Abstract
Background The production of inflammatory cytokines is reportedly increased in patients with coronavirus disease 2019 (COVID-19), causing the migration of neutrophils and monocytes to lung tissues. This disrupts the air–blood barrier by damaging the bronchial epithelial and vascular endothelial cells. As multiorgan dysfunction in sepsis is considered to be partly caused by complement activation, which can cause lung injury in patients with COVID-19. There are limited studies examining the link between complement activation in patients with COVID-19. This study aimed to AQinvestigate the association of complement activation with the pathophysiology of COVID-19. Twenty-seven patients with COVID-19 were enrolled in this study and classified into two groups depending on the indication for mechanical ventilation. Plasma complement factors (C3a, C5a, Ba, and sC5b-9), complement regulators (sCD59 and factor H), interleukin-6 (IL-6), and syndecan-1 levels were measured using Enzyme-linked immunosorbent assay (ELISA). Results: All complement factors and regulators, IL-6, and syndecan-1 levels were significantly elevated in patients with COVID-19 compared with those in healthy controls. C5a and sC5b-9 levels were decreased significantly in the invasive mechanical ventilation (IMV) group compared with those in the non-IMV group. Syndecan-1 levels were significantly increased in the IMV group compared with those in the non-IMV group. Conclusions: Complement activation is an exacerbating factor for lung injury in patients with COVID-19. Complement factors are nonessential predictors of mechanical ventilation; however, syndecan-1 could be a biomarker of COVID-19 severity in patients.
Keywords: Complement system, COVID-19, Invasive mechanical ventilation, Syndecan-1
Subject terms: Immunology, Translational research
Introduction
Four years since the world’s first case, the coronavirus disease 2019 (COVID-19) continues to threaten healthcare systems. COVID-19 can cause life-threatening pneumonia and multiorgan dysfunction, believed to be triggered by the activation of the innate immune response1. The time between the onset of symptoms and hospitalization for patients with COVID-19 is typically 7 (range, 3–9) days2. Approximately 17–35% of hospitalized patients with COVID-19 require intensive care unit (ICU) treatment. Among them, 29–91% of patients require invasive mechanical ventilation (IMV)2. Respiratory failure in patients with COVID-19 may result from cytokine storm syndrome (CSS), where proinflammatory cytokines cause inflammatory cell recruitment, inducing lung inflammation and damage3. However, a recent study investigated the role of CSS in respiratory failure in patients with COVID-193 and demonstrated that patients with COVID-19 had lower cytokine levels than those with influenza; moreover, elevated levels of interleukin-6 (IL-6), Granulocyte colony-stimulating factor (G-CSF), Interleukin-1 receptor antagonist (IL-1RA), and Monocyte chemoattractant protein1 (MCP1) were associated with mortality in patients with COVID-19; however, the levels were not significantly higher in patients with COVID-19 than in those with influenza3. Therefore, CSS alone cannot underline the mechanism of respiratory failure in patients with COVID-19.
The complement system is crucial in the immune response to sepsis and trauma4,5. These conditions trigger rapid activation of the complement cascade6,7, causing the formation of the C5b-9 complex. This complex binds to target cells and causes cell death or dysfunction. Complementary activation produces inflammatory mediators that recruit other immune cells and cause widespread tissue and organ damage4–6. Although the complement system benefits the host, it can be harmful when overactivated or depleted7. Therefore, the complement system should be tightly regulated to prevent excessive activation. The complement system has been implicated in the pathogenesis of COVID-198–13. Elevated levels of specific complement markers (sC5b-9 and C5a) are associated with COVID-19 severity and the need for IMV14. Additionally, sC5b-9 and C4d levels increase significantly in patients with respiratory failure and not in those with other complement factors (C5a, C3bc, C3bBbP, and MBL)9. Another study demonstrated that low properdin levels were associated with mechanical ventilation, suggesting enhanced alternative pathway activation15. Although several studies have reported an association between complement activation and respiratory failure in patients with COVID-19, no consensus has been reached.
African American/Black and Hispanic populations reportedly experience a disproportionate burden of severe-acute-respiratory-syndrome-related coronavirus (SARS-CoV-2) infections and COVID-19-related mortality16, suggesting that racial differences should be considered when discussing COVID-19. There are limited studies in the Asian population, especially in the Japanese population, to probe the link between complement activation and respiratory failure in patients with COVID-19. Therefore, this study aimed to understand the association of complement activation in patients with COVID-19 from Japan. This is the first Japanese report to investigate the association of complement activation with the pathophysiology of COVID-19.
Methods
Study design and patient enrollment
In this single-center prospective observational study, patients presenting to the hospital with SARS-CoV-2 infection were confirmed using a polymerase chain reaction from a nasopharyngeal swab. This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Juntendo University, Urayasu Hospital (3–022). This study enrolled patients (≥ 18 y old) with COVID-19 (n = 27) admitted to the intensive care unit (ICU) of Juntendo University, Urayasu Hospital, a tertiary emergency medical center in Japan, between March 2020 and June 2021. Patients with malignant disease or chronic administration of steroids were excluded. The date of ICU admission (or the date of initial admission for patients transferred from another hospital) was designated as day 0. All patients were hospitalized in ICU and classified into severe cases according to Clinical Management of Patients with COVID-19: a Guide for Front-line Healthcare Workers ver.9.0. https://www.mhlw.go.jp/content/000936655.pdf. The 27 patients were classified into two groups depending on the indication for IMV on Day 0. The IMV group includes 14 patients, and the non-IMV group includes 13. Written informed consent was obtained from the patients or their legal representatives. Patients’ data, such as age, sex, body mass index (BMI), comorbidities, pneumonia, extracorporeal membrane oxygenation (ECMO), and mortality from electronic clinical records were examined. All patients underwent CT scan for diagnosis of pneumonia. The Acute Physiology and Chronic Health Evaluation II (APACHE II)17 and Sequential Organ Failure Assessment (SOFA)18 scores were determined. Moreover, white blood cell (WBC) counts, platelet counts, and C-reactive protein (CRP) levels were examined. Complement components 3a and 5a (C3a and C5a), Ba, soluble C5b-9 (sC5b-9), soluble factor H, soluble CD59 (sCD59), IL-6, and syndecan-1 were assessed on Days 0 and 2–4 for the same group patients to examine the serial change. Control samples were obtained from healthy volunteers. The healthy control (HC) group, made up of healthy volunteers, included male (n = 12) and female (n = 7) individuals aged 47 years (interquartile range [IQR], 41–51 years). There was no significant difference in the sex distribution between the patient and HC groups; however, individuals in the HC group were significantly younger than the patients.
Blood sample processing
A heparinized blood sample (10 mL) was collected from the peripheral vein of each healthy volunteer and the arterial line or peripheral vein of patients with COVID-19. Blood collection from all patients with COVID-19 was performed within 24 h of ICU admission and on Days 2–4. Blood samples were centrifuged at 724 ×g for 5 min at 4 °C, and plasma was dispensed and stored at − 80 °C before analysis.
Measurement of complement components in plasma
The plasma levels of C3a, C5a, sC5b-9, Ba, and factor H were measured using the MicroVue C3a Plus EIA kit (# A031), C5a EIA kit (# A021), sC5b-9 Plus EIA kit (# A020), Ba Fragment EIA kit (# A033), and factor H EIA kit (# A039), respectively, all of which were sourced from Quidel Corp. (San Diego, CA, USA). The plasma levels of sCD59 and syndecan-1 were measured using the RayBio® Human CD59 Enzyme-linked immunosorbent assay (ELISA) kit (ELH-CD59) and RayBio Human Syndecan-1 ELISA Kit (#ELH-Syndecan1) (RayBiotech, Inc., Norcross, GA, USA), respectively. IL-6 concentration was measured using Quantikine ELISA Human IL-6 (#D6050) (R&D Systems, Inc., Minneapolis, MN, USA). The assessments were performed according to the manufacturer’s instructions.
Statistical analysis
Data are presented as medians and IQRs for continuous variables unless stated otherwise. Statistical analyses were performed using GraphPad Prism version 6.03 Windows (GraphPad Software, San Diego, CA, USA). Data were analyzed for significant differences using the Mann–Whitney test or Wilcoxon matched-pairs signed-rank test to compare the two groups. Differences were considered statistically significant at P values < 0.05.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Juntendo University, Urayasu Hospital (3–022).
Written informed consent was obtained from the patients or their legal representatives.
Results
Clinical characteristics
Twenty-seven patients with COVID-19 were enrolled and classified into two groups according to indications for IMV. The patients included male (n = 21) and female (n = 6) individuals, with a median age of 64 years (IQR, 52–72 years), BMI of 24.6 (21.4–28.7), APACHE II score of 15 (12–23), SOFA score of 3 (2–8), WBC counts of 7 × 103/µL (5.2 × 103–9.3 × 103/µL), platelet counts of 215 × 103/µL (181 × 103–307 × 103/µL), and CRP levels of 7.0 (3.8–12.1). Pneumonia was observed in 26 patients, and ECMO was introduced in five patients. The median duration from onset to admission was 9 (7–11) days. Three patients died during hospitalization. Additional demographic and baseline characteristics are presented in Table 1.
Table 1.
Characteristics of COVID-19 patients.
| All patients (n = 27) |
Non-IMV (n = 13) |
IMV (n = 14) |
HC (n = 19) |
|
|---|---|---|---|---|
| Age (years), median (IQR) | 64 (57–72) | 66 (52–74) | 64 (56–73) | 47 (41–51) |
| Male | 21 | 10 | 11 | 12 |
| BMI, median (IQR) | 24.6(21.4–28.7) | 21.4 (20.5–26.0) | 26.4 (24.4–30.1) | 24.0 (22.6–24.6) |
| Comorbidities | ||||
| Smoking history | 12 | 5 | 7 | 1 |
| Diabetes | 10 | 3 | 7 | 0 |
| Hypertension | 12 | 6 | 6 | 2 |
| Chronic cardiac disease | 4 | 0 | 2 | 0 |
| Chronic lung disease | 5 | 2 | 3 | 0 |
| Cancer | 3 | 1 | 2 | 0 |
| Chronic kidney disease | 3 | 1 | 2 | 0 |
| APACHEII score | 15 (12–23) | 12 (11–13) | 22 (15–29) | – |
| SOFA score | 3 (2–8) | 2 (1–2) | 7 (2–9) | – |
| WBC (×103/µL) | 7 (5.2–9.3) | 7.7 (5.6–8.4) | 6.5 (4.5–11.1) | – |
| Neutrophil (%), median (IQR) | 83.1 (78.4–87.3) | 79.6(73.6–86.9) | 85.3 (81.6–88.1) | – |
| Lymphocyte (%), median (IQR) | 9.3 (7.3–15.2) | 12.0 (8.1–16.0) | 8.2 (5.7–11.7) | – |
| Monocyte (%), median (IQR) | 4.7 (2.7–7.4) | 5.5 (3.1–7.7) | 3.5(2.1–5.9) | – |
| Platelets (×103/µL), median (IQR) | 215 (181–307) | 215 (194 − 334) | 216 (172–311) | – |
| CRP (mg/dL), median (IQR) | 7.0 (3.8–12.1) | 7.0 (2.5–11.2) | 7.0 (4.1–14.0) | – |
| Pneumonia | 26 | 12 | 14 | – |
|
Duration from onset to admission (days), median (IQR) |
9 (7–11) | 10 (8–12) | 9 (6–10) | – |
| ECMO | 5 | 0 | 5 | – |
| In-Hospital Mortality | 3 | 1 | 2 | – |
Complement system is activated in patients with COVID-19
Levels of representative complement activation products, such as C3a, C5a, Ba, and sC5b-9, were measured to check complement activation in patients with COVID-19. C3a and C5a levels were significantly increased in patients with COVID-19 on Day 0 compared with those in HCs (C3a, P < 0.0001; C5a, P < 0.0001). Thus, the complement system was activated, and inflammation increased on admission (Fig. 1a and b). The level of sC5b-9, the final product of the complement cascade4–6, was significantly increased in patients with COVID-19 on Day 0 (P < 0.0001) (Fig. 1c). Similarly, the levels of Ba were significantly increased (P < 0.0001) (Fig. 1d). These findings suggest that the alternative complement pathway was activated in patients with COVID-19. Our results showed that C3a, C5a, Ba, and sC5b-9 levels were significantly elevated in patients with COVID-19 compared with those in HCs. Alterations in soluble and membrane-bound regulators of complement activity occur after trauma, sepsis, and hemorrhagic shock5–7. Hence, levels of factor H and sCD59, well-known representative complement regulators, were measured to investigate dysregulated complement regulators. The factor H and sCD59 levels were elevated on Day 0 in patients with COVID-19 compared with those in HCs (factor H; P = 0.0014, sCD59; P < 0.0001) (Fig. 1e and f).
Fig. 1.
Complement system is activated in patients with COVID-19. (a–f) Complement component 3a (C3a), complement component 5a (C5a), soluble complement C5b-9 (sC5b-9), Ba fragment of complement factor B (Ba), factor H, and soluble CD59 (sCD59) levels significantly increased in patients with COVID-19 on Day 0 compared with those in healthy controls (HCs). Data are presented as median (interquartile range), and the groups were compared using the Mann–Whitney test.
IL-6 and syndecan-1 increase in patients with COVID-19
Some reports showed that IL-6 levels in patients with COVID-19 increased significantly compared with those in HCs and that IL-6 can be a biomarker of severe COVID-1919–21. Plasma IL-6 levels were significantly increased in patients with COVID-19 compared with those in HCs (P < 0.0001) (Fig. 2a).
Fig. 2.
Interleukin-6 (IL-6) and syndecan-1 levels increase in patients with COVID-19. (a and b) Plasma IL-6 and syndecan-1 levels were increased significantly in patients with COVID-19 compared with those in HCs. Data are presented as median (interquartile range), and the groups were compared using the Mann–Whitney test.
The inner surface of the vascular endothelium is coated with vascular endothelial glycocalyx, which is vital in microvascular and endothelial physiology22–24. As a biomarker of endothelial injury, the plasma level of syndecan-1 was measured. Syndecan-1 levels were significantly increased in patients with COVID-19 compared with those in HCs (P < 0.0001) (Fig. 2b).
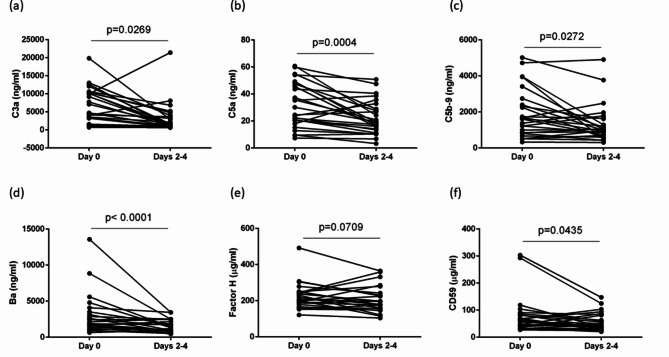
Serial changes in complement activation in patients with COVID-19 patients
Serial changes in complement activation in patients with COVID-19 were investigated. Besides Day 0, complement factors were measured on Days 2–4. The C3a and C5a levels on Days 2–4 were significantly lower than those on Day 0 (C3a, P = 0.0269; C5a, P = 0.0004) (Fig. 3a and b). The sC5b-9 levels on Days 2–4 were significantly lower than those on Day 0 (P = 0.0272) (Fig. 3c). Similarly, Ba levels were significantly reduced on Days 2–4 (P < 0.0001) (Fig. 3d). Our results showed that C3a, C5a, Ba, and sC5b-9 levels on Days 2–4 were significantly decreased in patients with COVID-19 compared with those on Day 0. Regarding complement regulators, although factor H levels showed no significant differences (P = 0.0709) (Fig. 3e), sCD59 levels on Days 2–4 were decreased compared with those on Day 0 (P = 0.0435) (Fig. 3f).
Fig. 3.
Serial changes in complement activation in patients with COVID-19. (a–d) Complement component 3a (C3a), complement component 5a (C5a), soluble complement C5b-9 (sC5b-9), and Ba fragment of complement factor B (Ba) levels on Days 2–4 were significantly decreased compared with those on Day 0. (e) Factor H levels showed no significant differences. (f) Soluble CD59 (sCD59) levels on Days 2–4 were significantly decreased compared with those on Day 0. The groups were compared using the Wilcoxon matched-pairs signed-rank test.
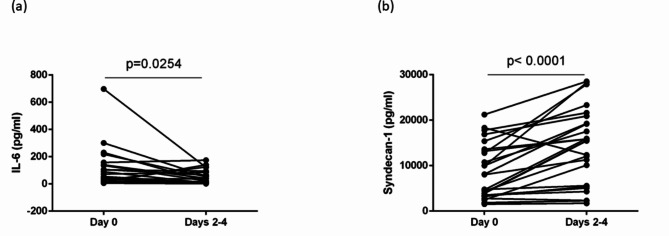
Serial changes in IL-6 and syndecan-1 levels in patients with COVID-19
Plasma IL-6 levels in patients with COVID-19 were significantly decreased on Days 2–4 compared with those in HCs, showing similar serial changes with complement factors (P = 0.0254) (Fig. 4a). In contrast, syndecan-1 levels on Days 2–4 were increased compared with those on Day 0, inconsistent with the changes in complement factors (P < 0.0001) (Fig. 4b).
Fig. 4.
Serial changes in IL-6 and syndecan-1 levels in patients with COVID-19. (a) Plasma IL-6 levels on Days 2–4 were significantly decreased in patients with COVID-19 compared with those in HCs. (b) In contrast, syndecan-1 levels on Days 2–4 were significantly increased compared with those on Day 0. The groups were compared using the Wilcoxon matched-pairs signed-rank test.
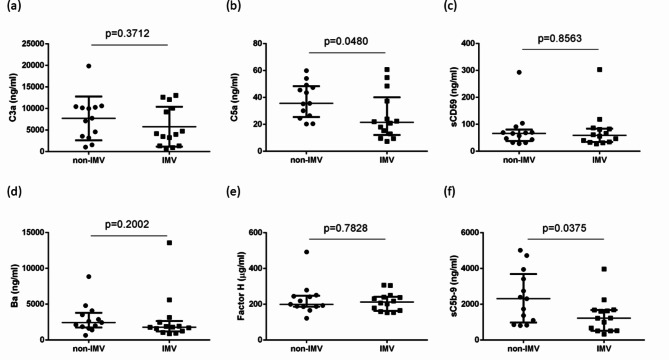
Comparison of complement activation between groups with and without IMV
To determine whether complement activation could be an indicator of IMV, the 27 patients were classified into two groups depending on the indication for IMV on Day 0. C3a levels did not differ significantly between the two groups (P = 0.3712) (Fig. 5a). C5a and sC5b-9 levels were significantly higher in the non-IMV group than in the IMV group (C5a, P = 0.0480; sC5b-9, P = 0.0375) (Fig. 5b and c). Ba levels did not differ significantly between the two groups (P = 0.2002) (Fig. 5d). Factor H and sCD59 levels did not differ between the two groups (factor H; P = 0.7828, sCD59; P = 0.8563) (Fig. 5e and f).
Fig. 5.
Comparison of complement activation between groups with and without IMV. (b and c) Complement component 5a (C5a) and soluble complement C5b-9 (sC5b-9) levels were elevated significantly in the non-IMV group compared with those in the IMV group. (a and d) In contrast, levels of complement component 3a (C3a) and Ba fragment of complement factor B (Ba) showed no significant differences between the two groups. (e and f) Factor H and soluble CD59 (sCD59) levels showed no differences between the two groups. Data are presented as median (interquartile range), and the groups were compared using the Mann–Whitney test.
Comparison of IL-6 and syndecan-1 between groups with and without IMV
IL-6 levels between the non-IMV and IMV groups did not differ significantly (P = 0.3450) (Fig. 6a); however, syndecan-1 levels were elevated in the IMV group compared with those in the non-IMV group (P = 0.0008) (Fig. 6b), indicating that endothelial injury could cause lung injury in patients with severe COVID-19.
Fig. 6.
Comparison of IL-6 and syndecan-1 levels between groups with and without IMV. (a) IL-6 levels between the non-IMV and IMV groups did not differ significantly. (b) Syndecan-1 levels were elevated in the IMV group compared with those in the non-IMV group. Data are presented as median (interquartile range), and the groups were compared using the Mann–Whitney test.
Discussion
COVID-19 induces acute respiratory distress syndrome, considered to result from a maladaptive immune response25–27. The activation of lung-resident immune cells via pattern recognition receptors drives the release of proinflammatory cytokines and the extravasation of blood neutrophils and monocytes into the bronchi25. These cells may disrupt the air–blood barrier by damaging airway epithelial and vascular endothelial cells26,27. Damage to vascular endothelial cells may account for thrombotic microangiopathies25–27. Although the pathophysiological mechanism remains unclear, the involvement of the complement system has been implicated in previous reports8–15,25–31.
In this study, patients with COVID-19 showed complement activation (C3a, C5a, Ba, and sC5b-9) and increased levels of complement inhibitors (soluble factor H and sCD59) on admission to the ICU. These findings are consistent with those of previous reports9–15,28,29. Holter et al. showed that the sC5b-9 and C4d levels were significantly higher in patients with respiratory failure than in those without respiratory failure on admission9. Ma et al. showed that complement activation (sC5b-9) was higher in patients hospitalized with COVID-19 than in those with influenza or other forms of non-COVID acute respiratory failure14. Boussier et al. investigated the RNA levels of 28 complement genes in the circulating whole blood of patients with COVID-19 and HCs and demonstrated complement activation in three complement pathways15. Detsika et al. showed that C3a and sC5b-9 might be prognostic tools for the progression and outcomes of COVID-1928. Hassan et al. showed that the survivors of COVID-19 had significantly higher serum C3 and C4 levels and significantly lower C3a and sC5b-9 levels29. We extensively demonstrated complement activation in patients with COVID-19 by measuring four representative complement factors (C3a, C5a, Ba, and sC5b-9) and two complement inhibitors (soluble factor H and sCD59), which is in more detail than that in other previous reports9–15,28,29 (Fig. 1).
To our knowledge, no previous reports have investigated serial changes in complement activation. In the current study, serial changes in complement factors showed that C3a, C5a, Ba, and sC5b-9 levels declined significantly on Days 2–4 compared with those on Day 0 (Fig. 3a–d). Similarly, sCD59 expression showed the same trend (Fig. 3e). However, factor H levels did not decrease on Days 2–4 (Fig. 3f). Intensive care, such as the administration of steroids, was started on Day 0, which could have contributed to the decline in complement factors in patients with COVID-19. The median duration from onset to admission was 9 days (Table 1). Therefore, complement system activation may have occurred earlier and passed its peak upon admission to the ICU. This was supported by the reduced IL-6 levels on Days 2–4 (Fig. 4a). Further investigations are required to answer this question.
Several studies have reported that complement activation is associated with the need for IVM in patients with COVID-199,14,28,29,31. Ma et al. showed that plasma sC5b-9 and C5a levels were significantly higher in patients with COVID-19 who require IVM than in those not requiring IMV14. Holter et al. showed that patients with respiratory failure had significantly higher sC5b-9 and C4d levels than those without respiratory failure9. Detsika et al. showed that C3a and C5b-9 levels were significantly higher in intubated patients than in non-intubated patients28. Charitos et al. analyzed complement system pathway activities and demonstrated that classical and alternative pathway activity levels increased in patients with COVID-19 who required mechanical ventilation or died during hospitalization compared with those who survived without mechanical ventilation30. Our study showed that C5a and sC5b-9 levels decreased significantly in patients with COVID-19 who required IMV compared with those in non-IMV patients with COVID-19, contradicting previous reports (Fig. 5). In previous reports, samples were obtained on admission to the emergency department, and patients were subsequently classified with or without IMV. However, in our study, patients were divided according to the presence or absence of IMV on the sampling day. Table 1 describes the median duration from onset to admission as 9 days. Gralinski et al. assessed the complement activation during SARS-CoV-2 infection31. C3 activation products (C3a, C3b, iC3b, C3c, and C3dg) were observed in the lungs as early as 1 day after SARS-CoV infection in a mouse model31. Therefore, complement activation may have already reached and passed its peak when our patients were admitted to the ICU after infection. Conversely, the complement factors are consumed when patients are admitted to the ICU.
African American/Black and Hispanic populations experience disproportionately high rates of SARS-CoV-2 infection and COVID-19-related mortality32. Although differences in healthcare access and exposure risk may drive higher infection and mortality rates, specific immune disparities could contribute to the greater risk of adverse outcomes in certain ethnicities32,33. Some authors have reported evidence of inherent differences in the immune system that may increase the predisposition of Black Americans to severe cytokine storms32,33. Therefore, ethnicity should be considered when discussing the immune systems of patients with COVID-19. Several studies have reported an association between complement activation and the need for IVM in patients with COVID-199,14,28,29,31. Ma et al. showed higher complement activation in the IMV group than in the non-IMV group; however, 79.1% of the patients included in their study were Black or African Americans14. These previous reports were from the United States and Europe. To our knowledge, this is the first such report in Japan. This could be one possibility that accounts for the discrepancy between our results and those of previous studies.
The complement system can be activated by classical, lectin, or alternative pathways4–7. Although the complement system is activated in patients with COVID-19, the underlying mechanism, which is essential as a treatment target, is not understood. Gao et al. showed that the N protein of coronaviruses triggers MASP2-mediated complement activation and drives disease severity via the lectin pathway34. Furthermore, Ali confirmed that the N protein of SARS-CoV-2 binds directly to the lectin pathway effector enzyme MASP-2 and activates the complement system35. Inhibition of the lectin pathway using a monoclonal antibody against MASP-2 effectively blocks lectin pathway-mediated complement activation35. Defendi et al. demonstrated that alternative and lectin pathways may be biomarkers of disease severity in patients with COVID-1936. Boussier et al. showed that the classical pathway is activated in all patients with COVID-19, whereas the hyperactivation of lectin and alternative pathways is associated with disease severity15. Our study demonstrated that Ba levels were elevated in patients with COVID-19 (Fig. 1d), suggesting the activation of an alternative pathway. However, no significant differences were observed between the non-IMV and IMV groups (Fig. 5d). Further investigations are required in a larger cohort of patients with SARS-CoV-2 infection to help rationalize therapeutic choices.
Endotheliopathy is a characteristic feature of COVID-192. The surface of the normal endothelium is covered by the endothelial glycocalyx, which is crucial in microvascular and endothelial physiology22–24,37. Syndecan-1 is the core proteo-glycan in the endothelial glycocalyx22–24,37. Astapenko et al. reported that syndecan-1 levels were significantly higher during ICU stay in the COVID-19 group than in the non-COVID group38. In our study, syndecan-1 levels were higher in patients with COVID-19 and increased on Days 2–4, different from the changes in complement factors. Notably, syndecan-1 levels were significantly elevated in the IMV group compared with those in the non-IMV group. Although mechanical ventilation has to be considered one of the causes of endothelial damage39, syndecan-1 could be a biomarker of COVID-19 severity.
A limitation of our study was the small patient population and the difference in age between the HC and patient groups. Our results showed that the age of patients with COVID-19 was higher than that of HCs, indicating the possibility of age-related variations in complement activation. Further studies with more patients having COVID-19 are required to ascertain the role of complement system activation.
Conclusions
Considering that complement activation is a cause of respiratory failure in patients with COVID-19, the complement system may be a promising target for treating patients with COVID-19. Vilobelimab, an anti-C5a monoclonal antibody, reportedly improves the survival of invasive mechanically ventilated patients with COVID-19 and significantly decreases mortality40. Further studies are required to establish valuable biomarkers and targets for treating patients with COVID-19.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- COVID-19
Corona virus disease-2019
- C3a
complement component 3a, C5a, complement component 5a
- sC5b-9
soluble complement C5b-9
- IMV
invasive mechanical ventilation
- ICU
intensive care unit
- HC
healthy control
- CSS
cytokine storm syndrome
- ECMO
extracorporeal membrane oxygenation
- APACHE
Acute Physiology and Chronic Health Evaluation
- SOFA
Sequential Organ Failure Assessment
- WBC
white blood cell
- CRP
C-reactive protein
Author contributions
K.S.; formal analysis and investigation. K.S.; conceptualization, formal analysis, investigation, writing-original draft, supervision writing-review and editing, and funding acquisition. Y.M.; investigation. Y.N.; investigation. T. I.; investigation. Y.K.; investigation. Y.K.; formal analysis and experiments. A.Y.; formal analysis and experiments. K.I.; supervision and conceptualization. K.O.; supervision and conceptualization, and H.T.; supervision, conceptualization, and funding acquisition.
Funding
This study was supported in part by JSPS KAKENHI [Grant Number 19H03764 and 23H03013].
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vabret, N. et al. Immunology of COVID-19: current state of the science. Immunity. 52, 910–941 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J. & Prescott, H. C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 324, 782–793 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Mudd, P. A. et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci. Adv. 6, eabe3024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fattahi, F., Zetoune, F. S. & Ward, P. A. Complement as a major inducer of harmful events in infectious sepsis. Shock. 54, 595–605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rognes, I. N. et al. Increased complement activation 3 to 6 h after trauma is a predictor of prolonged mechanical ventilation and multiple organ dysfunction syndrome: a prospective observational study. Mol. Med. 27, 35 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burk, A. M. et al. Early complementopathy after multiple injuries in humans. Shock. 37, 348–354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittirsch, D., Redl, H. & Huber-Lang, M. Role of complement in multiorgan failure. Clin. Dev. Immunol. 2012, 962927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Java, A. et al. The complement system in COVID-19: friend and foe. JCI Insight. 5, e140711 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holter, J. C. et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. U S A. 117, 25018–25025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skendros, P. et al. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 130, 6151–6157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magro, C. et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 220, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diao, B. et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 12, 2506 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramlall, V. et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 26, 1609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma, L. et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci. Immunol. 6, eabh2259 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boussier, J. et al. Severe COVID-19 is associated with hyperactivation of the alternative complement pathway. J. Allergy Clin. Immunol. 149, 550–556e2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey, K. et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann. Intern. Med. 174, 362–373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: a severity of disease classification system. Crit. Care Med. 13, 818–829 (1985). [PubMed] [Google Scholar]
- 18.Jones, A. E., Trzeciak, S. & Kline, J. A. The sequential organ failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 37, 1649–1654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, X. et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 71, 1937–1942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, J. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 55, 102763 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu, T. et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol. Med. 12, e12421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chelazzi, C., Villa, G., Mancinelli, P., De Gaudio, A. R. & Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care. 19, 26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frati-Munari, A. C. Medical significance of endothelial glycocalyx. Arch. Cardiol. Mex. 83, 303–312 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Woodcock, T. E. & Woodcock, T. M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 108, 384–394 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Risitano, A. M. et al. Complement as a target in COVID-19. Nat. Rev. Immunol. 20, 343–344 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson, B. et al. How the innate immune system of the blood contributes to systemic pathology in COVID-19-induced ARDS and provides potential targets for treatment. Front. Immunol. 13, 840137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo, M. W., Kemper, C. & Woodruff, T. M. COVID-19: complement, coagulation, and collateral damage. J. Immunol. 205, 1488–1495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detsika, M. G. et al. C3a and C5b-9 differentially predict COVID-19 progression and outcome. Life (Basel). 12, 1335 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan, A. E. et al. Prognostic significance of complement factors in severely ill patients with COVID-19. J. Investig Med. 70, 1466–1471 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Charitos, P. et al. Functional activity of the complement system in hospitalized COVID-19 patients: a prospective cohort study. Front. Immunol. 12, 765330 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gralinski, L. E. et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 9, e01753–e01718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tal, Y., Adini, A., Eran, A. & Adini, I. Racial disparity in Covid-19 mortality rates - a plausible explanation. Clin. Immunol. 217, 108481 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips, N., Park, I. W., Robinson, J. R. & Jones, H. P. The perfect storm: COVID-19 health disparities in US blacks. J. Racial Ethn. Health Disparities. 8, 1153–1160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao, T. et al. Highly pathogenic coronavirus N protein aggravates inflammation by MASP-2-mediated lectin complement pathway overactivation. Signal. Transduct. Target. Ther. 7, 318 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali, Y. M. et al. Lectin pathway mediates complement activation by SARS-CoV-2 proteins. Front. Immunol. 12, 714511 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Defendi, F. et al. Complement alternative and mannose-binding lectin pathway activation is associated with COVID-19 mortality. Front. Immunol. 12, 742446 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada, H., Yoshida, S., Hara, A., Ogura, S. & Tomita, H. Vascular endothelial injury exacerbates coronavirus disease 2019: the role of endothelial glycocalyx protection. Microcirculation. 28, e12654 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Astapenko, D. et al. Endothelial glycocalyx damage in patients with severe COVID-19 on mechanical ventilation – a prospective observational pilot study. Clin. Hemorheol Microcirc. 81, 205–219 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Pelosi, P. & Rocco, P. R. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med. 34, 631–639 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Vlaar, A. P. J. et al. Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 10, 1137–1146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.