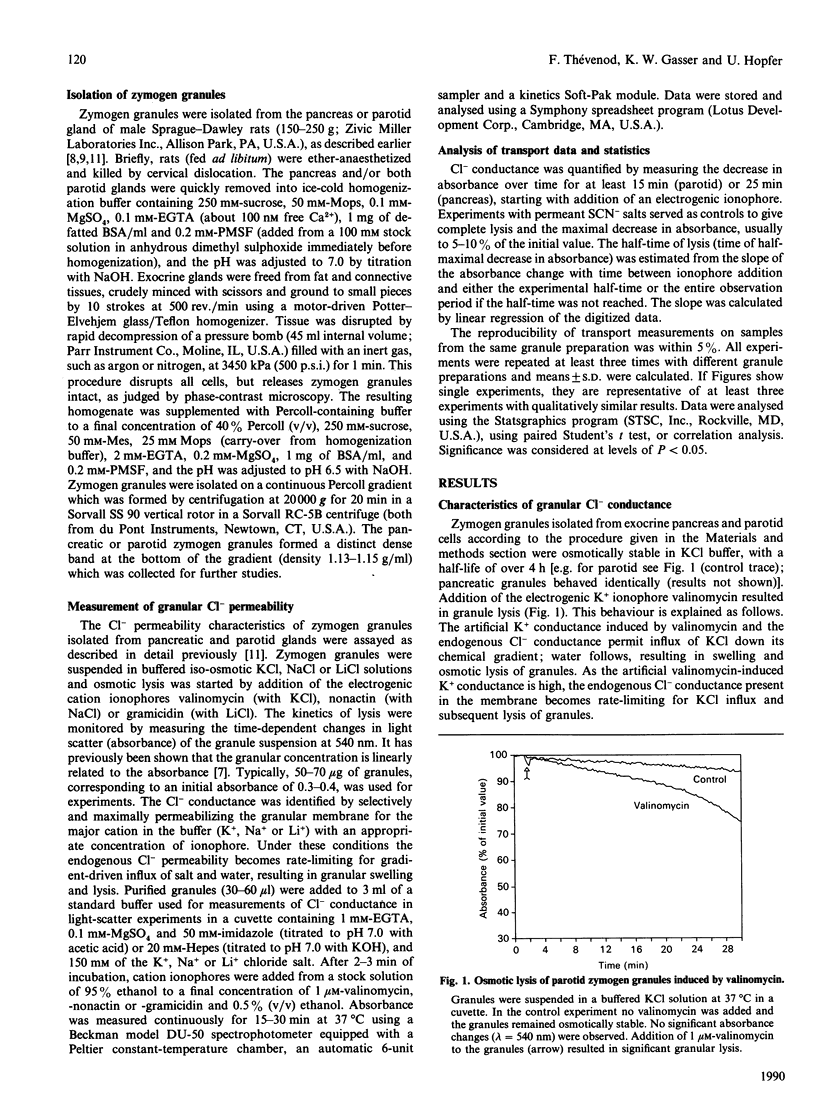
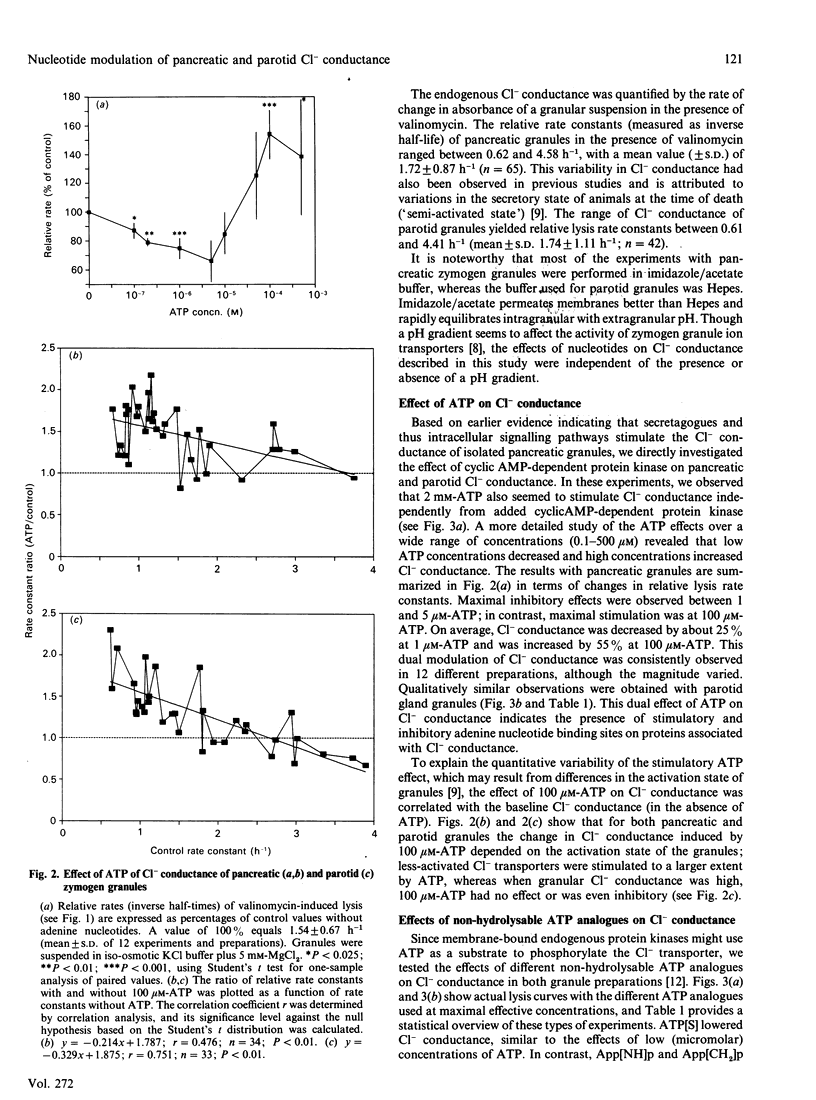
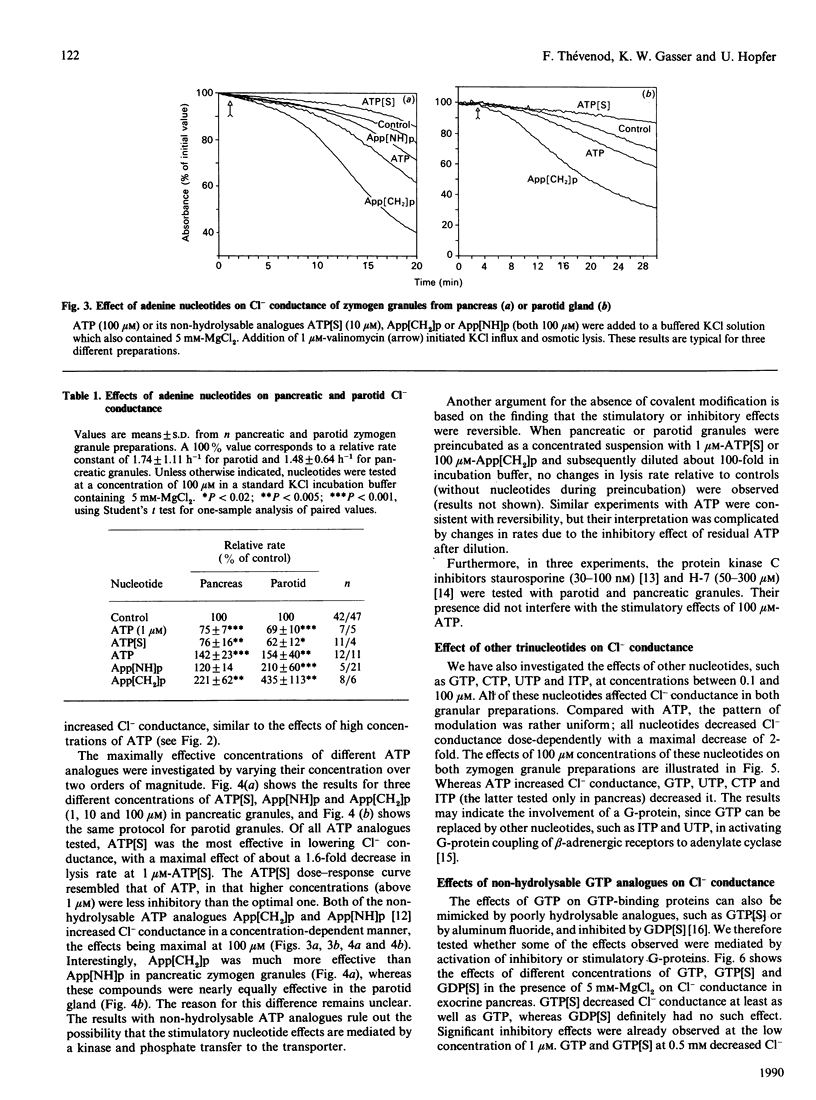
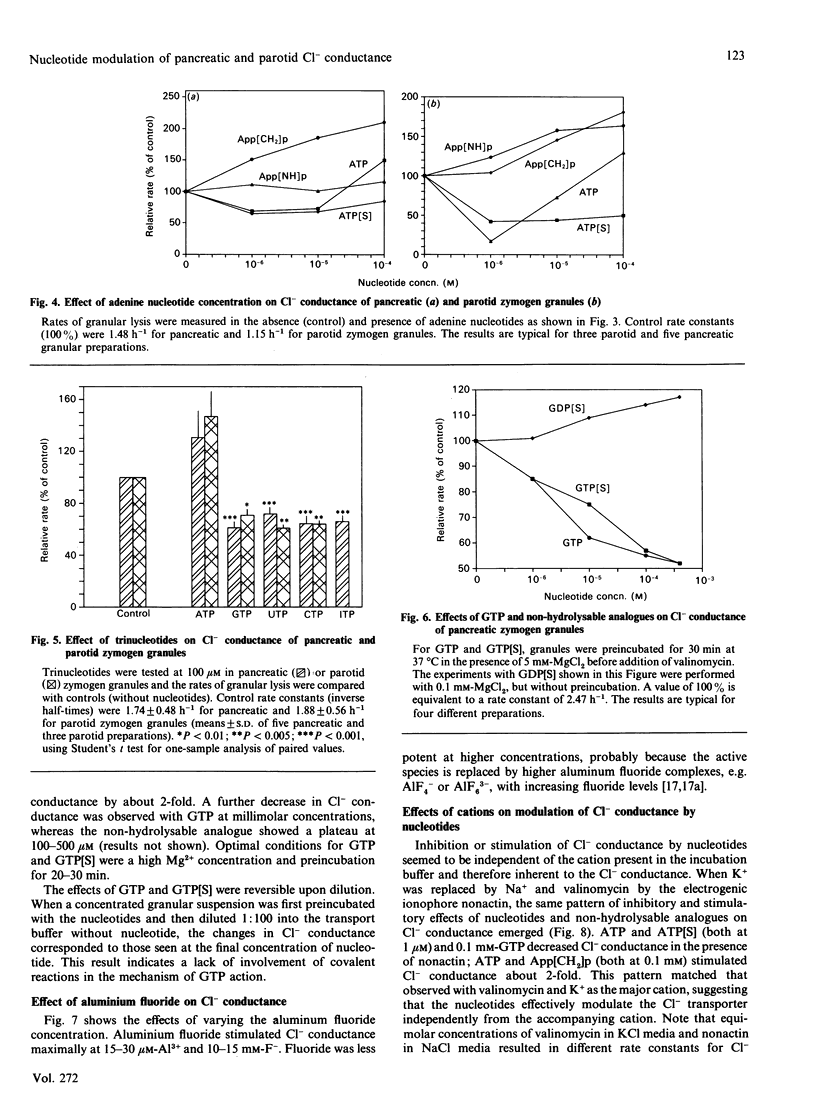
Abstract
The regulation of Cl- conductance by cytoplasmic nucleotides was investigated in pancreatic and parotid zymogen granules. Cl- conductance was assayed by measuring the rate of cation-ionophore-induced osmotic lysis of granules suspended in iso-osmotic salt solutions. Both inhibition and stimulation were observed, depending on the type and concentration of nucleotide. Under optimal conditions, the average inhibition measured in different preparations was 1.6-fold, whereas the average stimulation was 4.4-fold. ATP was inhibitory at 1-10 microM but stimulated Cl- conductance above 50 microM. Stimulation by ATP was more pronounced in granules with low endogenous Cl- conductance. The potency of nucleotides in terms of inhibition was ATP greater than adenosine 5'-[gamma-thio]triphosphate (ATP[S]) greater than UTP much greater than or equal to CTP much greater than or equal to GTP much greater than or equal to guanosine 5'-[gamma-thio]triphosphate (GTP[S]) much greater than or equal to ITP. The potency with respect to stimulation had the following order: adenosine 5'-[beta gamma-methylene]triphosphate (App[CH2]p) greater than ATP greater than guanosine 5'-[beta-thio]diphosphate (GDP[S]). Adenosine 5'-[beta gamma-imido]triphosphate (App[NH]p) was also stimulatory, and was more potent than ATP in the parotid granules, but less potent in the pancreatic granules. Aluminium fluoride stimulated Cl- conductance maximally at 15-30 microM-Al3+ and 10-15 mM-F. F was less effective at higher concentrations. Protein phosphorylation by kinases was apparently not involved, since the nucleotide effects (1) could be mimicked by non-hydrolysable analogues of ATP and GTP, (2) showed reversibility, and (3) were not abolished by the protein kinase inhibitors 1-(5-isoquinolinesulphonyl)-2-methylpiperazine (H-7) or staurosporine. The data suggest the presence of at least two binding sites for nucleotides, whereby occupancy of one induces inhibition and occupancy of the other induces stimulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Aurbach G. D. The effects of nucleotides on the expression of beta-adrenergic adenylate cyclase activity in membranes from turkey erythrocytes. J Biol Chem. 1974 Jan 10;249(1):157–161. [PubMed] [Google Scholar]
- Burnham D. B., Munowitz P., Thorn N., Williams J. A. Protein kinase activity associated with pancreatic zymogen granules. Biochem J. 1985 May 1;227(3):743–751. doi: 10.1042/bj2270743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Tsuruo T., Gottesman M. M., Pastan I. ATP-binding properties of P glycoprotein from multidrug-resistant KB cells. FASEB J. 1987 Jul;1(1):51–54. doi: 10.1096/fasebj.1.1.2886389. [DOI] [PubMed] [Google Scholar]
- De Lisle R. C., Hopfer U. Electrolyte permeabilities of pancreatic zymogen granules: implications for pancreatic secretion. Am J Physiol. 1986 Apr;250(4 Pt 1):G489–G496. doi: 10.1152/ajpgi.1986.250.4.G489. [DOI] [PubMed] [Google Scholar]
- De Lisle R. C., Schulz I., Tyrakowski T., Haase W., Hopfer U. Isolation of stable pancreatic zymogen granules. Am J Physiol. 1984 Apr;246(4 Pt 1):G411–G418. doi: 10.1152/ajpgi.1984.246.4.G411. [DOI] [PubMed] [Google Scholar]
- Deeg M. A., Graeff R. M., Walseth T. F., Goldberg N. D. A Ca2+-linked increase in coupled cAMP synthesis and hydrolysis is an early event in cholinergic and beta-adrenergic stimulation of parotid secretion. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7867–7871. doi: 10.1073/pnas.85.21.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine stimulates a Ca2+-dependent C1- conductance in mouse lacrimal acinar cells. Pflugers Arch. 1985 Mar;403(3):328–330. doi: 10.1007/BF00583609. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Zimmerberg J., Cohen F. S. Osmotic swelling of vesicles: its role in the fusion of vesicles with planar phospholipid bilayer membranes and its possible role in exocytosis. Annu Rev Physiol. 1986;48:163–174. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- Flockhart D. A., Freist W., Hoppe J., Lincoln T. M., Corbin J. D. ATP analog specificity of cAMP-dependent protein kinase, cGMP-dependent protein kinase, and phosphorylase kinase. Eur J Biochem. 1984 Apr 16;140(2):289–295. doi: 10.1111/j.1432-1033.1984.tb08100.x. [DOI] [PubMed] [Google Scholar]
- Gasser K. W., DiDomenico J., Hopfer U. Potassium transport by pancreatic and parotid zymogen granule membranes. Am J Physiol. 1988 Dec;255(6 Pt 1):C705–C711. doi: 10.1152/ajpcell.1988.255.6.C705. [DOI] [PubMed] [Google Scholar]
- Gasser K. W., DiDomenico J., Hopfer U. Secretagogues activate chloride transport pathways in pancreatic zymogen granules. Am J Physiol. 1988 Jan;254(1 Pt 1):G93–G99. doi: 10.1152/ajpgi.1988.254.1.G93. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gögelein H. Chloride channels in epithelia. Biochim Biophys Acta. 1988 Oct 11;947(3):521–547. doi: 10.1016/0304-4157(88)90006-8. [DOI] [PubMed] [Google Scholar]
- Hamada H., Hagiwara K., Nakajima T., Tsuruo T. Phosphorylation of the Mr 170,000 to 180,000 glycoprotein specific to multidrug-resistant tumor cells: effects of verapamil, trifluoperazine, and phorbol esters. Cancer Res. 1987 Jun 1;47(11):2860–2865. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Gasser K. Isolation of physiologically responsive secretory granules from exocrine tissues. Methods Enzymol. 1989;174:162–172. doi: 10.1016/0076-6879(89)74017-9. [DOI] [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Guanine nucleotides and Ca-dependent exocytosis. Studies on two adrenal cell preparations. FEBS Lett. 1985 Sep 23;189(2):345–349. doi: 10.1016/0014-5793(85)81053-x. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M. Regulation of chloride transport in epithelia. Annu Rev Physiol. 1989;51:143–160. doi: 10.1146/annurev.ph.51.030189.001043. [DOI] [PubMed] [Google Scholar]
- Martin R. B. Ternary hydroxide complexes in neutral solutions of Al3+ and F-. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1194–1200. doi: 10.1016/s0006-291x(88)81266-x. [DOI] [PubMed] [Google Scholar]
- Mellado W., Horwitz S. B. Phosphorylation of the multidrug resistance associated glycoprotein. Biochemistry. 1987 Nov 3;26(22):6900–6904. doi: 10.1021/bi00396a005. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Fick R. B. Cystic fibrosis. J Clin Invest. 1987 Dec;80(6):1523–1526. doi: 10.1172/JCI113237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Wrenn R. W. Phosphorylation of a pancreatic zymogen granule membrane protein by endogenous calcium/phospholipid-dependent protein kinase. Biochim Biophys Acta. 1984 Aug 8;775(1):1–6. doi: 10.1016/0005-2736(84)90227-x. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- de Weille J. R., Schmid-Antomarchi H., Fosset M., Lazdunski M. Regulation of ATP-sensitive K+ channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2971–2975. doi: 10.1073/pnas.86.8.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]


