Abstract
Tea gray blight disease is a significant threat to the tea industry. In this study, a biological activity approach was utilized to investigate the efficacy of green fungicides from Magnolia officinalis stem bark against Neopestalotiopsis ellipsospora. The active compounds were isolated and purified, and their structures were elucidated. In vitro and in vivo activity screenings revealed that the n-hexane extract, which contained magnolol and honokiol, exhibited strong activity against N. ellipsospora, showing complete inhibition at 100 mg/L. The EC50 values of magnolol and honokiol were 5.11 and 6.09 mg/L, respectively. Mechanistically, magnolol was found to disrupt N. ellipsospora invasion by damaging the cell membrane, increasing permeability, and causing leakage of intracellular substances. Transcriptome analysis revealed that magnolol treatment downregulates membrane-related genes and leads to the enrichment of lipid metabolism pathway genes. This study revealed that magnolol inhibits N. ellipsospora growth by affecting lipid metabolism and compromising cell membrane integrity.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75310-7.
Keywords: Magnolia Officinalis, Magnolol, Antifungal activity, Neopestalotiopsis Ellipsospora, Transcriptome analysis, Membrane lipid metabolism
Subject terms: Drug discovery, Microbiology
China, a leading tea-producing nation, recognizes the crucial role of the tea industry in its economic development1. However, ensuring the safety of tea leaves is essential for maintaining industry growth. Tea plants are typically cultivated in warm and humid environments, and such environments are also favorable for pathogen growth2. Consequently, various diseases can affect tea trees, leading to significant damage3–5. One primary leaf disease6,7 is tea gray blight disease, which is characterized by irregular ring-shaped spots on infected leaves8,9. In severe cases, this disease can cause extensive leaf loss, resulting in substantial economic tea production losses6.
Chemical control10,11 remains the primary method for preventing and managing tea gray blight disease. For example, some of the most commonly used pesticides on the market include thiophanate-methyl, difenoconazole, benomyl, and polyantimycin. Nonetheless, the distinctive features of tea production and consumption12 have led to concerns about pesticide residues. Consequently, there is an increasing interest in researching environmentally friendly pesticides13,14 that can effectively control tea gray blight disease. Natural products15, with their complex structures16, diverse biological activities17, low toxicity18,19, and ease of degradation20, hold promise in agricultural disease prevention and treatment. Magnolia officinalis21, which is traditionally used for treating gastrointestinal ailments22, contains magnolol23 as its active ingredient, which is known for its antioxidant24, anti-inflammatory, and antiulcer properties25. Studies have shown that magnolol and honokiol26, isomers that are the main active components of M. officinalis, exhibit potent antimicrobial properties against human pathogens27,28 such as Candida albicans, Helicobacter pylori, and Staphylococcus aureus, making these compounds valuable in clinical settings. However, limited information is available regarding their antifungal activity against tea gray blight.
The aim of this study was to evaluate the inhibitory activity of an ethanol extract of M. officinalis stem bark against Neopestalotiopsis ellipsospora. Bioassay-guided fractionation method can be utilized to identify the active components in an extract and subsequently isolate, purify, and characterize them. Additionally, the effects of magnolol on N. ellipsospora mycelial growth, pathogenicity, and mycelial accumulation were assessed both in vitro and in vivo. Moreover, the effects of magnolol on the N. ellipsospora cell wall and cell membrane integrity were determined. Furthermore, the transcriptomic response of N. ellipsospora to magnolol was investigated to explore potential molecular mechanisms and related genes in key pathways. The primary objective of these studies is to expand the application range of magnolol and enhance our understanding of the underlying mechanisms, thereby facilitating the development and synthesis of novel compounds with antifungal properties.
Results
M. officinalis stem bark extract
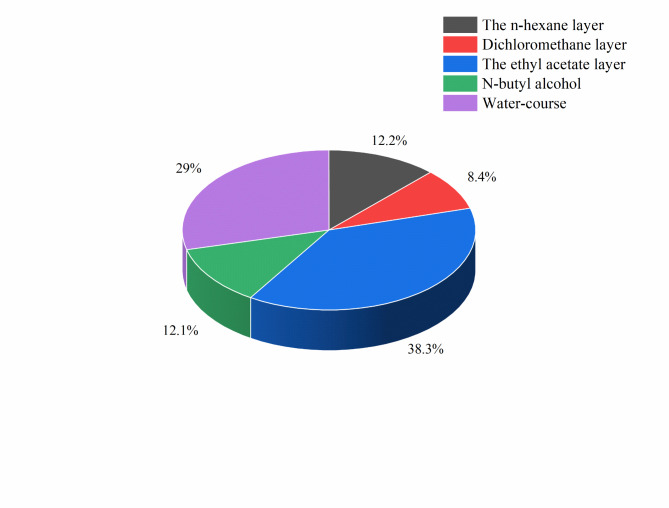
After pretreatment, a black semisolid mass was obtained from the 65% ethanolic extract, weighing 3.3241 g. The proportions of the extract that separated into different extraction layers are shown in Fig. 1. The extract showed good solubility in ethyl acetate, and this layer had the most extracted material.
Fig. 1.
Proportions of the 65% ethanolic extract that separated into different extraction layers.
Inhibition of N. ellipsospora by the M. officinalis stem bark extract
The effects of the different M. officinalis extract layers on the growth of N. ellipsospora mycelia were investigated, and the findings are summarized in Fig. 2. After 120 h of treatment at a concentration of 100 mg/L, the crude (65% ethanol) extract of M. officinalis exhibited an inhibition rate of 57.58%. Notably, the n-hexane extract displayed the highest antifungal activity, achieving a growth inhibition rate of 100%, indicating its superior effectiveness in combating tea gray blight disease. Conversely, the extracts from the water layer and n-butanol layer exhibited limited antifungal effects, suggesting that the components of the M. officinalis stem bark extract with potent inhibitory effects on N. ellipsospora were primarily extracted by solvents with low polarity.
Fig. 2.
Inhibition of N. ellipsospora by the M. officinalis extracts.
Identification of the active constituents in the M. officinalis bark extract
The liquid phase diagram in Fig. 3 depicts the presence of two main compounds in the n-hexane layer, with retention times for compounds 1 and 2 of 7.633 and 9.716 min, respectively.
Fig. 3.
Liquid chromatograph of the crude hexane layer.
Two main peaks were separated and purified to obtain compounds 1 and 2, yielding 98 mg and 86 mg, respectively. Compounds 1 and 2 were recrystallized from different solvents. Compound 1 formed needle-like crystals when it was recrystallized from methanol, whereas compound 2 formed tablet-like crystals when it was recrystallized from hexane. The crystals were characterized by nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS). The NMR spectra and HRMS data for compounds 1 and 2 are detailed below.
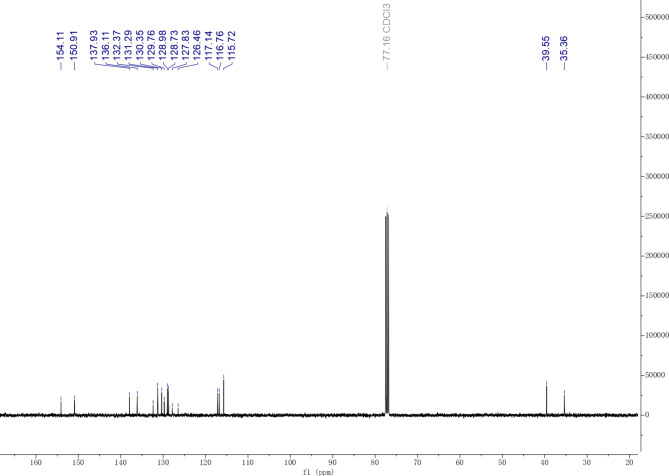
Compound 1:1H NMR (400 MHz, CDCl3) δ 7.25–7.18 (m, 2 H), 7.08–6.99 (m, 2 H), 6.91 (dd, J = 7.9, 5.6 Hz, 2 H), 6.21–5.81 (m, 2 H), 5.27–5.16 (m, 2 H), 5.12–5.04 (m, 4 H), 3.47 (d, J = 6.5 Hz, 2 H), 3.35 (d, J = 6.7 Hz, 2 H)13. C NMR (101 MHz, CDCl3) δ 154.11, 150.91, 137.93, 136.11, 132.37, 131.29, 130.35, 129.76, 128.98, 128.73, 127.83, 126.46, 117.14, 116.76, 115.72, 39.55, 35.36. HRMS (ESI): calculated for C18H18O2 ([M-H]−) 265.12340, found: 265.12432.
Compound 2:1H NMR (400 MHz, CDCl3) δ 7.18–7.04 (m, 4 H), 6.99–6.92 (m, 2 H), 6.03–5.89 (m, 2 H), 5.15–5.03 (m, 4 H), 3.37 (d, J = 6.7 Hz, 4 H)13. C NMR (101 MHz, CDCl3) δ 151.29, 137.64, 133.36, 131.34, 130.16, 123.75, 116.79, 115.99, 39.49. HRMS (ESI): calculated for C18H18O2 ([M -H]−) 265.12340, found: 265.12432.
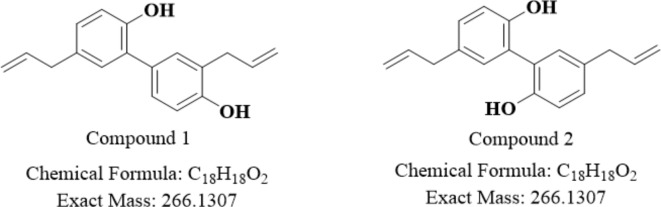
The carbon NMR spectrum of compound 2 displayed 9 peaks, despite the HRMS spectrum showing 18 carbon atoms, suggesting symmetry. Both compounds 1 and 2 had a molecular mass of 265.1243, confirming that they are isomers. The NMR and HRMS data aligned with that in the literature. Thus, compound 1 was identified as honokiol, and compound 2 was identified as magnolol, as shown in Fig. 4.
Fig. 4.
Structural formulae of the main active components involved in fungal inhibition.
Evaluation of the potency of Magnolol and Honokiol against N. ellipsospora
On the basis of the preliminary screening results, polyantimycin was selected as the control agent to further test the activities of magnolol and honokiol against N. ellipsospora. Table 1 shows that the EC50 values of honokiol, magnolol, and polyantimycin against N. ellipsospora were 6.07 mg/L, 5.11 mg/L, and 322.42 mg/L, respectively. Both magnolol and honokiol demonstrated significantly greater inhibitory activity against the pathogen causing tea gray blight disease than did the control agent polyantimycin.
Table 1.
Toxicity of magnolol and honokiol to N. Ellipsospora.
| Tested substances | Regression equation | EC50 (mg/L) | R2 |
|---|---|---|---|
| Honokiol | y = 2.6306 + 3.0267× | 6.07 ± 0.71 | 0.990 9 |
| Magnolol | y = 2.5470 + 3.4640× | 5.11 ± 0.57 | 0.975 2 |
| Chlorothalonil | y = 0.5118 + 1.7893× | 322.42 ± 58.59 | 0.981 6 |
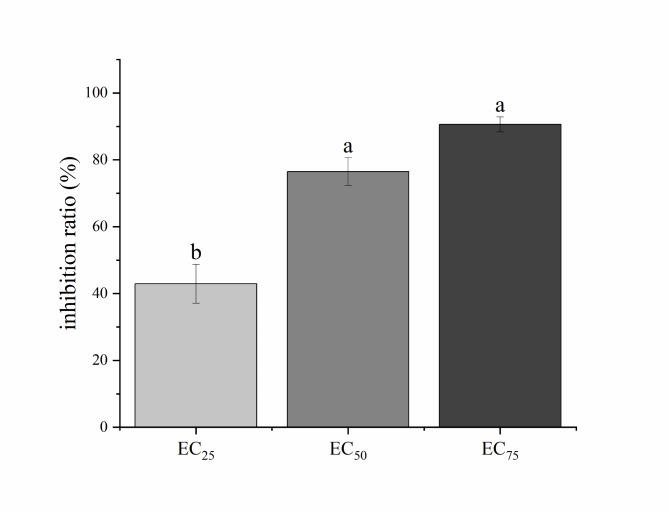
Indoor control effect of magnolol on tea gray blight disease
The results of the semi-isolated leaf experiments with different concentrations of N. ellipsospora are shown in Figs. 5 and 6, reveal a notable decrease in the disease affected area on magnolol-treated leaves compared with the control group. The inhibition rates at the magnolol EC25, EC50, and EC75 were 42.93%, 76.46%, and 90.61%, respectively, indicating the great ability of magnolol to hinder the invasion of Fuding Dabai tea leaves by N. ellipsospora and demonstrating its promising protective effects.
Fig. 5.
Control of N. ellipsospora by magnolol indoors. CK, 0 mg/L; EC25, 3.26 mg/L; EC50, 5.11 mg/L; and EC75, 8.00 mg/L.
Fig. 6.
Effects of different magnolol concentrations on N. ellipsospora. CK, 0 mg/L; EC25, 3.26 mg/L; EC50, 5.11 mg/L; and EC75, 8.00 mg/L.
Effects of Magnolol on the amount of N. ellipsospora mycelia
As shown in Fig. 7, following 120 h of cultivation, the mycelial mass in the control group was 623.56 mg. In contrast, the mycelial masses in the EC25 (3.26 mg/L), EC50 (5.11 mg/L), and EC75 (8.00 mg/L) magnolol treatment groups were 372.26 mg, 280.13 mg, and 204.13 mg, respectively. These values were significantly different from those of the control group, with reductions of 251.30 mg, 343.43 mg, and 419.43 mg, respectively. These results suggest that magnolol has a notable inhibitory effect on the growth of N. ellipsospora mycelia and leads to decreased mycelial accumulation.
Fig. 7.
Effects of different concentrations of magnolol on the amount of N. ellipsospora mycelia. There were significant differences between each magnolol group and the control group. CK, 0 mg/L; EC25, 3.26 mg/L; EC50, 5.11 mg/L; and EC75, 8.00 mg/L.
Effects of Magnolol on N. ellipsospora mycelial morphology
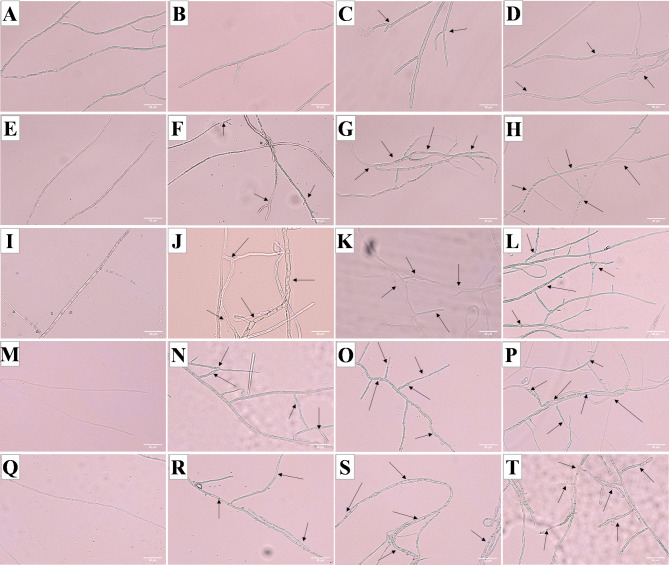
This study investigated the effects of varying concentrations of magnolol on the mycelial morphology of N. ellipsospora over different durations. The results depicted in Fig. 8 indicate that the mycelial morphology in the control group remained relatively stable and healthy across various time points. In contrast, the mycelia in the magnolol treatment groups exhibited progressively greater damage with increasing magnolol concentrations and extended exposure durations. The affected mycelia displayed abnormal protrusions, thickening, thinning, twisting, and a reduction in internal contents, suggesting that magnolol disrupted the integrity of the cell membrane.
Fig. 8.
Morphological changes in the mycelia under different treatments. Twenty-four hours: A (CK, 0 mg/L), B (EC25, 3.26 mg/L), C (EC50, 5.11 mg/L), and D (EC75, 8.00 mg/L); 48 h: E (CK, 0 mg/L), F (EC25, 3.26 mg/L), G (EC50, 5.11 mg/L), and H (EC75, 8.00 mg/L); 72 h: I (CK, 0 mg/L), J (EC25, 3.26 mg/L), K (EC50, 5.11 mg/L), and L (EC75, 8.00 mg/L); 96 h: M (CK, 0 mg/L), N (EC25, 3.26 mg/L), O (EC50, 5.11 mg/L), and P (EC75, 8.00 mg/L); and 120 h: Q (CK, 0 mg/L), R (EC25, 3.26 mg/L), S (EC50, 5.11 mg/L), and T (EC75, 8.00 mg/L). The scale bar is 30 μm.
CFW staining
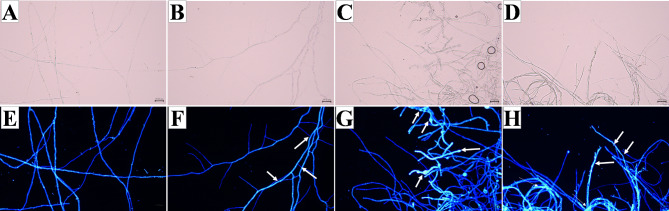
CFW can bind to the cell wall components chitin and glucan, resulting in fluorescence emission. As shown in Fig. 9, compared to the control group (Fig. 9E), the mycelial cells of N. ellipsospora in the treatment group exhibited high sensitivity to CFW binding. Moreover, as the concentration of magnolol increased, the fluorescence intensity also increased. Interestingly, magnolol was found to cause swelling and morphological changes in the mycelia. CFW staining indicated that the fluorescence intensity was greater in the swollen parts of the mycelia under dark field conditions, suggesting that magnolol may induce abnormal mycelial growth by thickening the cell wall. These findings indicate that the cell wall of N. ellipsospora is a target of magnolol.
Fig. 9.
Fluorescence and nonfluorescence effects of CFW on mycelia after treatment with magnolol for 72 h. (A, E): CK, 0 mg/L; (B, F): EC25, 3.26 mg/L; (C, G): EC50, 5.11 mg/L; and (D, F): EC75, 8.00 mg/L. The scale bar is 30 μm.
PI fluorescence
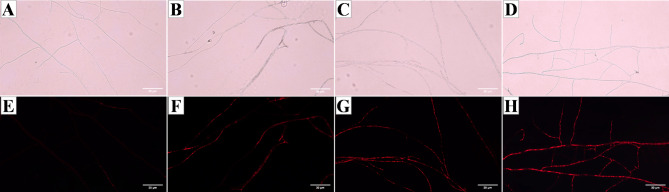
When the cell membrane is damaged pl dye can bind to nucleic acids to emit red fluorescence, so the intensity of the fluorescence can reflect the degree of damage to the cell membrane. Figure 10 illustrates PI fluorescence under identical exposure conditions. Compared with the control group, all the treatment groups presented noticeable red fluorescence, with the intensity increasing with increasing magnolol concentration. These findings suggest that magnolol has the ability to disrupt the integrity of the N. ellipsospora cell membrane.
Fig. 10.
Fluorescence and nonfluorescence effects of PI on mycelia after treatment with magnolol for 72 h. (A, E): CK, 0 mg/L; (B, F): EC25, 3.26 mg/L; (C, G): EC50, 5.11 mg/L; and (D, F): EC75, 8.00 mg/L. The scale bar is 30 μm.
Effect of Magnolol on N. ellipsospora mycelial cell membrane permeability
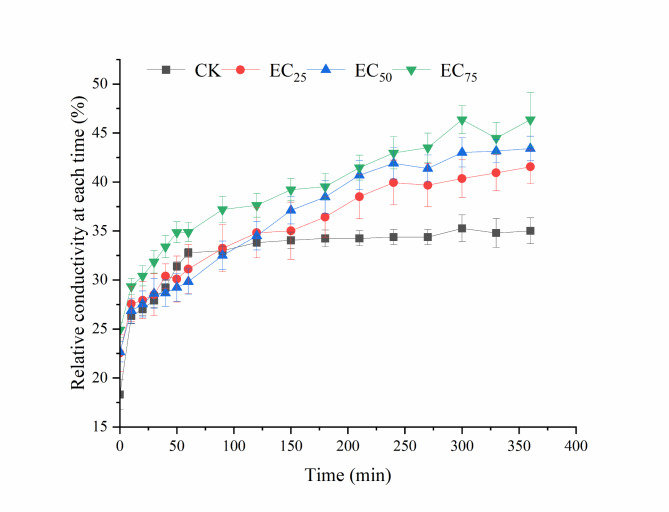
Relative conductivity is a crucial metric for assessing cell membrane permeability. As shown in Fig. 11, from 0 to 600 min, the relative conductivity of mycelia in the various treatment groups increased rapidly, after which different samples showed different trends. After 60 min, the mycelia in the control group reached a relative conductivity, in contrast with those treated with magnolol, whose relative conductivity continued to increase. Moreover, as the concentration of magnolol increased, so did the relative conductivity. By the 360-minute mark, the relative conductivities in the EC25, EC50, and EC75 treatment groups were 41.55%, 43.41%, and 46.37%, respectively, whereas that of the control group was 35.01%. Notable variations in relative conductivities were detected among the EC50, EC75, and control groups, suggesting that magnolol caused harm to the cell membrane of N. ellipsospora.
Fig. 11.
Effect of magnolol on the N. ellipsospora mycelial cell membrane permeability. CK, 0 mg/L; EC25, 3.26 mg/L; EC50, 5.11 mg/L; and EC75, 8.00 mg/L.
Effects of Magnolol on extracellular nucleic acids and proteins
Following cell membrane damage, alterations in the intracellular contents are likely. To explore the potential link between cell membrane damage and the release of intracellular contents, we assessed the levels of extracellular nucleic acids and proteins. The data presented in Fig. 12 indicate a significant increase in the extracellular nucleic acid and protein contents within the initial 8 h, which was followed by a decrease at the 24 h mark. Further examination revealed that higher concentrations of magnolol correlated with elevated extracellular nucleic acid and protein levels. Specifically, the EC75 group presented higher levels than did the control group at different time points, while the disparities between the EC25 and EC50 groups and the control group were relatively minor, although significant variations were observed at 4 h and 24 h. Overall, the analysis suggested that magnolol treatment can induce the release of nucleic acids and proteins from N. ellipsospora by compromising the integrity of the cell membrane.
Fig. 12.
Effects of magnolol on the contents of extracellular nucleic acids (OD260) and proteins (OD280) from the N. ellipsospora mycelia.
SEM
Under a scanning electron microscope (Fig. 13), the N. ellipsospora mycelial thickness in the control group appeared uniform, with a smooth and intact surface and clear separation. In contrast, the N. ellipsospora mycelial surfaces in the magnolol group displayed signs of slight atrophy, deformation, abnormal enlargement, wrinkling, indentations, uneven thickness, and a flattened appearance. These findings suggest that magnolol adversely affects the morphology and structural integrity of N. ellipsospora mycelia.
Fig. 13.
Morphology of the mycelia after 72 h of treatment with different concentrations of magnolol. (A, C): CK, 0 mg/L; and (B, D): EC50, 5.11 mg/L.
TEM
The ultrastructure of N. ellipsospora was examined by TEM to investigate the effects of various treatments (Fig. 14). In the control group, the N. ellipsospora mycelial cells displayed a regular morphology, a uniform texture, and consistent cell wall thickness. These cells were closely connected to the cell membrane, and the organelles were evenly distributed in the cytoplasm, resulting in a clear and complete structure. However, in the magnolol group, the cells exhibited deformities, with the cytoplasmic wall being separated and extravasated. The intracellular structures appeared blurred, and the thickness of the cell wall was inconsistent. Furthermore, the cell membrane had collapsed and undergone lysis. These results suggest that magnolol could be specifically affecting the mycelial membrane of N. ellipsospora.
Fig. 14.
Ultrastructure of N. ellipsospora mycelial cells after 72 h of treatment with different concentrations of magnolol. (A, C): CK, 0 mg/L; and (B, D): EC50, 5.11 mg/L.
RNA-seq and DEG analyses
Summary of the sequencing data quality
Transcriptome sequencing of the control and magnolol treatment groups (each containing 3 biological replicates) yielded an average of 40.8 million reads per sample (Table S1). The original reads in both groups presented a Q30% exceeding 93%. After trimming, approximately 37–43 million clean reads were obtained. A total of 12,873 contigs, with an average size of 1609 bp and an N50 of 1609 bp, were assembled from the clean short reads. These findings underscore the high reliability of the RNA-Seq data, supporting its suitability for further DEG analysis.
Analysis of the significantly DEGs
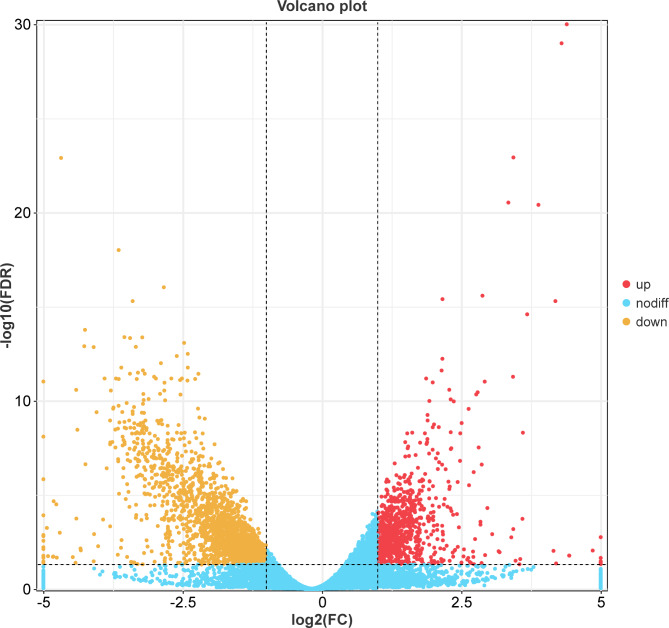
The criteria for selecting DEGs in this experiment were p < 1 and |log2FoldChange|>1. A volcano plot of the DEGs (Fig. 15) revealed that 1923 genes were upregulated and 852 were downregulated in response to magnolol.
Fig. 15.
Volcano plot indicating the gene expression levels in the control and treatment groups.
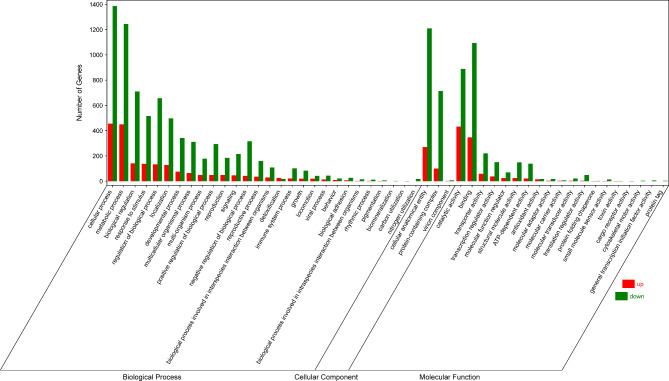
DEG annotation was performed with the GO database (Fig. 16). The majority of these genes are associated with various biological processes. Specifically, there was enrichment of genes in cellular processes, metabolic processes, biological regulation, regulation of biological processes, response to stimulus, localization, developmental processes, multicellular organismal processes, and negative regulation of biological processes. With respect to molecular function, binding, catalytic activity, transporter activity, transcription regulatory activity, and ATP-dependent activity were enriched in these genes. In terms of cellular components, organelles, membrane-bound organelles, the cytoplasm, the nucleus, the membrane, the membrane-enclosed lumen, and nonmembrane-bound organelles, including components related to the cell membrane, were enriched. These findings suggest that magnolol may impact the functions of the cell membrane in N. ellipsospora.
Fig. 16.
Plot of the GO functional annotation of the DEGs.
Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis of the DEGs
To further investigate the biological functions of the DEGs, we utilized pathway information from the KEGG database (Fig. 17) to identify key biochemical metabolic and signal transduction pathways associated with the DEGs. There was enrichment of the N. ellipsospora DEGs following magnolol treatment predominantly in pathways related to amino acid metabolism, carbohydrate metabolism, cofactor and vitamin metabolism, lipid metabolism, other amino acid metabolism, and energy metabolism.
Fig. 17.
Plot of the KEGG pathway enrichment of the DEGs.
Analysis of the DEGs related to cell membrane pathways
The physiological, biochemical, and transcriptomic experiments have suggested that magnolol primarily targets the cell membrane of N. ellipsospora, while affecting both the cell wall and the membrane. To validate these findings, the DEGs associated with the cell membrane have been identified. Among these 53 DEGs, 17 are upregulated, and 36 are downregulated. KEGG enrichment analysis has highlighted enrichment in pathways such as fatty acid metabolism, glycerophospholipid metabolism, glycerol metabolism, unsaturated fatty acid biosynthesis, and the MAPK signaling pathway (Fig. 18). Phospholipids are important components of cell membranes, so we have paid special attention to the potential effects of magnolol on phospholipid biosynthesis in N. ellipsospora. The results showed that during the phospholipid biosynthesis process in N. ellipsospora, the expression of genes involved in sn-Glycerol-3P29 synthesis (NTE1 and GDE1) and Acyl-CoA synthase genes (FUM18 and FUM17) is inhibited by magnolol. These findings suggest that magnolol hinders the synthesis of phospholipids in N. ellipsospora cell membranes, interferes with the transmembrane transport of membrane lipids and disrupts the integrity of cell membranes, thereby achieving an antifungal effects.
Fig. 19.
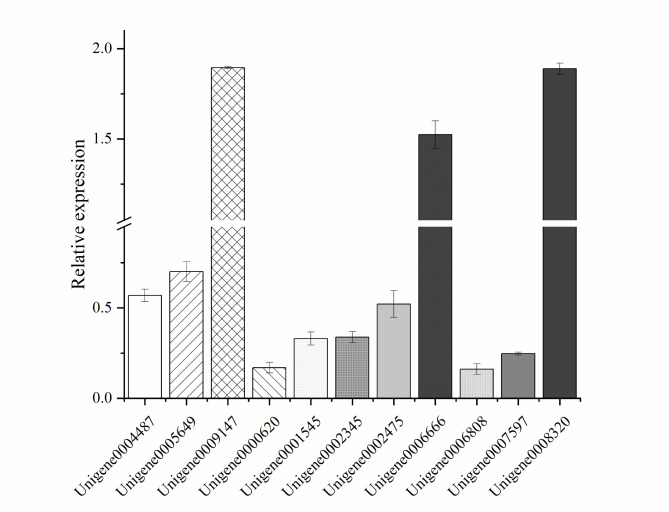
Verification of the DEGs. Genes with expression levels lower than 1 were considered downregulated, whereas those with levels greater than 1 were considered upregulated.
Fig. 18.
Analysis of DEGs related to cell membrane pathways. (A) Differential gene upregulation and downregulation expression profiles. (B) Differential gene KEGG pathway enrichment plot.
Discussion
Tea gray blight is a common fungal disease that affects tea trees, leading to notable yield losses ranging from 10 to 20% in tea gardens30,31. Currently, the use of chemical fungicides is the main method for managing this disease32. However, the use of these chemicals has raised concerns regarding environmental pollution, drug resistance, and food safety33,34. As a result, there is a growing interest in natural products that offer safe, effective, and environmentally-friendly alternatives.
Magnolia officinalis35,36 is a traditional Chinese medicine. In Sun’s study37, the root extract of Magnolia officinalis inhibited the growth of methicillin-resistant Staphylococcus aureus by disrupting its cell walls and cell membranes and cutting off the nutrient supply. In addition, its extract showed good bacteriostatic activity against food-borne pathogens such as Listeria monocytogenes, Enterococcus faecalis, Escherichia coli, Salmonella typhimurium, Staphylococcus aureus, and Bacillus anthracis38. The results of the study showed that Magnolia officinalis has a good prospect for developing bacteriostatic agents. In this study, different polar solvents were selected to extract the stem bark of Magnolia officinalis, and their antibacterial activity was investigated. It was found that the n-hexane extract exhibited the highest activity, with an inhibition rate of 100% when the concentration was 100 mg/L. To further explore the inhibitory effects of the n-hexane extract, the two compounds with the highest content were isolated and purified. Based on NMR and HRMS data, these two compounds were identified as honokiol and magnolol. This study indicates that honokiol and magnolol, as the main bioactive components of Magnolia officinalis, demonstrate higher activity, consistent with the findings of Jiang et al.39. It is well known that honokiol and magnolol possess a wide range of pharmacological effects40, including antibacterial, anti-inflammatory, antitumor, muscle-relaxant, cholesterol-lowering, and anti-aging properties. They also exhibit good inhibitory activity against plant pathogenic fungi such as Phytophthora sojae41 and Botrytis cinerea42. However, research on the use of natural products to treat tea gray mold disease is relatively limited. This study determined the EC50 values of these two compounds against N. ellipsospora to be 5.11 mg/L and 6.07 mg/L, respectively, which are significantly better than those of the positive control agents. Given the strong activity and safety43 of the natural products magnolol and honokiol, they are expected to become environmentally-friendly compounds for managing diseases in tea plantations, reducing reliance on chemical pesticides and potentially improving the quality and safety of tea.
In recent years, many studies have investigated the mechanisms of magnolol’s action on fungi. For example, magnolol can damage the mitochondria of pathogens44, disrupting respiratory metabolism. Magnolol has also been shown to inhibit biofilm formation in Candida albicans45. Additionally, magnolol may interfere with the normal mitochondrial function and lipid metabolism of fungi46,47. In our study, magnolol inhibits the development of Tea gray blight disease on tea leaves by reducing mycelial accumulation and destroying mycelial morphology, leading to abnormal changes in these cells. SEM further confirms this result. The inhibitory mechanisms of plant extracts against fungi typically include the destruction of the cell wall48–50 and cell membrane51–53. In this study, the relative electrical conductivity of the mycelia treated with magnolol was higher than that of the control group, which indicates the leakage of intracellular proteins and nucleic acids. This conclusion was supported by PI staining and TEM. It suggests that magnolol compromised the integrity of the cell membrane of N. ellipsospora. The integrity of the cell wall was assessed using CFW staining. Compared to the control group, the mycelia treated with magnolol exhibited mutations and distortions, making them abnormally sensitive to CFW. This indicates that the stability of the cell wall was affected.
Subsequently, transcriptome analysis revealed that magnolol mainly acts on the cell membrane of N. ellipsospora, but has limited effects on its cell membrane. The cell wall and cell membrane play a crucial role in providing structural support, maintaining the internal stability of cell activities, and preserving cell integrity54,55, and these functions are essential for the normal growth of cells. Therefore, these components have always been important targets for antifungal drugs56,57. The cell membrane58 is mainly composed of lipids such as glycerophospholipids, sphingolipids, and sterols, which form the structural basis of the membrane and regulate fluidity, thereby playing a key role in signal transduction. In this study, treatment with magnolol led to significant changes in the expression of membrane-related genes. There were 53 membrane-related differentially expressed genes (DEGs), with 17 genes up-regulated and 36 genes down-regulated. KEGG enrichment analysis was performed on these DEGs, and the most significantly enriched pathway was the lipid metabolism pathway. It inhibited the expression of phospholipid synthesis genes (NTE1, GDE1, FUM18, and FUM17), hindering sn-Glycerol-3P synthesis and membrane-related signal transduction (Fig. 20). In recent years, transcriptome analysis has been commonly used to study the molecular mechanisms by which active substances prevent plant pathogens59. Previous studies have investigated the mechanism of action of honokiol on Aspergillus flavus60 and R. solani61 through transcriptome analysis. The research results showed significant down-regulation related to spore development, cell wall and cell membrane integrity, oxidative stress, and energy metabolism. In addition, in the study on the influence of honokiol on Botryosphaeria dothidea by Zhou62, similar results were obtained. This indicates that honokiol and magnolol have similar mechanisms of action. However, further research is still required for clarification.
Fig. 20.
Diagram of the mechanism of action of magnolol against N. ellipsospora.
Conclusion
In this study, the antifungal properties of magnolia extract was explored, and it was revealed that magnolol, which is a component of the magnolia extract, is the most effective antifungal component within magnolia. Moreover, the antifungal mechanism of magnolol against N. ellipsospora, the causative agent of tea leaf spot disease, was systematically investigated. The results demonstrated that magnolol displayed strong antifungal activity against N. ellipsospora, reducing mycelial accumulation and effectively inhibiting pathogen infection, suggesting that magnolol is a potential ecofriendly solution for managing tea leaf spot disease. Moreover, this study revealed that disruption of cell membrane integrity is the primary mode of antifungal action of magnolol. Transcriptome analysis confirmed that magnolol hindered phospholipid synthesis in N. ellipsospora, leading to significant impairment of cell membrane function and disruption of normal mycelial physiological processes. This investigation elucidated the antifungal mechanism of magnolol against N. ellipsospora, the pathogen responsible for tea leaf spot disease, laying the groundwork for the development of magnolol-based antifungal treatments.
Materials and methods
M. officinalis, N. ellipsospora, media, tea leaves, and reagents
The experiment utilized dry bark from M. officinalis purchased from Huaxi, Guizhou. The N. ellipsospora test strain was isolated and identified at the Guizhou Tea Research Institute. PDA (Potato Dextrose Agar Medium) and PDB (Potato Dextrose Broth) culture media were sourced from Guangdong Huankai. Fuding Dabai tea leaves were selected, grown at a temperature between 25 and 28 °C under 70 to 90% humidity. Polyantimycin was obtained from Fujian Kaili Bioproducts Co., Ltd.
Magnolia bark extraction
The dried stem bark of M. officinalis was ground into a powder with a grinder. Subsequently, 20 g of the powder was placed in a 1 L round bottom flask, to which 300 g of 65% ethanol was added for one week of extraction. The mixture underwent three cycles of heating and refluxing for 2 h each. The resulting extracts were then filtered, combined, and concentrated under reduced pressure at 50 °C. The crude extract obtained was dispersed in 500 mL of water, followed by the addition of 500 mL of n-hexane. Ultrasound-assisted extraction was performed with shaking, and this process was repeated three times. The combined extract was concentrated under reduced pressure. Sequential extraction was performed using solvents of increasing polarity, including dichloromethane, ethyl acetate, and n-butanol, following the same procedure as that for the n-hexane extraction. Each layer of the extract was concentrated separately under reduced pressure to obtain its respective crude extract.
Antifungal activity of the crude Magnolia bark extract
The mycelial growth rate method63 was utilized to assess antifungal activity. The crude extracts from each layer were dissolved in dimethyl sulfoxide (DMSO) to prepare a stock solution, which was subsequently diluted with sterile water and mixed with PDA. The concentration of the culture medium was adjusted to 100 mg/L. PDA medium containing 1% DMSO was utilized as a blank control. Once the mycelia of the blank control reached two-thirds of the medium, the colony diameter was measured according to the cross method to calculate the inhibitory activity.
Isolation and Purification of the crude M. officinalis extract and component structural identification
The organic layer derived from the crude M. officinalis bark extract was isolated and purified due to its substantial antifungal activity. The concentrated layer was analyzed by liquid chromatography. The n-hexane layer was concentrated and analyzed with a C18 chromatographic column (particle size: 5 μm; dimensions: 250 mm × 4.6 mm; Dalianilit) under optimized conditions: a methanol-water ratio of 70:30 (V/V), a flow rate of 1.0 mL/min, a detection wavelength of 294 nm, a column temperature of 35 °C, and an injection volume of 4 µL. Following this, the main products were purified by chromatography and their were subsequently structurally identified.
Antifungal activities of Magnolol and Honokiol against N. ellipsospora
Both compounds were dissolved separately in DMSO to generate stock solutions, which were subsequently diluted with sterile water to generate five different concentration gradients. These solutions were then combined with PDA and poured into plates. A 5 mm diameter N. ellipsospora colony was positioned at the center of the PDA medium. The negative control consisted of PDA with 1% DMSO, while the positive control utilized a commercial biological pesticide containing polyantimycin. After inoculation, the plates were incubated at 28 °C for 7 days. The colony diameter was measured according to the cross method to calculate the inhibition rate, and the toxicity regression equation and the EC25, EC50, and EC75 values were subsequently determined.
Evaluation of the indoor effectiveness
The experiment utilized the Fuding Dabaicha tea variety, which is known for being extensively cultivated in Guizhou. The experiment was performed according to Zhou’s research method64 with modifications. The leaves, which were uniform in size, were disinfected with a 75% alcohol solution before being inoculated with N. ellipsospora colonies. The leaves were subsequently treated with varying concentrations of magnolol (0, EC25, EC50, or EC75) and placed in a cultivation chamber in the temperature of 25 °C and a humidity of 80%. After 7 days of observation, the lesion area was measured to calculate the relative inhibition rate and assess the inhibitory effect.
Quantity of N. ellipsospora mycelia
Magnolol was dissolved in DMSO and diluted with sterile water and added to PDA to the 0, EC25, EC50, and EC75 concentrations. The PDA was poured into the plates, and after cooling, sterile filter paper was placed on the media. A 5 mm diameter N. ellipsospora colony was subsequently inoculated in the center of the PDA medium. The samples were incubated at 28 °C in the dark for 7 days, after which the mycelia were weighed.
Observation of the mycelial morphology
Following Zhou’s method64, magnolol was dissolved in DMSO, diluted with sterile water and added to PDA to the 0, EC25, EC50, and EC75 concentrations. Each mixture was poured into plates and allowed to solidify. Then, a 5 mm diameter N. ellipsospora colony was placed in the center of the PDA medium, and the area around the colony was covered with a sterile cover glass. The samples were incubated at 28 °C in the dark, and the morphology of the mycelia was observed under an optical microscope at 24, 48, 72, 96, and 120 h.
Calcofluor white (CFW) staining
CFW65 is a dye that fluoresces upon binding to chitin and glucan and indicate cell wall damage. According to the procedure described in the mycelium observation study, mycelium culture for 72 h, harvested and washed three times with distilled water. A small portion of the mycelia was then combined with 5 µL of HEPENGBIO staining solution (HEPENGBIO Biotechnology Co., Ltd., Shanghai, China) and incubated in the dark for 5 min. Next, 500 µL of HEPENGBIO washing solution was added, and this washing process was repeated 5–7 times. Next, 100 µL of HEPENGBIO washing solution was added with gentle mixing, and 10 µL of the suspension was extracted. The mycelia were mounted on a slide, covered with a coverslip, and examined under a fluorescence microscope (excitation wavelength, 355 nm; and emission wavelength, 440 nm).
Propidium iodide (PI)
PI66 is a fluorescent dye similar to ethidium bromide that emits red fluorescence upon intercalation into double-stranded DNA, allowing the visualization of cell membrane damage. Mycelia were obtained according to the procedure in CFW Next, 0.5 mL of 30 mg/L PI solution (Beijing Sunbio Biotechnology Co., Ltd., Beijing, China) was added, and the mixture was incubated for 20 min. The mycelia were subsequently rinsed three times with water, mounted on a glass slide, and observed under a fluorescence microscope (excitation wavelength, 535 nm; and emission wavelength, 615 nm).
Determination of mycelial cell membrane permeability
Relative conductivity can serve as an indicator of cell membrane permeability67. Mycelia (0.1 g) were added to 30 mL aqueous solutions of magnolol at the 0, EC25, EC50, and EC75 concentrations and thoroughly mixed. Liquid control samples were also prepared using 30 mL of aqueous solutions of magnolol at the 0, EC25, EC50, and EC75 concentrations without mycelia. The conductivity was measured at various time points (0, 10, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, and 360 min) with a DDS-307 conductivity meter. The conductivity at each of these times was denoted as R1, and the initial conductivity of the solution without mycelia was denoted as R1-0. After 360 min, the samples were boiled for 10 min in a water bath, and after cooling, the measured conductivity was considered the absolute conductivity (denoted as R2); additionally, the absolute conductivity of the solution without mycelia was denoted as R2-0, as shown in Eq. (1).
 |
1 |
Determination of extracellular nucleic acids and proteins
The specific experimental methods used were previously described66,68. A total of 0.1 g of mycelia was weighed and added to aqueous solutions of magnolol at the 0, EC25, EC50, and EC75 concentrations. The supernatants of these solutions from the different treatment groups were collected at 0, 4, 8, and 24 h. The absorbance (OD value) of the filtrate was then measured at 260 nm (for nucleic acids) and 280 nm (for proteins).
Scanning electron microscopy (SEM) analysis
Following established research protocols, three 5 mm N. ellipsospora colonies were inoculated into PDB and incubated at 28 °C and 160 rpm for 2 days. Subsequently, magnolol was added to the culture medium at the EC50 concentration or not, and culture was maintained for an additional 72 h. The mycelia were then harvested, rinsed three times with PBS, and subjected to gradient dehydration using ethanol concentrations of 30%, 50%, 70%, 80%, 95%, and 100% (twice) for 15 min each. This was followed by immersion in an ethanol-isopentyl acetate solution (1:1, V/V) for 20 min, followed by immersion in 100% isopentyl acetate for 20–30 min twice. A small portion of the sample was extracted with tweezers, spread onto a coverslip, and dried with a critical point dryer. The dried sample was fixed to conductive adhesive on a sample holder with tweezers, placed in a plasma sputter coater, and placed under vacuum. Upon reaching the optimal conditions, ion sputtering was carried out for 60 s, with additional sputtering performed in cases of severe charging. Following their preparation, the samples were examined under a scanning electron microscope for analysis.
Transmission electron microscopy (TEM) analysis
The mycelia were obtained according to the procedure in SEM. The samples were fixed in 2.5% glutaraldehyde for 12 h at 4 °C, rinsed with 0.1 M PBS three times (15 min each) and then postfixed in a 2% osmium tetroxide (OsO4) solution for 2 h at 4 °C. Next, the samples were rinsed again with 0.1 M PBS three times (15 min each). Dehydration was performed using a gradient of ethanol solutions (30%, 50%, 70%, 80%, 90%, and 95%) for 20 min each, followed by 90% ethanol and 90% acetone (V/V = 1/1) for 20 min each. The samples were further dehydrated with 90% acetone for 20 min; all of these steps were performed at 4 °C. The samples were subsequently dehydrated with 100% acetone three times at room temperature for 20 min each time. The samples were then embedded as follows. First, the samples were immersed in pure acetone and embedding solution (V/V = 3/1) at room temperature for 3–4 h, followed by immersion in pure acetone and embedding solution (V/V = 1/1) at room temperature for 12 h. This was followed by immersion in pure acetone and embedding solution (V/V = 1/3) at room temperature. The final step involved immersion in pure embedding solution at 37 °C three times (2–3 h each time). The samples were then incubated in an oven at 37 °C for 12 h, 45 °C for 12 h, and 60 °C for 24 h. The samples were subsequently sliced into 70–90 nm ultrathin sections and stained with 3% uranium acetate and lead citrate. After drying, the slices were observed via TEM (Hitachi, JEM-1010, Japan).
RNA-seq and differentially expressed gene (DEG) analyses
Three N. ellipsospora colonies were placed in PDB and cultivated for 72 h at 28 °C with shaking at 160 rpm. Subsequently, magnolol was added to the culture medium to reach the EC50 concentration, and cultivation was continued for another 72 h. The mycelia were then filtered and washed three times with sterile water. A total of 0.1 g of mycelia was promptly frozen in liquid nitrogen and stored at -80 °C. The samples were transported on dry ice to a sequencing platform (Guangzhou Kidio Biotechnology Co., Ltd.) for reference-free transcriptome sequencing.
RT-qPCR validation was conducted to confirm the transcriptional-level findings from the RNA-Seq data analysis. A total of 11 DEGs associated with the cell membrane were randomly selected for validation. The sequences of the primers, including those for the internal reference gene GAPDH (ID: Unigene0011038), are listed in Table 2. Total RNA extraction for RT-qPCR analysis was performed with the RNAprep Pure Plant Kit (Tiangen Biotech, Beijing, China).
Table 2.
Primers used for qRT-PCR analysis.
| Gene ID | Gene name | Description | log2(fc) | Primer sequence (5’-3’) |
|---|---|---|---|---|
| Unigene0011038 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase 1 | - | AGGAGCATCTTCTGTTGACCAT |
| GGACCTCTGCGTGTATAAGTTG | ||||
| Unigene0000620 | atrD | Hypothetical protein | -1.94 | CCACCATCACCATTGCTCAC |
| TCATTCTCCGCCTGTTCATCT | ||||
| Unigene0001545 | GDE1 | Hypothetical protein | -1.93 | GCCGACTGCCGTTACATTATG |
| GTCTTGCCATTCACTGCCATC | ||||
| Unigene0002345 | rga4 | Hypothetical protein | -1.34 | CGCCGAGCAGAATAACAACAT |
| TCAGGACAGCAGCATCATCAT | ||||
| Unigene0002475 | gld1 | Glycerol-3-phosphate dehydrogenase | -1.14 | GCAACAGCCACTTCCTTCC |
| CGATGACGGTGACCTTGTG | ||||
| Unigene0004487 | flbA | Hypothetical protein | -1.63 | TGCTCTGAGACTGCTGTTCC |
| CGAATTGCCTGCCGACATC | ||||
| Unigene0005649 | cki1 | Casein kinase I | -1.20 | CCGACTACGACTACCTGAGAG |
| CGTGCCGTGAAGTTCCATC | ||||
| Unigene0006666 | phqN | Hypothetical protein | 1.01 | CCAAGAGCAACCGCAACTAC |
| CCGTGTTCGTGTCAATCCATT | ||||
| Unigene0006808 | TAZ1 | Hypothetical protein | -1.17 | CAACCATACCGCAGGCAATC |
| GATGACGACGATGACGACAAT | ||||
| Unigene0007597 | CYP505 | Hypothetical protein | -1.39 | GTGATTACCTGGCTGTGCTAC |
| TGGTGCTTGCTGGAAGAGAT | ||||
| Unigene0008320 | wis1 | Hypothetical protein | 1.25 | CGCCTCGCCATACATTATAGAT |
| GTGCTTGTCCTTGAGTTCCTT | ||||
| Unigene0009147 | DGAT2A | Diacylglycerol O-acyltransferase 1 | 1.21 | GGTCAGAGGAGGTTGGATACT |
| GTGGTTGATGCGTGTGGAT |
For cDNA synthesis, 2.0 µg of RNA was reverse transcribed with FastKing gDNA Dispelling RT SuperMix (Tiangen, Beijing, China) in a 20 µL reaction volume according to the manufacturer’s instructions. PCR was subsequently conducted via the SYBR Green method and the Bio-Rad CFX96 RT-qPCR system (Bio-Rad, Berkeley, CA, USA) in a 96-well plate, with 3 biological replicates for each sample. The final reaction volume of 20 µL included 0.8 µL of cDNA template, 10 µL of 2× SG Fast qPCR Master Mix (Sangon, Shanghai, China), 0.6 µL of forward primer (200 nM), 0.6 µL of reverse primer (200 nM), and 8 µL of PCR-grade water. The reaction conditions included an initial step at 95 °C for 1 min, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 56 °C for 10 s, and extension at 72 °C for 15 s. Finally, relative gene expression was quantified by the 2–∆∆Ct method.
Statistical analysis
All the experiments were conducted at least three times, and the results are presented as the means ± standard errors. Significant differences among the groups were assessed via one-way analysis of variance with Tukey’s honest significant difference (HSD) test. A p value of less than 0.05 was considered to indicate statistical significance. Statistical analyses were carried out with SPSS version 24.0. All figures were generated with the Origin 2021 software package.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by Natural Science Foundation of Guizhou Province (zk[2021]032), by the the earmarked fund for GZMARS-Tea (GZCYCYJSTX-05), and by the Department of Agriculture and Rural Affairs of Guizhou Province (GZNYGJHX-2023009), and by the Guizhou Provincial Basic Research Program (Natural Science No. Qiankehe foundation ZK[2022]224).
Author contributions
Zhiwei Lei (L) and Wen Yang (Y) conceptualized and planned the study, obtained funding, coordinated project operations, supervised the research, and revised the manuscript. Jiying Zhang (Z) formulated the experimental design, conducted experiments, and authored the manuscript, including the initial draft and subsequent revisions. Furthermore, Jiying Zhang (Z), Jianmei Yao (Y), Chiyu Ma (M), and Huifang Liu (L) analyzed and structured the data, developed visual representations, and made contributions to revising the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wen Yang, Email: yangwen3409@126.com.
Zhiwei Lei, Email: leizhiwei816@163.com.
References
- 1.Phan, P., Chen, N., Xu, L., Dao, D. M. & Dang, D. Ndvi variation and yield prediction in growing season: A case study with tea in Tanuyen Vietnam. Atmosphere-Basel. 12 (8), 962 (2021). [Google Scholar]
- 2.Rui, L., Su, J., Li, T., Sun, J. & Wu, G. Molecular regulation of immunity in tea plants. Mol. Biol. Rep.50, 2883–2892 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Huang, Y. et al. Biosynthetic Pathway and Bioactivity of Vanillin, a highly abundant metabolite distributed in the Root Cortex of Tea Plants (Camellia Sinensis). J. Agr Food Chem.72 (3), 1660–1673 (2024). [DOI] [PubMed] [Google Scholar]
- 4.Jian, Y. Cloud platform system for the diagnosis of typical tea Plant diseases based on neural network. J. Phys: Conf. Ser.1648, 042086 (2020). [Google Scholar]
- 5.Ponmurugan, P., Manjukarunambika, K. & Gnanamangai, B. M. Impact of various Foliar diseases on the biochemical, volatile and Quality Constituents of Green and Black Teas. Australas Plant. Path. 45, 175–185 (2016). [Google Scholar]
- 6.Tan, R. et al. Comparative transcript profiling of resistant and Susceptible Tea Plants in response to Gray Blight Disease. Agronomy. 14 (3), 565 (2024). [Google Scholar]
- 7.Xu, G., Ying, F., Wu, H. & Tang, X. Biocontrol Potential of Two Deep-Sea Microorganisms against Gray Blight Disease of Tea. Egypt. J. Biol. Pest Co.33(1), (2023).
- 8.Nozawa, S., Togawa, M. & Watanabe, K. Reidentification of Pestalotiopsis Sensu Lato Causing Gray Blight of Tea in Japan. J. Gen. Plant. Pathol.88, 293–299 (2022). [Google Scholar]
- 9.Gan, D. et al. Phytotoxic Meroterpenoids with Herbicidal activities from the Phytopathogenic Fungus Pseudopestalotiopsis Theae. Phytochemistry. 206, 113522 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Sanjay, R., Ponmurugan, P. & Baby, U. I. Evaluation of fungicides and biocontrol agents against grey blight disease of tea in the field. Crop Prot.27(3–5), 689–694 (2008). [Google Scholar]
- 11.Pandey, A. K., Sinniah, G. D., Babu, A. & Tanti, A. How the global tea industry copes with fungal diseases-challenges and opportunities. Plant. Dis.105 (7), 1868–1879 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Khanal, D. et al. Insecticide Residue Analysis on Vegetable Crops through Rapid Bioassay of Pesticide Residue (Rbpr) technique in Nepal. J. King Saud University-Science. 35 (5), 102671 (2023). [Google Scholar]
- 13.Zhang, M. et al. Synthesis, antibacterial and antifungal activity of myricetin derivatives containing Piperidine and amide fragments. Pest Manag Sci.79 (12), 4795–4808 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Huang, S. et al. The latest research progress on the Prevention of Storage pests by Natural products: species, mechanisms, and sources of inspiration. Arab. J. Chem.15 (11), 104189 (2022). [Google Scholar]
- 15.Moloney, M. G. Natural products as a source for Novel Antibiotics. Trends Pharmacol. Sci.37 (8), 689–701 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., Mathai, N. & Kirchmair, J. Scope of 3D shape-based approaches in Predicting the Macromolecular targets of structurally Complex Small molecules Including Natural products and Macrocyclic Ligands. J. Chem. Inf. Model.60 (6), 2858–2875 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shady, N. H. et al. Bioactive Natural products of Marine sponges from the Genus Hyrtios. Molecules. 22 (5), 781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto, S. et al. Growth suppression of Cancer spheroids with mutated Kras by Low-Toxicity compounds from Natural products. Anticancer Res.41 (8), 4061–4070 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Liu, Y. et al. Discovery of a low toxicity O-Glcnac transferase (ogt) inhibitor by structure-based virtual screening of Natural products. Sci. Rep. -Uk. 7, 12334 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobbens, E. S. et al. The Inhibitory Effect of Natural products on protein fibrillation may be caused by Degradation Products–A study using aloin and insulin. Plos One11(2), e0149148 (2016). [DOI] [PMC free article] [PubMed]
- 21.Li, J. et al. Zebrafish-based screening of antiseizure plants used in traditional Chinese medicine: Magnolia Officinalis Extract and its constituents magnolol and honokiol exhibit potent anticonvulsant activity in a therapy-resistant Epilepsy Model. Acs Chem. Neurosci.11 (5), 730–742 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Wu, X. et al. Protective effect of a polyphenolic Rich Extract from Magnolia Officinalis Bark on Influenza Virus-Induced Pneumonia in mice. J. Ethnopharmacol.134 (1), 191–194 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Yang, Y. et al. Identification and validation of Magnolol Biosynthesis genes in Magnolia Officinalis. Molecules. 29 (3), 587 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharanya, J., Purushothaman, A., Janardanan, D. & Koley, K. Theoretical exploration of the antioxidant activity of Honokiol and Magnolol. Comput. Theor. Chem.1232, 114460 (2024). [Google Scholar]
- 25.Sarrica, A., Kirika, N., Romeo, M., Salmona, M. & Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med.84 (16), 1151–1164 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Ming-Xin Guo, M. M., Wu, X., Feng, Y. & Hu, Z. Research Progress on the structural modification of Magnolol and Honokiol and the Biological activities of their derivatives. Chem. Biodivers.20, e202300754 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Guo, Y. et al. Development of Membrane-Active Honokiol/Magnolol Amphiphiles as potent Antibacterial agents against Methicillin-Resistant Staphylococcus Aureus (Mrsa). J. Med. Chem.64 (17), 12903–12916 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Zhou, P., Fu, J., Hua, H. & Liu, X. In Vitro Inhibitory activities of Magnolol against Candida Spp. Drug. Des. Devel. Ther.6 (11), 2653–2661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morbidoni, H. R., de Mendoza, D. & Cronan, J. J. E. Synthesis of Sn-Glycerol 3-Phosphate, a key precursor of membrane lipids, in Bacillus Subtilis. J. Bacteriol.177, 5899–5905 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng, S. et al. Metabolic rewiring in tea plants in response to Gray Blight Disease unveiled by Multi-omics Analysis. Metabolites. 13 (11), 1122 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey, A. K., Kumar, A., Samota, M. K. & Tanti, A. Trichoderma Reesei as an Elicitor triggers defense responses in Tea Plant and Delays Gray blight symptoms. Pestic Biochem. Phys.188(6), 105279 (2022). [DOI] [PubMed] [Google Scholar]
- 32.BORA, P. & BORA, L. C. Revisiting non-chemical modes of diseases and pests Management in Tea (Camellia Sinensis): A review. Indian J. Agricultural Sci.93 (1), 3–14 (2022). [Google Scholar]
- 33.Gustavo, H. S. et al. Coffee Leaf Rust in Brazil: historical events, current Situation, and Control measures. Agronomy. 12(2), 496 (2022). [Google Scholar]
- 34.Togola, A. et al. Measurement of pesticide residues from Chemical Control of the Invasive Spodoptera Frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria. Int. J. Env Res. Pub He. 15(5), 849 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, H. et al. A review of the Phytochemistry and pharmacological activities of Magnoliae Officinalis cortex. J. Ethnopharmacol.23 (236), 412–442 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Woodbury, A., Yu, S. P., Wei, L. & García, P. Neuro-modulating effects of Honokiol: a review. Front. Neurol.4, 130 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, J. et al. Antimicrobial activity and mechanism of Magnolia Officinalis Root Extract against Methicillin-Resistant Staphylococcus Aureus based on Mannose Transporter. Ind. Crop Prod.201, 116953 (2023). [Google Scholar]
- 38.Hu, Y., Qiao, J., Zhang, X. I. & GE, C. Antimicrobial activity of Magnolia Officinalis extracts in Vitro and its effects on the preservation of Chilled Mutton. J. Food Biochem.35 (2), 425–441 (2011). [Google Scholar]
- 39.Jiang, X. et al. Screening and evaluating Honokiol from Magnolia Officinalis against Nocardia Seriolae Infection in Largemouth Bass (Micropterus Salmoides). J. Fish. Dis.45 (11), 1599–1607 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., Liu, Q., Shi, L., Qin, P. & Wang, Q. Honokiol triggers receptor–interacting protein kinase 3–Mediated cell death of Neuroblastoma cells by upregulating reactive oxygen species. Mol. Med. Rep.16 (6), 8525–8529 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Thuerig, B. et al. Efficacy of a Magnolia Officinalis Bark Extract against Grapevine Downy Mildew and Apple Scab under Controlled and Field conditions. Crop Prot.114, 97–105 (2018). [Google Scholar]
- 42.Cui, X. et al. Magnolol Inhibits Gray Mold on Postharvest Fruit by inducing autophagic activity of Botrytis Cinerea. Postharvest Biol. Tec.180, 111596 (2021). [Google Scholar]
- 43.Huang, Y. et al. In Vitro Metabolism of Magnolol and Honokiol in Rat liver microsomes and their interactions with seven cytochrome P substrates. Rapid Commun. Mass. Sp. 180, 111596 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Liao, K. & Sun, L. Roles of the Hsp90-Calcineurin pathway in the antifungal activity of Honokiol. J. Microbiol. Biotechn. 28(7), 1086–1093 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Sun, L. & Liao, K. The Effect of Honokiol on Ergosterol biosynthesis and vacuole function in Candida Albicans. J. Microbiol. Biotechn. 30 (12), 1835–1842 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun, L., Ye, X., Ding, D. & Kai, L. Opposite effects of vitamin C and vitamin E on the antifungal activity of Honokiol. J. Microbiol. Biotechn. 29 (4), 538–547 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Sun, L., Liao, K., Hang, C. & Wang, D. Honokiol induces reactive oxygen species-mediated apoptosis in Candida Albicans through mitochondrial dysfunction. Plos One12(2), e0172228 (2017). [DOI] [PMC free article] [PubMed]
- 48.Delso, I. et al. Inhibitors against fungal cell wall remodeling enzymes. Chemmedchem. 33 (2), 229–238 (2017). [DOI] [PubMed] [Google Scholar]
- 49.John, E. J. et al. Fungal cell Wall-Associated effectors: sensing, Integration, suppression, and Protection. Mol. Plant. Microbe in. 13 (2), 128–132 (2024). [DOI] [PubMed] [Google Scholar]
- 50.Azam, R., Asghar, R., Shahram, S., Mehrdad, N. & Saeid, E. Antifungal activity of Thymol against the Main Fungi Causing Pomegranate Fruit rot by suppressing the activity of cell wall degrading enzymes. Lwt-Food Sci. Technol.161, 113303 (2022). [Google Scholar]
- 51.Wang, W. et al. Inhibition of three Citrus pathogenic Fungi by peptide Paf56 involves cell membrane damage. Foods. 10 (9), 2031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sant, D. G., Tupe, S. G., Ramana, C. V. & Deshpande, M. V. Fungal cell membrane-promising drug target for antifungal therapy. J. Appl. Microbiol.121 (6), 1498–1510 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Zhang, S., Wu, Z., Yang, Y. & Li, K. Antifungal action of antifungalmycin N2 against Rhizoctonia Solani by disrupting cell membrane and inhibiting succinate dehydrogenase. Curr. Microbiol.77 (2), 254–260 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Tada, R., Latgé, J. & Aimanianda, V. Undressing the fungal cell Wall/Cell membrane–the antifungal drug targets. Curr. Pharm. Des.51 (3), 333–339 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Fan, K. et al. Antifungal activity and action mechanisms of 2,4-Di-Tert-Butylphenol against Ustilaginoidea Virens. J. Agr Food Chem.71 (46), 17723–17732 (2023). [DOI] [PubMed] [Google Scholar]
- 56.Lee, H. & Kim, Y. Myricetin disturbs the cell Wall Integrity and increases the membrane permeability of Candida Albicans. J. Microbiol. Biotechn. 32 (1), 37–45 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee, H. & Kim, Y. Aucklandia Lappa Causes Cell Wall Damage in Candida Albicans by reducing chitin and (1,3)-β-D-Glucan. J. Microbiol. Biotechn. 30 (7), 967–973 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng, Y. & Chen, B. Role of cell membrane homeostasis in the pathogenicity of pathogenic filamentous Fungi. Virulence. 15 (1), 2299183 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahoo, R. & Kadoo, N. Transcriptome analysis and structure-based drug Discovery identifies potential Biofungicides for Controlling Fusarium Wilt in Chickpea. J. Mol. Liq. 399 (11), 124364 (2024). [Google Scholar]
- 60.Zhang, W. et al. Transcriptomic Analysis Shows the antifungal mechanism of Honokiol against Aspergillus Flavus. Int. J. Food Microbiol.384, 109972 (2022). [DOI] [PubMed] [Google Scholar]
- 61.Yan, Y. et al. Bioassay-guided isolation of two antifungal compounds from Magnolia Officinalis, and the mechanism of action of Honokiol. Pestic Biochem. Phys.170, 104705 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Zhang, Z. et al. Honokiol inhibits Botryosphaeria Dothidea, the Causal Pathogen of Kiwifruit Soft Rot, by targeting membrane lipid biosynthesis. Pest Manag Sci.80 (4), 1779–1794 (2023). [DOI] [PubMed] [Google Scholar]
- 63.Ren, Y. et al. Integration of Transcriptomic and Proteomic Data reveals the possible action mechanism of the Antimicrobial Zhongshengmycin against Didymella Segeticola, the Causal Agent of Tea Leaf Spot. Phytopathology. 111 (12), 2238–2249 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Zhou, A. A. et al. Natural product Citronellal can significantly disturb Chitin Synthesis and Cell Wall Integrity in Magnaporthe Oryzae. J. Fungi (Basel). 8 (12), 1310 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su, X. et al. Calcofluor White Hypersensitive Proteins Contribute to stress tolerance and pathogenicity in Entomopathogenic Fungus, Metarhizium Acridum. Pest Manag Sci.77 (4), 1915–1924 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Huang, J. et al. Mechanisms of Litsea Cubeba Essential Oil in the Control of Colletotrichum Scovillei in Pepper (Capsicum Annuum L.): cell Membrane/Wall perspective. Physiol. Mol. Plant. P. 127, 102103 (2023). [Google Scholar]
- 67.Wei, H. et al. Utilization of straw-based phenolic acids as a Biofugicide for a Green Agricultural production. J. Biosci. Bioeng.131 (1), 53–60 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Yasir, M., Dutta, D. & Willcox, M. D. P. Comparative Mode of Action of the Antimicrobial Peptide Melimine and its derivative Mel4 against Pseudomonas Aeruginosa. Sci. Rep. -Uk. 9 (1), 7063 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).