Abstract
The association between inflammatory markers (IMs) and bone turnover markers (BTMs) in osteoporotic fracture patients has not been comprehensively studied. Therefore, this study examined the correlation between the platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), or Monocyte-to-lymphocyte ratio (MLR) and BTMs in osteoporosis (OP) fracture patients. This retrospective cross-sectional study analyzed 740 OP fracture patients admitted to the hospital from January 2017 to July 2022. MLR, NLR, and PLR were calculated based on each patient’s complete blood count. The relationship between IMs and BTMs was assessed using three models by adjusting variables. Furthermore, the potential curve relationship between IMs and BTMs was also determined via the threshold effect analysis and curve fittings. In addition, stratified analysis was performed on each adjusted variable to confirm the stability of the results. After adjusting the variables, the results showed that NLR was negatively correlated with procollagen type 1 N-terminal propeptide (P1NP) (β = -1.1788, 95% CI: -1.7230 to -0.6345, P-value < 0.0001) and β-C-terminal telopeptide of type I collagen (β-CTX) (β = -0.0104, 95% CI: -0.0145 to -0.0062, P-value < 0.0001), Furthermore, MLR was negatively correlated with P1NP (β = -17.4523, 95% CI: -27.7335 to -7.1710, P-value = 0.0009) and β-CTX (β = -0.1327, 95% CI: -0.2211 to -0.0443, P-value = 0.0034). However, PLR indicated a positive correlation with P1NP (β = 0.0326, 95% CI: 0.0007 to 0.0645, P-value = 0.0458) and β-CTX (β = 0.0003, 95% CI: 0.0001 to 0.0006, P-value = 0.0204). The threshold effect analysis and curve fittings revealed the presence of a turning point between NLR, MLR, and P1NP, β-CTX. In addition, the stratified analysis validated the result’s stability. In conclusion, this study indicates a negative correlation between NLR and MLR with P1NP, while PLR shows a positive correlation with P1NP. Additionally, NLR and MLR exhibit a negative correlation with β-CTX, whereas PLR demonstrates a positive correlation with β-CTX. Further research is required to assess the intricate mechanisms linking IM with bone metabolism.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75704-7.
Keywords: P1NP, β-CTX, Osteoporotic fractures, Inflammatory markers, Bone turnover markers
Subject terms: Orthopaedics, Osteoporosis, Inflammation, Inflammation, Epidemiology
Introduction
Osteoporosis (OP) is characterized by a gradual decline in bone mass, reduced bone strength, and a breakdown of bone microarchitecture, which significantly increases the risk of fractures1. Clinically, OP is defined by a bone mineral density (BMD) of < 2.5 standard deviations (SD) of the average peak bone mass of healthy adults2. Hip and vertebral fractures are the most prevalent and debilitating outcomes of OP, and these are associated with substantial healthcare costs and an increased mortality rate of 20% within the first year of post-fracture3. The International Osteoporosis Foundation has reported that, for adults over 50 years old, 1 in every 5 men and 1 in every 3 women are at risk of osteoporotic fracture during their lifetime4,5. In the United States, the prevalence of OP in 50-year-old adults has been observed to increase from 9.4% in 2007–2008 to 12.6% in 2017–2018, with women being more susceptible6. Furthermore, in 2019, an estimated 32 million individuals (6.5 million men and 25.5 million women) were diagnosed with OP across 29 European countries6,7. Moreover, the global expenditures for osteoporotic fractures, which was $10 billion in 2010, have been expected to reach $17 billion by 20308. Therefore, OP represents a significant threat to human health and well-being.
The recommended diagnostic tool for OP is BMD9. However, bone turnover markers (BTMs), released during the bone remodeling process, are also employed for OP diagnosis10. These BTMs are mainly the products of bone proteins, such as type I collagen, that are significantly modified at the post-translational level during bone synthesis11. Some of these biomarkers include N-terminal propeptide of type I procollagen (PINP), cross-linked C-terminal telopeptide of type I collagen (fragments beta-CTX, alpha-CTX), cross-linked C-terminal of type I collagen (ICTP), C-terminal propeptide of type I procollagen (PICP), osteocalcin (OC), and alkaline phosphatase (AP)12. Of these, β-CTX and P1NP are the only recommended biomarkers for investigating bone metabolism in adults13.
Several studies have indicated a strong association between systemic immunity and inflammation with OP8,14,15. For instance, lymphocyte dysfunction can initiate an inflammatory cascade that accumulates macrophages and neutrophils, thereby disrupting the dynamic balance in bone formation8,16. Moreover, circulating immune cells, as well as increased levels of pro-inflammatory cytokines, can directly or indirectly impact osteoclastogenesis, thus altering bone resorption17.
Recently, it was proposed that the inflammatory markers (IMs), such as the platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and Monocyte-lymphocyte ratio (MLR), which are determined during a full blood cell count (FBC), can offer insight into a body’s immune response and inflammatory condition18,19. Furthermore, these markers are simple, economical, and reliable indicators of inflammation because of the simplicity and low cost of blood tests.
The literature has indicated that these IMs are associated with different conditions, such as depression20, infertility21, spontaneous preterm birth22, and type II diabetes in patients with chronic hepatitis C-related cirrhosis23. However, despite the significant evidence supporting the association of inflammation with OP development, the correlation of NLR, MLR, and PLR with OP has not been comprehensively studied. Currently, no conclusive evidence supports a direct relationship between these biomarkers and OP. Therefore, this study aimed to elucidate the correlation between IMs and BTMs.
Materials and methods
Research participants and design
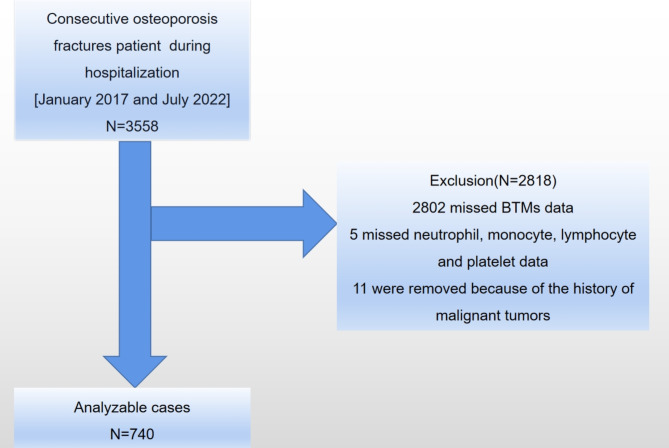
This study was carried out at the Affiliated Kunshan Hospital of Jiangsu University (AKHJU) in Jiangsu Province, China. This university was subsequently known as a tertiary-level A Medical Hospital in the Kunshan area with more than 3,000,000 admitted patients. This study analyzed more than 50 years old OP fracture (proximal humerus, vertebra, hip, fracture of the wrist) patients who were hospitalized between January 1, 2017, and July 27, 2022. Their electronic records were retrieved. Of these, 3,558 patients were included in this study. The exclusion criteria excluded patients who did not have BTMs, neutrophil, monocyte, lymphocyte, and platelet data, were < 50 years old, and had a history of malignant tumors8. Based on the inclusion criteria, 740 patients were selected for the current analysis (Fig. 1). This study followed the Helsinki Declaration and acquired ethical approval from the AKHJU (2021-06-015-K01). All the patients provided written informed consent before the study, and the investigators were blinded to the patient’s data during the analysis.
Fig. 1.
A diagrammatic representation of the study’s design. BTMs: bone turnover markers.
BTMs examination
The primary outcome variables of this study included β-CTX (reference ranges for males aged 50–70 years: 0.104–0.504 ng/mL, for males over 70 years: 0.164–0.624 ng/mL, for postmenopausal females: 0.330–0.782 ng/mL) and P1NP (reference ranges: 20–80 ng/mL) were measured by electrochemiluminescence using the Roche Cobas s8000 instrument. For laboratory analysis of β-CTX and P1NP, morning fasting venous blood samples were collected from all the participants.
Serum analysis
For routine laboratory tests, all participants fasted for at least 8 h and remained at rest without engaging in vigorous physical activity before blood collection. Then, neutrophils (reference range: 2.0–7.5 × 109/L), lymphocytes (reference range: 1.0–3.0 × 109/L), and monocytes (reference range: 0.2–0.8 × 109/L) were quantified using the Sysmex XN-10 (B4) hematology analyzer after flow cytometric staining. Moreover, platelet (reference range: 100–300 × 109/L) levels were measured via impedance counting. Based on the results, PLR was calculated as the ratio of platelet count to lymphocyte count, while NLR and MLR were calculated as the ratios of neutrophil count to lymphocyte count and monocyte count to lymphocyte count, respectively.
According to the National Health Commission’s Clinical Laboratory Internal Quality Assessment Criteria, for routine tests, it is imperative to ensure that the intra- and inter-batch coefficient of variation (CV) for quantitative items were ≤ 1/3 of the Total Error Allowance (TEa), respectively. The intra- and inter-batch CV for white blood cell and platelet counts should both be ≤ 6.0% and ≤ 8.0%, respectively. For biochemical tests, internal quality control standards primarily reference the performance standards; in the absence of these, they refer to 1/3 of the TEa. For quantitative items, the intra- and inter-batch CV should be < 1/4 and < 1/3 of the TEa, respectively.
Covariates
The covariates included age, sex, smoking, drinking, calcium (Ca), magnesium (Mg), potassium (K), sodium (Na), phosphorus (P), uric acid (UA), urea nitrogen (UN), creatinine (CR), homocysteine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A (Apo A), fibrinogen, neutrophil, monocyte, the score of American society of anesthesiologists (ASA) and body mass index (BMI), with the latter determined by dividing the weight (in kg) by the height (in meters) squared. Current or former smokers within the past 12 months were identified as ever-smokers, whereas participants who consumed alcohol at least once a week within the past 12 months were considered current drinkers. All patients fasted for 8 h before blood sampling, and all laboratory results were obtained within this period.
Statistical analyses
In this study, categorical data were analyzed by univariate analyses using Fisher’s exact tests or Pearson’s chi-square tests and represented as frequencies (percentages). Whereas continuous data, such as demographic, laboratory, and clinical information, were displayed as medians (including the Min and Max) or means with standard deviations (SD). The normally or not normally distributed continuous data were analyzed via the Mann-Whitney U tests and independent sample t-tests, respectively.
To evaluate the independent association between IMs and BTMs in OP fracture patients, covariates were adjusted using the generalized estimating equation. Furthermore, 3 models were developed to assess the association between IMs and BTMs in OP patients, including an unadjusted Model 1, a minimally-adjusted Model 2, and a fully-adjusted Model 3. To identify collinearity among the covariates, a variance inflation factor (VIF) analysis was performed. The covariates were then adjusted and included or excluded from the baseline or full model based on the (1) Inclusion criteria: the standardized regression coefficient (β) or matched odds ratio (OR) changed by > 10% and (2) Exclusion criteria: a covariate with a P-value of ≤ 0.1 in model 1 or in the univariate analysis. In Model 2, adjustments were mainly focused on rudimentary demographic data, whereas the fully adjusted Model 3 was established based on the two criteria mentioned above.
Potential non-linear correlations were also identified using a generalized additive model (GAM). In cases where an apparent correlation existed, the threshold effect of the resulting smoothed curve was determined using a two-segment linear regression model. Then, a recursive method was used to independently compute the inflection point, applying a maximum likelihood model in situations where these curves exhibited a distinct ratio. In addition, subgroup analyses were conducted on clinically relevant participant subsets to enhance the findings’ reliability, with a P-value of ≤ 0.05 in two-tailed tests indicating the results’ significance. Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc, MA, USA) and R packages (http://www.R-project.org, The R Foundation) were employed for all the statistical analyses. The significance threshold was set at a two-sided P-value of ≤ 0.05.
Results
Participants’ baseline characteristics
The OP fracture patients were comprehensively analyzed, and based on the inclusion criteria, 740 patients were included in the study. The mean age of the patients was 68.65 years old, with 50 and 103 years indicating the youngest and oldest age, respectively. There were 33.51% males and 66.49% females, which indicates a higher prevalence of OP fractures among female patients. The mean (SD) and median (Min-Max) of MLR, NLR, PLR, P1NP, and β-CTX were 0.41 (0.26) and 0.33 (0.00–2.50), 5.64 (4.79) and 4.16 (0.77–47.19), 161.30 (83.97) and 140.29 (32.86–717.59), 58.15 (35.78) and 51.00 (11.00–565.00) and 0.53 (0.28) and 0.48 (0.07–2.13), respectively (Table 1).
Table 1.
The baseline characteristics of the participants.
| Variables | Mean (SD) / N (%) | Median (Min-Max)a |
|---|---|---|
| Age, years | 68.65 (11.32) | 68.00 (50.00-103.00) |
| BMI, kg/m2 | 23.02 (3.37) | 23.14 (13.52–33.20) |
| Na, mmol/L | 141.25 (2.75) | 141.50 (116.40-153.70) |
| Mg, mmol/L | 0.91 (0.09) | 0.91 (0.62–1.43) |
| K, mmol/L | 3.87 (0.43) | 3.86 (2.36–6.76) |
| P, mmol/L | 1.08 (0.19) | 1.08 (0.31–1.87) |
| Ca, mmol/L | 2.22 (0.12) | 2.22 (1.76–2.62) |
| Fibrinogen, g/L | 3.05 (0.81) | 2.96 (0.83–6.10) |
| HDL, mmol/L | 1.33 (0.29) | 1.30 (0.55–2.44) |
| LDL, mmol/L | 2.66 (0.74) | 2.60 (0.79–5.89) |
| Apo A, g/L | 1.23 (0.22) | 1.21 (0.61–1.95) |
| Homocysteine, µmol/L | 13.28 (6.98) | 11.47 (0.00-68.87) |
| ALT, U/L | 22.81 (16.35) | 19.00 (5.00-226.00) |
| AST, U/L | 25.81 (16.23) | 22.00 (8.00-266.00) |
| UA, umol/L | 280.72 (91.17) | 266.00 (83.00-780.00) |
| Cr, µmol/L | 63.22 (23.91) | 59.00 (10.00-395.00) |
| UN, mmol/L | 6.30 (5.38) | 5.80 (2.40-139.81) |
| Neutrophil, × 109/L | 5.64 (2.65) | 4.97 (1.10–17.20) |
| Monocyte, × 109/L | 0.45 (0.21) | 0.40 (0.00-1.85) |
| Lymphocyte, × 109/L | 1.26 (0.52) | 1.20 (0.30–3.90) |
| Platelet, × 109/L | 177.01 (61.40) | 169.00 (42.40–540.00) |
| β-CTX, ng/mL | 0.53 (0.28) | 0.48 (0.07–2.13) |
| P1NP, ng/mL | 58.15 (35.78) | 51.00 (11.00-565.00) |
| MLR | 0.41 (0.26) | 0.33 (0.00-2.50) |
| NLR | 5.64 (4.79) | 4.16 (0.77–47.19) |
| PLR | 161.30 (83.97) | 140.29 (32.86-717.59) |
| N (%) | ||
| Sex, N (%) | ||
| Female | 492 (66.49%) | |
| Male | 248 (33.51%) | |
| Smoking, N (%) | ||
| No | 688 (92.97%) | |
| Yes | 52 (7.03%) | |
| Drinking, N (%) | ||
| No | 709 (95.81%) | |
| Yes | 31 (4.19%) | |
| ASA, N (%) | ||
| 1 | 64 (8.65%) | |
| 2 | 513 (69.32%) | |
| ≥3 | 163 (22.03%) | |
SD, standard deviation; BMI, body mass index; NA, sodium; Mg, magnesium; K, potassium; P, phosphorus; Ca, calcium; HDL, high density lipoprotein; LDL, low density lipoprotein; Apo A, apolipoprotein A; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UA, uric acid; CR, creatinine; UN, urea nitrogen; β-CTX, β-C-terminal telopeptide of type I collagen; P1NP, procollagen type 1 N-terminal propeptide; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, plateletto-lymphocyte ratio; ASA, the score of american society of anesthesiologists.
aFor continuous variables.
Association between IMs and BTMs
To explore the potential relationship between IMs and BTMs, three models were constructed: an unadjusted Model 1, a Model 2 with adjustments of demographic features (including drinking, smoking, BMI, age, and sex), and a fully-adjusted Model 3.
The data in Model 3 (Table 2) indicated that NLR was negatively correlated with BTMs. For every 1-unit increase in NLR, the corresponding change in β-CTX and P1NP was − 0.0104 (95% CI: -0.0145 to -0.0062; P-value < 0.0001) and − 1.1788 (95% CI: -1.7230 to -0.6345; P-value < 0.0001), respectively. Furthermore, MLR was negatively correlated with BTMs as for every 1-unit increase in MLR, the corresponding change in β-CTX and P1NP were − 0.1327 (95% CI: -0.2211 to -0.0443; P-value = 0.0034) and − 17.4523 (95% CI: -27.7335 to -7.1710; P-value = 0.0009), respectively. However, there was no negative correlation between PLR and BTMs as in Model 3; for every 1-unit increase in PLR, there was a significant increase of 0.0003 (95% CI: 0.0001 to 0.0006; P-value = 0.0204) and 0.0326 (95% CI: 0.0007 to 0.0645; P-value = 0.0458) in β-CTX and P1NP levels, respectively.
Table 2.
Associations between inflammatory markers and BTMs in different models.
| P1NP | β-CTX | |||
|---|---|---|---|---|
| NLR | ||||
|
Model 1 β (95% CI) P-value |
-1.0293 (-1.5627, -0.4960) | 0.0002 | -0.0112 (-0.0153, -0.0070) | < 0.0001 |
|
Model 2 β (95% CI) P-value |
-1.0419 (-1.5776, -0.5062) | 0.0001 | -0.0114 (-0.0155, -0.0072) | < 0.0001 |
|
Model 3a β (95% CI) P-value |
-1.1788 (-1.7230, -0.6345) | < 0.0001 | -0.0104 (-0.0145, -0.0062) | < 0.0001 |
| MLR | ||||
|
Model 1 β (95% CI) P-value |
-15.1813 (-25.1430, -5.2197) | 0.0029 | -0.1431 (-0.2210, -0.0653) | 0.0003 |
|
Model 2 β (95% CI) P-value |
-15.1736 (-25.1922, -5.1551) | 0.0031 | -0.1407 (-0.2189, -0.0626) | 0.0004 |
|
Model 3b β (95% CI) P-value |
-17.4523 (-27.7335, -7.1710) | 0.0009 | -0.1327 (-0.2211, -0.0443) | 0.0034 |
| PLR | ||||
|
Model 1 β (95% CI) P-value |
-0.0151 (-0.0458, 0.0156) | 0.3363 | 0.0000 (-0.0002, 0.0002) | 0.9811 |
|
Model 2 β (95% CI) P-value |
-0.0159 (-0.0467, 0.0149) | 0.3127 | -0.0000 (-0.0002, 0.0002) | 0.9673 |
|
Model 3c β (95% CI) P-value |
0.0326 (0.0007, 0.0645) | 0.0458 | 0.0003 (0.0001, 0.0006) | 0.0204 |
Model 1: no covariates were adjusted.
Model 2 was adjusted for age, sex, BMI, smoking and drinking.
Model 3a was adjusted for age, sex, BMI, smoking, drinking, Ca, UA, CR, AST and Monocyte.
Model 3b was adjusted for age, sex, BMI, smoking, drinking, Ca, UA, UN, CR, HDL and LDL.
Model 3c was adjusted for age, sex, BMI, smoking, drinking, K, Mg, Na, P, Fibrinogen, Neutrophil, Apo A, HDL, Homocysteine, ALT and ASA.
P1NP, procollagen type 1 N-terminal propeptide; β-CTX, β-C-terminal telopeptide of type I collagen; NLR, neutrophil-to-lymphocyte ratio; CI, confidence interval; MLR, monocyte-to-lymphocyte ratio; PLR, plateletto-lymphocyte ratio; BMI, body mass index; Ca, calcium; UA, uric acid; CR, creatinine; AST, aspartate aminotransferase; UN, urea nitrogen; HDL, high density lipoprotein; LDL, low density lipoprotein; K, potassium; Mg, magnesium; Na, sodium; P, phosphorus; Apo A, apolipoprotein A; ALT, alanine aminotransferase; ASA, the score of american society of anesthesiologists.
Spline smoothing plot and threshold analyses
This study also performed curve fitting and threshold effect analyses to explore the potential relationship between IMs and BTMs. The results revealed that MLR and NLR had a curvilinear relationship with BTMs, with the presence of a turning point. In contrast, PLR showed a linear relationship with BTMs (Table 3).
Table 3.
Threshold effect analysis examining the relationship between inflammatory markers and BTMs.
| NLR, Model 3a | MLR, Model 3b | PLR, Model 3c | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PINP β (95% CI) |
P-value | β-CTX β (95% CI) |
P-value | PINP β (95% CI) |
P-value | β-CTX β (95% CI) |
P-value | PINP β (95% CI) |
P-value | β-CTX β (95% CI) |
P-value | |
| Model Ad | ||||||||||||
| One line slope | -1.1788 (-1.7230, -0.6345) | < 0.0001 | -0.0104 (-0.0145, -0.0062) | < 0.0001 | -17.4523 (-27.7335, -7.1710) | 0.0009 | -0.1327 (-0.2211, -0.0443) | 0.0034 | 0.0326 (0.0007, 0.0645) | 0.0458 | 0.0003 (0.0001, 0.0006) | 0.0204 |
| Model Be | ||||||||||||
| Turning point (K) | 6 | 7.1625 | 0.1364 | 0.8226 | 244.2857 | 311.6667 | ||||||
| < K | -5.6203 (-7.3378, -3.9028) | < 0.0001 | -0.0336 (-0.0442, -0.0230) | < 0.0001 | 202.4161 (20.1683, 384.6639) | 0.0299 | -0.2185 (-0.3415, -0.0954) | 0.0005 | 0.0470 (-0.0059, 0.1000) | 0.0823 | 0.0002 (-0.0001, 0.0006) | 0.2229 |
| > K | 0.1640 (-0.5633, 0.8912) | 0.6587 | 0.0001 (-0.0059, 0.0062) | 0.9640 | -20.5459 (-31.1007, -9.9910) | 0.0002 | 0.0472 (-0.1533, 0.2477) | 0.6446 | 0.0141 (-0.0488, 0.0769) | 0.6615 | 0.0005 (-0.0002, 0.0013) | 0.1478 |
| Slope 2-Slope 1 | 5.7843 (3.6585, 7.9101) | < 0.0001 | 0.0338 (0.0195, 0.0480) | < 0.0001 | -222.9620 (-407.4822, -38.4418) | 0.0182 | 0.2657 (-0.0002, 0.5315) | 0.0506 | -0.0330 (-0.1294, 0.0634) | 0.5029 | 0.0003 (-0.0007, 0.0013) | 0.5282 |
| LRTf | < 0.001 | < 0.001 | 0.017 | 0.048 | 0.495 | 0.521 | ||||||
NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, plateletto-lymphocyte ratio; P1NP, procollagen type 1 N-terminal propeptide; β-CTX, β-C-terminal telopeptide of type I collagen; CI, confidence interval; BMI, body mass index; Ca, calcium; UA, uric acid; CR, creatinine; AST, aspartate aminotransferase; UN, urea nitrogen; HDL, high density lipoprotein; LDL, low density lipoprotein; K, potassium; Mg, magnesium; Na, sodium; P, phosphorus; Apo A, apolipoprotein A; ALT, alanine aminotransferase; ASA, the score of american society of anesthesiologists.
aAdjusted for age, sex, BMI, smoking, drinking, Ca, UA, CR, AST and Monocyte.
bAdjusted for age, sex, BMI, smoking, drinking, Ca, UA, UN, CR, HDL and LDL.
cAdjusted for age, sex, BMI, smoking, drinking, K, Mg, Na, P, Fibrinogen, Neutrophil, Apo A, HDL, Homocysteine, ALT and ASA.
dLinear analysis, P-value < 0.05 indicates a linear relationship.
eNonlinear analysis.
fP-value < 0.05 means Model B is significantly different from Model A, which indicates a nonlinear relationship.
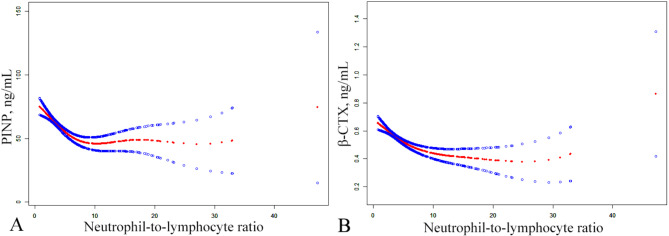
The threshold effect analysis for NLR and BTMs revealed that the P-value for LRT was < 0.05, suggesting a turning point at k = 6 for P1NP and k = 7.1625 for β-CTX. The left side of the curve indicated a negative correlation between NLR and P1NP or β-CTX, with effect sizes, 95% CI and a P-value = -5.6203 (95% CI: -7.3378 to -3.9028; P-value < 0.0001) and − 0.0336 (95% CI: -0.0442 to -0.0230; P-value < 0.0001), respectively. However, on the right side of the curve, the effect sizes, 95% CI, and P-value were 0.1640 (95% CI: -0.5633 to 0.8912; P-value = 0.6587) and 0.0001 (95% CI: -0.0059 to 0.0062; P-value = 0.9640), respectively (Fig. 2).
Fig. 2.
The smoothed adjusted curves for NLR, β-CTX and P1NP. The red line denotes the non-linear connection between NLR and both β-CTX and P1NP, whereas the blue line indicates the CI at 95%. Models were adjusted for age, sex, BMI, smoking, drinking, Ca, UA, CR, AST and monocyte; NLR, neutrophil-to-lymphocyte ratio; P1NP, procollagen type 1 N-terminal propeptide; β-CTX, β-C-terminal telopeptide of type I collagen; CI, confidence interval; BMI, body mass index; Ca, calcium; UA, uric acid; CR, creatinine; AST, aspartate aminotransferase.
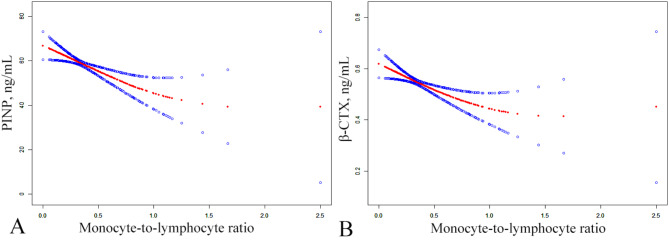
The threshold effect analysis for MLR and BTMs revealed a turning point at k = 0.1364 and 0.8226 for PINP and β-CTX, respectively. In this case, the left side of the curve indicated that the effect sizes, 95% CI, and P-value between MLR and P1NP or β-CTX were 202.4161 (95% CI: 20.1683 to 384.6639; P-value = 0.0299) and − 0.2185 (95% CI: -0.3415 to -0.0954; P-value = 0.0005), respectively. In contrast, on the right side of the curve, a negative correlation was observed between MLR and P1NP, whereas a positive correlation was noted between MLR and β-CTX, with effect sizes, 95% CI, and P-value of -20.5459 (95% CI: -31.1007 to -9.9910; P-value = 0.0002) and 0.0472 (95% CI: -0.1533 to 0.2477; P-value = 0.6446), respectively (Fig. 3).
Fig. 3.
The smoothed adjusted curves for MLR, β-CTX and P1NP. The red line denotes the non-linear connection between the MLR and both β-CTX and P1NP, whereas the blue line indicates the CI at 95%. Models were adjusted for age, sex, BMI, smoking, drinking, Ca, UA, UN, CR, HDL and LDL; MLR, monocyte-to-lymphocyte ratio; P1NP, procollagen type 1 N-terminal propeptide; β-CTX, β-C-terminal telopeptide of type I collagen; CI, confidence interval; BMI, body mass index; Ca, calcium; UA, uric acid; CR, creatinine; UN, urea nitrogen; HDL, high density lipoprotein; LDL, low density lipoprotein.
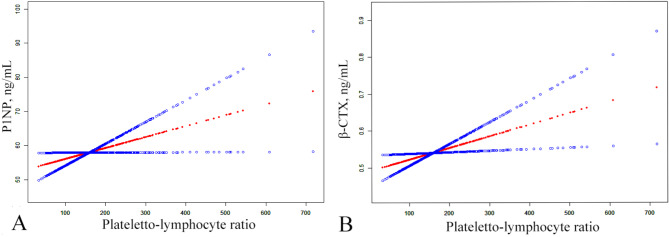
The threshold effect analysis for PLR and BTMs showed that the P-value for LRT was > 0.05, thus indicating a lack of non-linear relationship between the two variables (Fig. 4).
Fig. 4.
The smoothed adjusted curves for PLR, β-CTX and P1NP. The red line denotes the non-linear connection between the PLR and both β-CTX and P1NP, whereas the blue line indicates the CI at 95%. Models were adjusted for age, sex, BMI, smoking, drinking, K, Mg, Na, P, fibrinogen, neutrophil, Apo A, HDL, homocysteine, ALT and ASA; PLR, plateletto-lymphocyte ratio; P1NP, procollagen type 1 N-terminal propeptide; β-CTX, β-C-terminal telopeptide of type I collagen; CI, confidence interval; BMI, body mass index; K, potassium; Mg, magnesium; Na, sodium; P, phosphorus; Apo A, apolipoprotein A; HDL, high density lipoprotein; ALT, alanine aminotransferase; ASA, the score of american society of anesthesiologists.
Subgroup analysis
Subgroup analysis was conducted using the fully adjusted Model 3 to confirm the robustness of the results. All analysis variables were adjusted except for those used for subgroup classification, and the results were consistent in all subgroups (Tables S1, S2, and S3).
Discussion
This study analyzed the data of 740 osteoporotic fracture patients admitted at Kunshan First People’s Hospital to investigate the association between IMs and BTMs. The adjustment of confounding factors revealed a negative correlation between BTMs and MLR or NLR. Furthermore, a positive correlation was found between PLR and BTMs. Moreover, the threshold effect analyses and curve-fitting graphs revealed the presence of a turning point between MLR, NLR, and BTMs. To confirm the stability of the data, a stratified analysis was performed, which revealed that the results were consistent across the different strata.
In recent years, several studies have investigated the association between IMs and BMD. Öztürk et al.24 found a significant negative correlation between lumbar spine (L2-L4) and femoral neck scores with NLR in the elderly population. Furthermore, Tang et al.15 observed a negative correlation between NLR and BMD in postmenopausal women, which was positively associated with the risk of OP. Moreover, according to Yolaçan et al.25, the NLR, PLR, MLR, and systemic immune-inflammation index (SII) values were negatively correlated with BMD changes in postmenopausal Turkish women. Lee et al.26 identified a negative correlation between NLR quartiles and the average lumbar spine BMD in postmenopausal patients in South Korea. In addition, Song et al.27 revealed that postmenopausal women with rheumatoid arthritis have high baseline NLR, PLR, and MLR, which were independently associated with a higher risk of incidental vertebral fractures. Overall, these studies highlighted the detrimental impact of elevated inflammation levels on patients’ bone health, which is consistent with the conclusions of the present study that high NLR and MLR levels are detrimental to bone health. However, Ma et al.28 research revealed a positive correlation between circulating platelet concentration and BMD of the lumbar spine, left hip joint, and right hip joint. Platelets are cytoplasmic fragments derived from megakaryocytes and play a crucial role in bone homeostasis, formation, and resorption29,30. It has been observed that platelets have a supportive role in bone formation, particularly in platelet-derived growth factors (PDGFs), which promote bone formation by influencing cell proliferation, chemotaxis, differentiation, and extracellular matrix synthesis31,32. Increased platelets and decreased lymphocytes increase the PLR and also platelet’s supportive effect on bone formation. Therefore, there is a positive correlation between PLR and BTMs.
Osteoclasts and osteoblasts predominantly modulate the bone remodeling process; however, dendritic cells, macrophages, monocytes, B cells, and T cells are also observed to be involved in inflammation and bone loss33,34. Oxidative and inflammatory stimuli, such as NO, IL-1β, IL-6, IL-8, IL-18, IL-15, IL-17, IL-32, and TNF-α, can activate osteoclasts-mediated bone resorption, and many of these stimuli induce osteoclastogenesis by enhancing RANKL release35. The literature has indicated that during the initial stages of bone healing, neutrophils exert a protective effect on bone formation36. However, excessive activation of neutrophils, as a result of infections or injuries, can produce chemokines that inhibit B lymphocytes or recruit pro-inflammatory cells such as Th17 cells, thereby causing inflammation-associated bone loss37. Moreover, activated neutrophils can secrete RANKL, which promotes osteoclast formation and maturation and results in bone loss38. Th17 cells are a subtype of T cells that promote osteoclastogenesis, and their numbers increase in the bone marrow, and IL-17 levels increase in the peripheral blood39. It has been reported that blocking the IL-17 pathway could exert a protective effect against bone loss in OVX mice39.
The pathological pathways of inflammation are similar to those related to primary OP causes, such as aging and estrogen deficiency, as they all contribute to progressive bone density loss40,41. At low levels, estrogen strongly stimulates immune cells to produce inflammatory mediators34,41. Furthermore, low estrogen levels can activate T cells and macrophages, which, in turn, release inflammatory cytokines such as TNF-α, IL-6, and IL-1β, thereby inhibiting osteoblasts and stimulating osteoclasts to collectively promote bone resorption34,35. As estrogen levels fall with age, IL-1, IL-6, and TNF-α levels rise, contributing to the “inflammaging” observed in older adults42. In postmenopausal women, employing TNF-α inhibitors to block TNF-α has demonstrated a reduction in the serum levels of carboxy-terminal cross-linking telopeptide of type I collagen (CTX), a bone resorption marker, indicating the pivotal role of TNF-α in bone resorption34. In addition to increasing the inflammatory mediators, estrogen deficiency also enhances oxidative stress markers, further accelerating bone aging effects43.
The experimental model data have indicated that aging can significantly impact the response of CD8 + and CD4 + T cells44. The lower diversity of CD8 + T cells markedly limits the initiation of an effective immune response, leading to chronic inflammation38. Inflammatory cells such as T lymphocytes produce a large number of cytokines that stimulate the RANKL/RANK/OPG pathway, thereby promoting osteoclast differentiation and bone homeostasis disruption45. Moreover, B lymphocytes can affect bone metabolism by regulating the balance between RANKL and OPG46. In a normal state, B lymphocytes inhibit osteoclast activity and slow bone resorption. However, under inflammatory conditions, B lymphocytes may also express RANKL and promote osteoclastogenesis27,47,48. Osteoclastogenesis can be regulated with RANKL from both B cells and B cell-derived plasma cells49. In addition, T cell subgroups may also influence the role of B cells. For instance, when activated by Th1 cytokines, the B cells can inhibit osteoclastogenesis. Furthermore, Th2 cytokines can also stimulate B cells to promote osteoclastogenesis50,51. Although most studies focus on B cell’s association with osteoclastogenesis, a recent study found that B cells can inhibit osteoblast differentiation by activating extracellular signal-regulated kinase (ERK) and nuclear factor κ B (NF-kB) signaling pathways, thus inhibiting bone formation52.
These results may have significant clinical implications. Firstly, the negative correlation between MLR, NLR, and BTMs suggests that inflammation impairs bone turnover, thereby increasing patient’s susceptibility to OP. However, given the supportive effect of platelets on bone formation, PLR showed a positive correlation with BTMs. Furthermore, the threshold effect analysis indicated the presence of inflection points (k = 0.1364, k = 0.8226) between MLR, P1NP, and β-CTX. On the left side of the inflection point, MLR exhibited a significantly positive correlation with P1NP, while on the right side, MLR was significantly negatively correlated with P1NP. This implies that controlling the patient’s MLR within 0.1364 may enhance bone formation and, to some extent, improve the patient’s bone health. Moreover, MLR was significantly negatively correlated with β-CTX on the left side of the inflection point and positively correlated on the right side. In addition, an inflection point (k = 6, k = 7.1625) was also observed between NLR and P1NP or β-CTX, which was significantly negatively correlation on the left side, indicating that NLR could decrease P1NP and β-CTX to induce bone turnover damage. Whereas on the right side of the inflection point, the correlation was not significant (P-value > 0.05). The negative correlations observed between NLR, MLR, and BTMs may suggest the detrimental impact of inflammation on bone metabolism. This association provides a novel perspective for clinical strategies, suggesting that monitoring inflammation levels could be crucial when assessing the risk of osteoporotic fractures in elderly patients. Furthermore, the positive correlation between PLR and BTMs provides potential therapeutic avenues, indicating that platelet levels or function modulation might ameliorate the risk of OP in elderly individuals. These findings might offer new avenues for tailored assessment and preventive strategies for osteoporotic fractures. By integrating the relationship between IMs and BTMs, clinicians can more accurately evaluate the bone health status of elderly patients and implement appropriate interventions to reduce fracture risk, enhance their quality of life, and advance the development of preventive and therapeutic strategies for osteoporotic fractures.
This study has several strengths. For instance, three separate models were established to investigate the relationship between IMs and BTMs, and the effects of potential confounding variables were also considered. The results showed a negative correlation between MLR, NLR, and BTMs in patients with OP fractures, suggesting that high levels of MLR and NLR may impair BTMs in older populations. In addition, PLR was positively correlated with BTMs, possibly due to the supportive effect of platelets on bone formation. Therefore, monitoring the level of IMs in patients may be beneficial in reducing the high incidence and mortality rates associated with OP and fractures. In addition, these laboratory markers are simpler, more convenient, and more cost-effective compared with other tests that assess bone density. However, there are certain limitations in this study as well. Firstly, given its cross-sectional design, the study inherently harbors limitations, notably potential biases such as recall and selection biases. The researchers’ inability to manipulate exposure timing posed a challenge in definitively establishing causal relationships. Data collection, susceptible to constraints from measurement tools or data sources, may introduce distortions in information. Moreover, definitively establishing the temporal relationship between IMs and the onset of OP remains a complex endeavor. Secondly, there is potential involvement of genetic and non-genetic factors in the development of OP, but the data in this study were only adjusted for certain demographic, lifestyle, and laboratory variables during analysis. Moreover, the sample size was small and only comprised 740 identifiable samples. In addition, this study did not compare the PLR, NLR, and MLR with standard inflammation markers such as proinflammatory cytokines. Therefore, their capacity to accurately reflect the inflammatory status might be restricted. Lastly, these results may not be applicable to individuals of other races, as the study was conducted at a single institute on a relatively small patient population. To ensure the consistency and validity of these findings, additional and more extensive studies should be conducted using a multi-center randomized design, a wider range of biochemical markers, and a more diverse ethnic population.
Conclusion
In conclusion, this study, the relationship between IM and BTMs in osteoporotic fracture patients was investigated. The results indicate that both the NLR and MLR have adverse effects on bone formation and metabolism, thereby increasing the risk of osteoporotic fractures. In contrast, due to the supportive role of platelets in bone formation, the PLR shows a positive correlation with P1NP and β-CTX, indicating a beneficial effect on bone formation and metabolism to some extent. However, further prospective cohort studies and mechanistic research are necessary to validate these findings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
Study design: KL. Study conduct: CL and KL. Data collection: JX, SHG, MZX, KL, YQG and YQG, Data analysis: JX and SHG. Data interpretation: JX and KL. Drafting manuscript: JX. Revising manuscript content: SHG, MZX and KL. Approving the final version of the manuscript: CL, YQG and KL. JX and KL take responsibility for the integrity of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
The study was supported by National Natural Science Foundation of China (CN) (82172441), Suzhou City Major Disease Multicenter Clinical Research Project (CN) (DZXYJ202312), Special Funding for Jiangsu Province Science and Technology Plan (Key Research and Development Program for Social Development) (CN) (BE2023737), Kunshan Key Research and Development Program Project (CN) (KS2126) and Gusu Health Talent Plan Scientific Research Project (CN) (GSWS2022109).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jian Xu and Yue-qin Guo contributed equally to this work.
References
- 1.Delitala, A. P., Scuteri, A. & Doria, C. Thyroid hormone diseases and osteoporosis. J. Clin. Med.9(4) (2020). [DOI] [PMC free article] [PubMed]
- 2.Park, H. et al. Bone Mineral Density Screening interval and transition to osteoporosis in Asian women. Endocrinol. Metab. (Seoul)37(3), 506–512 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, Z. et al. Identification of diagnostic genes and effective drugs associated with osteoporosis treatment by single-cell RNA-Seq analysis and network pharmacology. Mediat. Inflamm. 2022, 6830635 (2022). [DOI] [PMC free article] [PubMed]
- 4.Sozen, T., Ozisik, L. & Basaran, N. C. An overview and management of osteoporosis. Eur. J. Rheumatol.4(1), 46–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu, D. et al. Romosozumab in osteoporosis: Yesterday, today and tomorrow. J. Transl. Med.21(1), 668 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kedzia, G. et al. Impact of Dietary protein on osteoporosis development. Nutrients15(21) (2023). [DOI] [PMC free article] [PubMed]
- 7.Kanis, J. A. et al. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos.16(1), 82 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, S. et al. Association between inflammatory markers and bone mineral density: A cross-sectional study from NHANES 2007–2010. J. Orthop. Surg. Res.18(1), 305 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, K. H. et al. Prevalence, clinical implication, and cause of spine hip discordance in Elderly patients with fragility hip fracture. J. Bone Metab.29(1), 51–57 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu, P. Y. et al. Role of fracture risk assessment tool and bone turnover markers in predicting all-cause and cardiovascular mortality in hemodialysis patients. Front. Med. (Lausanne)9, 891363 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kakali, L. et al. Fluctuation of bone turnover markers’ levels in samples of gingival crevicular fluid after orthodontic stimulus: A systematic review. Syst. Rev.11(1), 3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almalki, A. et al. Association between chronological age and IGF-1, IGFBP-3, and CTX levels in saliva of children through younger adult population with varying periodontal status. Children (Basel)9(9) (2022). [DOI] [PMC free article] [PubMed]
- 13.Bilinski, W. J. et al. Relationships between bone turnover markers and Factors Associated with metabolic syndrome in Prepubertal girls and boys. Nutrients14(6) (2022). [DOI] [PMC free article] [PubMed]
- 14.Zhang, S. & Ni, W. High systemic immune-inflammation index is relevant to osteoporosis among middle-aged and older people: A cross-sectional study. Immun. Inflamm. Dis.11(8), e992 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang, Y. et al. Systemic immune-inflammation index and bone mineral density in postmenopausal women: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2007–2018. Front. Immunol.13, 975400 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner, J., Rudi, T. & von Baehr, V. Osteoimmunology of tumor necrosis factor-alpha, IL-6, and RANTES/CCL5: A review of known and poorly understood inflammatory patterns in osteonecrosis. Clin. Cosmet. Investig. Dent.10, 251–262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J. et al. Alendronate-induced perturbation of the bone proteome and Microenvironmental Pathophysiology. Int. J. Med. Sci.18(14), 3261–3270 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosidlo, J. W. et al. Clinical significance and diagnostic utility of NLR, LMR, PLR and SII in the Course of COVID-19: A literature review. J. Inflamm. Res.16, 539–562 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, F. et al. Monocyte-to-lymphocyte ratio: A potential novel predictor for acute kidney injury in the intensive care unit. Ren. Fail.44(1), 1004–1011 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mongan, D. et al. Associations between plasma inflammatory markers and psychotic disorder, depressive disorder and generalised anxiety disorder in early adulthood: A nested case-control study. Brain Behav. Immun.111, 90–100 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Duan, Y. et al. Inflammatory markers in women with infertility: A cross-sectional study. Int. J. Gen. Med.16, 1113–1121 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuce, E. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) can predict spontaneous Preterm Birth? J. Inflamm. Res.16, 2423–2429 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, D. et al. NLR and PLR combined detection prognosis of patients with chronic hepatitis C-associated cirrhosis complicated by T2DM. J. Infect. Dev. Ctries.17(10), 1356–1361 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Ozturk, Z. A. et al. Inverse relationship between neutrophil lymphocyte ratio (NLR) and bone mineral density (BMD) in elderly people. Arch. Gerontol. Geriatr.57(1), 81–85 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Yolacan, H. & Guler, S. Inverse correlation between bone Mineral density and systemic Immune inflammation index in postmenopausal Turkish women. Cureus15(4), e37463 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, S. H. et al. The relationship of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio with bone Mineral Density in Korean Postmenopausal women. Chonnam Med. J.55(3), 150–155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song, B. W. et al. Associations of Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and monocyte-to-lymphocyte ratio with osteoporosis and incident vertebral fracture in Postmenopausal Women with Rheumatoid Arthritis: A single-Center Retrospective Cohort Study. Medicina (Kaunas)58(7) (2022). [DOI] [PMC free article] [PubMed]
- 28.Ma, W. C. et al. Circulating platelet concentration is associated with bone mineral density in women. Arch. Osteoporos.17(1), 44 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Deng, T. et al. Application of PRP in Chloasma: A meta-analysis and systematic review. Comput. Intell. Neurosci.2022, 7487452. (2022). [DOI] [PMC free article] [PubMed]
- 30.Liu, T. et al. Chondroitin sulfate alleviates osteoporosis caused by calcium deficiency by regulating lipid metabolism. Nutr. Metab. (Lond)20(1), 6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salamanna, F. et al. Platelet features and derivatives in osteoporosis: A rational and systematic review on the best evidence. Int. J. Mol. Sci.21(5) (2020). [DOI] [PMC free article] [PubMed]
- 32.Shindo, S. et al. Dual-function semaphorin 4D released by platelets: Suppression of Osteoblastogenesis and Promotion of Osteoclastogenesis. Int. J. Mol. Sci.23(6) (2022). [DOI] [PMC free article] [PubMed]
- 33.Long, C. L. & Humphrey, M. B. Osteoimmunology: The expanding role of immunoreceptors in osteoclasts and bone remodeling. Bonekey Rep.1 (2012). [DOI] [PMC free article] [PubMed]
- 34.Damani, J. J. et al. The role of Prunes in modulating Inflammatory pathways to improve Bone Health in Postmenopausal Women. Adv. Nutr.13(5), 1476–1492 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sapir-Koren, R. & Livshits, G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone103, 102–115 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Kovtun, A. et al. Neutrophils in tissue trauma of the skin, bone, and lung: Two sides of the same Coin. J. Immunol. Res.2018, 8173983 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajishengallis, G. et al. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin. Immunol.28(2), 146–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, W. et al. Role of immune system in the pathophysiology of different types of osteoporosis. Front. Endocrinol. (Lausanne)13, 965258 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao, R. Immune regulation of bone loss by Th17 cells in oestrogen-deficient osteoporosis. Eur. J. Clin. Investig.43(11), 1195–1202 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Lin, Z. et al. Protective effect of alpha-lipoic acid against antimycin A cytotoxicity in MC3T3-E1 osteoblastic cells. Cell. Stress Chaperones22(1), 5–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, J. et al. Systemic immune-inflammation index is associated with decreased bone mass density and osteoporosis in postmenopausal women but not in premenopausal women. Endocr. Connect.12(2) (2023). [DOI] [PMC free article] [PubMed]
- 42.Koelman, L. et al. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing16, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manolagas, S. C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev.31(3), 266–300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stojic-Vukanic, Z. et al. Age and sex determine CD4 + T cell stimulatory and polarizing capacity of rat splenic dendritic cells. Biogerontology21(1), 83–107 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Walsh, M. C. & Choi, Y. Regulation of T cell-associated tissues and T cell activation by RANKL-RANK-OPG. J. Bone Min. Metab.39(1), 54–63 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla, P. et al. Interleukin 27 (IL-27) alleviates bone loss in estrogen-deficient conditions by induction of early growth Response-2 gene. J. Biol. Chem.292(11), 4686–4699 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piemontese, M. et al. Cortical bone loss caused by glucocorticoid excess requires RANKL production by osteocytes and is associated with reduced OPG expression in mice. Am. J. Physiol. Endocrinol. Metab.311(3), E587–E593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yi, L. et al. Gene modification of transforming growth factor beta (TGF-beta) and interleukin 10 (IL-10) in suppressing Mt Sonicate Induced Osteoclast formation and bone absorption. Med. Sci. Monit.24, 5200–5207 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komatsu, N. et al. Plasma cells promote osteoclastogenesis and periarticular bone loss in autoimmune arthritis. J. Clin. Invest.131(6) (2021). [DOI] [PMC free article] [PubMed]
- 50.Choi, Y. & Kim, J. J. B cells activated in the presence of Th1 cytokines inhibit osteoclastogenesis. Exp. Mol. Med.35(5), 385–392 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Zhao, X. et al. Delayed allogeneic skin graft rejection in CD26-deficient mice. Cell. Mol. Immunol.16(6), 557–567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun, W. et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun.9(1), 5127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.