The subterranean estuary: A reaction zone of ground water and sea water. Mar. Chem.65, 111–125. 10.1016/S0304-4203(99)00014-6 (1999). [Google Scholar]
- 6.Sadeghfam, S. et al. Chapter 7 - hydrochemical analysis of seawater intrusion by graphical techniques in coastal aquifers to delineate vulnerable areas, Groundwater Contamination in Coastal Aquifers, Elsevier, 91–104, 10.1016/B978-0-12-824387-9.00011-6, (2022).
- 7.Gowtham, B. et al. Chapter 17 - geochemical assessment of high salinity in groundwater along Ramanathapuram Coast, Southern Tamil Nadu, Groundwater Contamination in Coastal Aquifers, Elsevier, 213–231, 10.1016/B978-0-12-824387-9.00014-1, (2022).
- 8.Burnett, W. C., Taniguchi, M. & Oberdorfer, J. Measurement and significance of the direct discharge of groundwater into the coastal zone. J. Sea Res.46, 109–116. 10.1016/S1385-1101(01)00075-2 (2001). [Google Scholar]
- 9.Burnett, B. et al. Assessing methodologies for measuring groundwater discharge to the ocean. Eos Trans. Am. Geophys. Union. 83, 117. 10.1029/2002EO000069 (2002). [Google Scholar]
- 10.Charette, M. A. & Sholkovitz, E. R. Oxidative precipitation of groundwater-derived ferrous iron in the subterranean estuary of a coastal bay. Geophys. Res. Lett.29, 85. 10.1029/2001GL014512 (2002). [Google Scholar]
- 11.Charette, M. A., Sholkovitz, E. R. & Hansel, C. M. Trace element cycling in a subterranean estuary: Part 1. Geochemistry of the permeable sediments. Geochim. Cosmochim. Acta. 69, 2095–2109. 10.1016/j.gca.2004.10.024 (2005). [Google Scholar]
- 12.Spiteri, C., Regnier, P., Slomp, C. P. & Charette, M. A. pH-Dependent iron oxide precipitation in a subterranean estuary. J. Geochemical Explor.88, 399–403. 10.1016/j.gexplo.2005.08.084 (2006). [Google Scholar]
- 13.Michael, H. A., Mulligan, A. E. & Harvey, C. F. Seasonal oscillations in water exchange between aquifers and the coastal ocean. Nature. 436, 1145–1148. 10.1038/nature03935 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Krest, J. M., Moore, W. S., Gardner, L. R. & Morris, J. T. Marsh nutrient export supplied by groundwater discharge: Evidence from radium measurements. Global Biogeochem. Cycles. 14, 167–176. 10.1029/1999GB001197 (2000). [Google Scholar]
- 15.Charette, M. A., Buesseler, K. O. & Andrews, J. E. Utility of radium isotopes for evaluating the input and transport of groundwater-derived nitrogen to a Cape Cod estuary. Limnol. Oceanogr.46, 465–470. 10.4319/lo.2001.46.2.0465 (2001). [Google Scholar]
- 16.Valiela, A. I. et al. Nitrogen Loading from Coastal watersheds to receiving estuaries: New Method and Application. Ecol. Appl.7, 358–380. 10.1890/1051-0761(1997)007 (1997). [0358:NLFCWT]2.0.CO;2. [Google Scholar]
- 17.Slomp, C. P. & Van Cappellen, P. Nutrient inputs to the coastal ocean through submarine groundwater discharge: Controls and potential impact. J. Hydrol.295, 64–86. 10.1016/j.jhydrol.2004.02.018 (2004). [Google Scholar]
- 18.Li, L., Barry, D. A., Stagnitti, F. & Parlange, J. Y. Submarine groundwater discharge and associated chemical inputs to a coastal sea. Water Resour. Res.35, 3253–3259. 10.1029/1999WR900189 (1999). [Google Scholar]
- 19.International Institute for Applied Systems Analysis (IIASA). Chemical time bombs: Definition, concepts, and examples in Executive Report 16 (CTB Basic Document 1) (ed. Stigliani, W.) Novographic, Vienna, Austria, ISBN 3704501034; (1991). https://pure.iiasa.ac.at/id/eprint/3510/1/ER-91-016.pdf
- 20.Ali, H. & Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’ – proposal of a comprehensive definition. Toxicol. Environ. Chem.100 (1), 6–19. 10.1080/02772248.2017.1413652 (2018). [Google Scholar]
- 21.Gambrell, R. P. Trace and toxic metals in Wetlands-A Review. J. Environ. Qual.23, 883. 10.2134/jeq1994.00472425002300050005x (1994). [DOI] [PubMed] [Google Scholar]
- 22.Colombani, N., Mastrocicco, M. & Dinelli, E. Trace elements mobility in a saline coastal aquifer of the Po River lowland (Italy). J. Geochemical Explor.159, 317–328. 10.1016/j.gexplo.2015.10.009 (2015). [Google Scholar]
- 23.Knee, K. L., Paytan, A. Submarine groundwater discharge a source of nutrients, metals, and pollutants to the coastal Ocean in Treatise on Estuarine and Coastal Science (Eds Wolanski. E., McLusky, W.) 4.08, 4, 205–233 (Elsevier, 2011).
- 24.Kumar, M. et al. Review of perspective, problems, challenges, and future scenario of metal contamination in the urban environment.J. Hazard. Toxic Radioact. Waste. 21, 04017007 (2017). 10.1061/(ASCE)HZ.2153-5515.0000351 (2017).
- 25.El-Kholy, R. A. et al. Groundwater quality assessment using water quality index and multivariate statistical analysis: case study-east Matrouh, Northwestern Coast, Egypt. Environ. Sci. Pollut Res.29, 65699–65722. 10.1007/s11356-022-19761-3 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Norrström, A. C. Metal mobility by de-icing salt from an infiltration trench for highway runoff. Appl. Geochem.20, 1907–1919. 10.1016/j.apgeochem.2005.06.002 (2005). [Google Scholar]
- 27.Langmuir, D. Aqueous Environmental Geochemistry (Prentice-Hall, 1997).
- 28.Appelo, C. A. J., Postma, D. & Geochemistry Groundwater and Pollution 2nd edn (CRC, 2005). 10.1201/9781439833544
- 29.Bäckström, M., Karlsson, S., Bäckman, L., Folkeson, L. & Lind, B. Mobilisation of heavy metals by deicing salts in a roadside environment. Water Res.38, 720–732. 10.1016/j.watres.2003.11.006 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Acosta, J. A., Jansen, B., Kalbitz, K., Faz, A. & Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere. 85, 8, 1318–1324. 10.1016/j.chemosphere.2011.07.046 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Gambrell, R. P., Wiesepape, J. B. & Duff, P. Jr. The effects of pH, redox, and salinity on metal release from a contaminated sediment. Water Air Soil. Pollut. 57, 359–367. 10.1007/BF00282899 (1991). [Google Scholar]
- 32.Grassi, S. & Netti, R. Sea water intrusion and mercury pollution of some coastal aquifers in the province of Grosseto (Southern Tuscany — Italy). J. Hydrol.237 (4), 3. 10.1016/S0022-1694(00)00307-3 (2000). [Google Scholar]
- 33.Sun, H., Alexander, J., Gove, B. & Koch, M. Mobilization of arsenic, lead, and mercury under conditions of sea water intrusion and road de-icing salt application. J. Contam. Hydrol.180, 12–24. 10.1016/j.jconhyd.2015.07.002 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Pucci, A. A., Harriman, D. A., Ervin, E. M., Bratton, L. & Gordon, A. lead and cadmium associated with saltwater intrusion in a new jersey aquifer system. J. Am. Water Resour. Assoc.25, 1267–1272. 10.1111/j.1752-1688.1989.tb01339.x (1989). [Google Scholar]
- 35.Lee, Y., Rahman, M. D. M., Guebuem, K. & Seunghee, H. Mass balance of total mercury and monomethylmercury in coastal embayments of a volcanic Island: Significance of submarine groundwater discharge. Environ. Sci. Technol.45, 9891–9900. 10.1021/es202093z (2011). [DOI] [PubMed] [Google Scholar]
- 36.Bone, S., Gonneea, M. E. & Charette, M. Geochemical cycling of arsenic in a coastal aquifer. Environ. Sci. Technol.40, 3273–3278. 10.1021/es052352h(2006). [DOI] [PubMed] [Google Scholar]
- 37.Szymczycha, B., Kroeger, K. D. & Pempkowiak, J. Significance of groundwater discharge along the coast of Poland as a source of dissolved metals to the southern Baltic Sea. Mar. Pollut Bull.109, 151–162. 10.1016/j.marpolbul.2016.06.008 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Burton, E. D., Phillips, I. R. & Hawker, D. W. Factors controlling the geochemical partitioning of trace metals in estuarine sediments. Soil. Sediment. Contamination: Int. J.15, 3, 253–276. 10.1080/15320380600646290 (2006). [Google Scholar]
- 39.Jia, Y. et al. Sources of groundwater salinity and potential impact on arsenic mobility in the western Hetao Basin, Inner Mongolia. Sci. Total Environ.601–602, 691–702. 10.1016/j.scitotenv.2017.05.196 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Nodefarahani, M. et al. Metal pollution assessment in surface sediments of Namak Lake, Iran. Environ. Sci. Pollut Res.27, 45639–45649. 10.1007/s11356-020-10298-x (2020). [DOI] [PubMed] [Google Scholar]
- 41.Mali, M. et al. Multivariate analyses for investigating highly polluted marine ecosystem: The case study of Mar Piccolo (Taranto, South Italy). Aquat. Ecosyst. Health Manag.23 (4), 436–444. 10.1080/14634988.2020.1807224 (2021). [Google Scholar]
- 42.Calabrese, A., Massarelli, C., Uricchio, V. F. & Campanale, C. Safeguarding drinking Water Use: Use and quality of water, case study of Taranto Province. Procedia Eng.89, 232–238. 10.1016/j.proeng.2014.11.182 (2014). [Google Scholar]
- 43.Mali, M., Dell’Anna, M. M., Notarnicola, M., Mastrorilli, P. & Damiani, L. Combining chemometric tools for assessing hazard sources and factors acting simultaneously in contaminated areas. Case study: Mar Piccolo Taranto (South Italy). Chemosphere. 184, 784–794. 10.1016/j.chemosphere.2017.06.028 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Cotecchia, F. et al. A geo-chemo-mechanical study of a highly polluted marine system (Taranto, Italy) for the enhancement of the conceptual site model. Sci. Rep.11, 4017. 10.1038/s41598-021-82879-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mali, M. et al. Are conventional statistical techniques exhaustive for defining metal background concentrations in harbour sediments? A case study: The Coastal Area of Bari (Southeast Italy) Chemosphere, 138, 708–717 (2015). 10.1016/j.chemosphere.2015.07.046 [DOI] [PubMed]
- 46.Mali, M. et al. Long-term monitoring programs to assess environmental pressures on coastal area: Weighted indexes and statistical elaboration as handy tools for decision-makers. Ecol. Ind.101, 838–850. 10.1016/j.ecolind.2019.01.085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mali, M. et al. Multivariate tools to investigate the spatial contaminant distribution in a highly anthropized area (Gulf of Naples, Italy). Env Sci. Poll. Res.29, 41, 62281–62298. 10.1007/s11356-022-19989-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Servizio Geologico d’Italia, Martinis, E. & Robba, F. Note Illustrative della Carta Geologica d’Italia (Illustrative Notes to the Geological Map of Italy), 1:100,000, Foglio (Folio) 202 Taranto, (1971). https://sgi.isprambiente.it/geologia100k/mostra_pdf.aspx?pdffile=202.pdf
- 49.Regione Puglia. Carta geo-litologica della Puglia (Geolithological map of Puglia), (in Italian). http://www.sit.puglia.it/portal/portale_cartografie_tecniche_tematiche/Cartografie%20tecniche/Carta%20Idrogeomorfologica (last accessed May 2024).
- 50.Kottek, M., Grieser, J., Beck, C., Rudolf, B. & Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z.15, 259–263. 10.1127/0941-2948/2006/0130 (2006). [Google Scholar]
- 51.Isa, N. M., Aris, A. Z. & Sulaiman, W. N. A. W. Extent and severity of groundwater contamination based on hydrochemistry mechanism of sandy tropical coastal aquifer. Sci. Total Environ.438, 414–425. 10.1016/j.scitotenv.2012.08.069 (2012). [DOI] [PubMed] [Google Scholar]
- 52.MD 471/1999 - Italian Ministerial Decree N. 471 *1999. (GU General Series no. 293 15/12/1999 - Suppl. Ordinario n. 218) accessed on 22/05/2024 (https://www.gazzettaufficiale.it/eli/id/1999/12/15/099G0540/sg).
- 53.Stuyfzand, P. & Stuurman, R. J. Recognition and genesis of various brackish to hypersaline groundwaters in the Netherlands. Proc. 13th salt water intrusion meeting: University of Cagliari Sardinia, 125–136 (1994).
- 54.Stuart, A., Ord, J. K. & Arnold, S. Kendall’s Advanced Theory of Statistics: Volume 2A—Classical Inference and the Linear Model, sixth edition, § 20.2–20.3 (Arnold). (1999).
- 55.Henderson, A., Comiskey, J., Dallmeier, F. & Alonso, A. Framework for Assessment and Monitoring of Biodiversity, Editor(s): Simon Asher Levin, Encyclopaedia of BiodiversityPages 1–11 (Elsevier, 2007). 10.1016/B0-12-226865-2/00129-2
- 56.Ferraro, A. et al. Characterizing contaminant distribution in marine-coastal sediments through multivariate and non-parametric statistical analyses: A complementary strategy supporting environmental monitoring and control. Environ. Monit. Assess.195, g59. 10.1007/s10661-022-10617-4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Communication of the Ministry of Health. accessed on May 2024: (2016). www.salute.gov.it/portale/temi/documenti/acquepotabili/parametri/CARBONIO_ORGANICO_TOTALE.pdf
- 58.LD 152/06 - Italian Legislative Decree No. 152/06, Parte IV., Titolo, V., Allegato 5 (2006). https://www.gazzettaufficiale.it/atto/serie_generale/caricaArticolo?art.versione=1&art.idGruppo=54&art.flagTipoArticolo=3&art.codiceRedazionale=006G0171&art.idArticolo=14&art.idSottoArticolo=1&art.idSottoArticolo1=10&art.dataPubblicazioneGazzetta=2006-04-14&art.progressivo=0.
- 59.McMahon, P. B. & Chapelle, F. H. Redox processes and water quality of selected principal aquifer systems: GroundWater, 46, 259–271 (2008). 10.1111/j.1745-6584.2007.00385.x [DOI] [PubMed]
- 60.Jia, C. et al. Ground water copper levels in the seawater intrusion area and the possible physical and chemical dynamics. Environ. Sci. : Processes Impacts. 23, 335. 10.1039/d0em00435a (2021). [DOI] [PubMed] [Google Scholar]
- 61.Cary, L. et al. Selenium mobility in a major chalk aquifer (Lille metropolis, northern France): Contaminants cycles driven by geology, redox processes and pumping. Chem. Geol.583, 120465. 10.1016/j.chemgeo.2021.120465 (2021). [Google Scholar]
- 62.Bailey, R. T. & Review Selenium contamination, fate, and reactive transport in groundwater in relation to human health. Hydrogeology Journal; Heidelberg, 25, Fasc. 4:1191–1217, (2017). 10.1007/s10040-016-1506-8
- 63.Gan, X. et al. Remediation of selenium-contaminated soil through combined use of earthworm Eisenia fetida and organic materials. J. Hazard. Mater.405, 124212. 10.1016/j.jhazmat.2020.124212 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Chen, Q. et al. Groundwater Selenium Level and its Enrichment dynamics in seawater intrusion area along the Northern Coastal zones of Shandong Province, China. Geochem. Int.57, 1236–1242. 10.1134/S0016702919110065 (2019). [Google Scholar]
- 65.Balistrieri, L. S. & Chao, T. T. Adsorption of selenium by amorphous iron oxyhydroxide and manganese dioxide. Geochim. Cosmochim. Acta. 54, 3, 739–751. 10.1016/0016-7037(90)90369-V (1990). [Google Scholar]
- 66.Fujii, R. & Deverel, S. Distribution of Selenium in soils of Agricultural Fields, Western San Joaquin Valley, California. Soil Sci. Soc. Am. J.10.2136/sssaj1988.03615995005200050011x (1988). [Google Scholar]
- 67.Wright, M. T., Stollenwerk, K. G. & Belitz, K. Assessing the solubility controls on vanadium in groundwater, northeastern San Joaquin Valley, CA. Appl. Geochem.48, 41–52. 10.1016/j.apgeochem.2014.06.025 (2014). [Google Scholar]
- 68.Eliopoulos, I. P. D., Eliopoulos, G. D. & Economou-Eliopoulos, M. The Cr(VI) stability in contaminated coastal groundwater: salinity as a driving force. Minerals, 11, 160. (2021). 10.3390/min11020160
- 69.Papazotos, P., Vasileiou, E. & Perraki, M. Elevated groundwater concentrations of arsenic and chromium in ultramafic environments controlled by seawater intrusion, the nitrogen cycle, and anthropogenic activities: The case of the Gerania Mountains, NE Peloponnese, Greece. Appl. Geochem.121, 104697. 10.1016/j.apgeochem.2020.104697 (2020). [Google Scholar]
- 70.Tian, Y. et al. Speciation of nickel (II) chloride complexes in hydrothermal fluids: In situ XAS study. Chem. Geol.334, 345–363. 10.1016/j.chemgeo.2012.10.010 (2012). [Google Scholar]
- 71.Gantayat, R. R. & Elumalai, V. Salinity-induced changes in heavy metal behaviour and mobility in semi-arid coastal aquifers: A comprehensive review. Water. 16, 7, 1052. 10.3390/w16071052 (2024). [Google Scholar]
- 72.Andersson, A. ‘Mercury in Soils’ in: (ed Nriagu, J. O.) The Biogeochemistry of Mercury in the Environment, Elsevier/North-Holland Biomedical, 79–106 (1979).
- 73.Barringer, J. L., Szabo, Z. & Reilly, P. Occurrence and mobility of mercury in groundwater, Published: 27 February, 10.5772/55487 (2013).
- 74.Wang, Y., Jiao, J. J. & Cherry, J. A. Occurrence and geochemical behaviour of arsenic in a coastal aquifer–aquitard system of the Pearl River Delta, China. Sci. Total Environ.427–428, 286–297. 10.1016/j.scitotenv.2012.04.006 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Zhi, C., Cao, W., Wang, Z., Li, Z. & Ren, Y. Genesis of as in the groundwater with extremely high salinity in the Yellow River Delta, China. Appl. Geochem.13910.1016/j.apgeochem.2022.105229 (2022).
- 76.Liu, C. et al. Identifying sources and controlling factors of arsenic release in saline groundwater aquifers. Hydrol. Earth Syst. Sci. Discuss.10, 10565–10603. 10.5194/hess-18-1089-2014 (2013). [Google Scholar]
- 77.Giambastiani, B. M. S., Colombani, N. & Mastrocicco, M. D. Small-scale variability of Trace Elements in a shallow aquifer. Water Air Soil. Poll.226, 2, 1–14. 10.1007/s11270-014-2283-4 (2015). [Google Scholar]
- 78.Kumar, A. R. & Riyazuddin, P. Seasonal variation of redox species and redox potentials in shallow groundwater: A comparison of measured and calculated redox potentials. J. Hydrol.444–445, 187–198. 10.1016/j.jhydrol.2012.04.018 (2012). [Google Scholar]
- 79.Greene, R., Timms, W., Rengasamy, P., Arshad, M. & Cresswell, R. Soil and aquifer salinization: Toward an Integrated Approach for Salinity Management of Groundwater. (Eds (eds Jakeman, A. J., Barreteau, O., Hunt, R. J., Rinaudo, J. D. & Ross, A.) Integrated Groundwater Management. Springer, Cham. 10.1007/978-3-319-23576-9_15 (2016). [Google Scholar]
- 80.Eurola, M., Hietaniemi, V. & Kontturi, M. Selenium content of Finnish oats in 1997–1999: Effect of cultivars and cultivation techniques. Agricultural Food Sci.13, 46–53. 10.2137/1239099041837941 (2004). [Google Scholar]
- 81.Buccolieri, A. et al. Heavy metals in marine sediments of Taranto Gulf (Ionian Sea, Southern Italy. Mar. Chem.99, 1–4. 10.1016/j.marchem.2005.09.009 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Obasi, I. A., Nnachi, E. E., Igwe, O. E. & Obasi, N. P. Evaluation of pollution status of heavy metals in the groundwater system around open dumpsites in Abakaliki urban, Southeastern Nigeria. Afr. J. Environ. Sci. Technol.9, 7, 600–609. 10.5897/AJEST2015.1780 (2015). [Google Scholar]
- 83.Fang, K. et al. Decreased soil cation exchange capacity across northern China’s grasslands over the last three decades. J. Geophys. Research: Biogeosciences. 122, 3088–3097. 10.1002/2017JG003968 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available upon request to the corresponding author.