Abstract
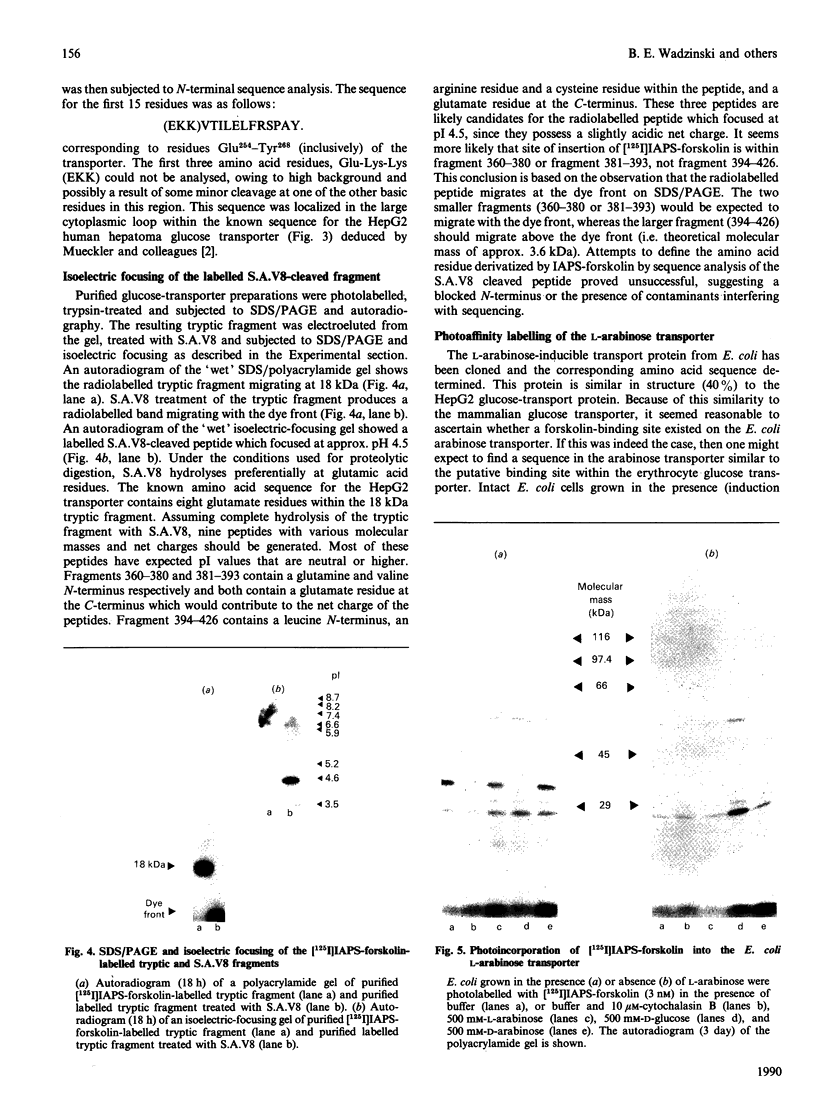
Chemical and proteolytic digestion of intact erythrocyte glucose transporter as well as purified transporter protein has been used to localize the derivatization site for the photoaffinity agent 3-[125I]iodo-4-azido-phenethylamino-7-O-succinyldeacetylforskol in [( 125I]IAPS-forskolin). Comparison of the partial amino acid sequence of the labelled 18 kDa tryptic fragment with the known amino acid sequence for the HepG2 glucose transporter confirmed that the binding site for IAPS-forskolin is between the amino acid residues Glu254 and Tyr456. Digestion of intact glucose transporter with Pronase suggests that this site is within the membrane bilayer. Digestion of labelled transporter with CNBr generated a major radiolabelled fragment of Mr approximately 5800 putatively identified as residues 365-420. Isoelectric focusing of Staphylococcus aureus V8 proteinase-treated purified labelled tryptic fragment identified two peptides which likely correspond to amino acid residues 360-380 and 381-393. The common region for these radiolabelled peptides is the tenth putative transmembrane helix of the erythrocyte glucose transporter, comprising amino acid residues 369-389. Additional support for this conclusion comes from studies in which [125I]APS-forskolin was photoincorporated into the L-arabinose/H(+)-transport protein of Escherichia coli. Labelling of this transport protein was protected by both cytochalasin B and D-glucose. The region of the erythrocyte glucose transporter thought to be derivatized with IAPS-forskolin contains a tryptophan residue (Trp388) that is conserved in the sequence of the E. coli arabinose-transport protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A., Henderson P. J. Homologies between sugar transporters from eukaryotes and prokaryotes. Annu Rev Physiol. 1989;51:459–471. doi: 10.1146/annurev.ph.51.030189.002331. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M. T., Alvarez J., Panico M., Gibbs A. F., Morris H. R., Chapman D., Baldwin S. A. Investigation of the structure and function of the human erythrocyte glucose transporter by proteolytic dissection. Biochim Biophys Acta. 1987 Dec 11;905(2):295–310. doi: 10.1016/0005-2736(87)90458-5. [DOI] [PubMed] [Google Scholar]
- Cairns M. T., Elliot D. A., Scudder P. R., Baldwin S. A. Proteolytic and chemical dissection of the human erythrocyte glucose transporter. Biochem J. 1984 Jul 1;221(1):179–188. doi: 10.1042/bj2210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Su C., Pessin J. E., Mora R., Gitomer W., Czech M. P. Photoaffinity labeling of the human erythrocyte D-glucose transporter. J Biol Chem. 1982 May 25;257(10):5419–5425. [PubMed] [Google Scholar]
- Daruwalla K. R., Paxton A. T., Henderson P. J. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem J. 1981 Dec 15;200(3):611–627. doi: 10.1042/bj2000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Meeran K., Cairns M. T., Baldwin S. A. Peptide-specific antibodies as probes of the orientation of the glucose transporter in the human erythrocyte membrane. J Biol Chem. 1987 Jul 5;262(19):9347–9352. [PubMed] [Google Scholar]
- Deziel M. R., Jung C. Y., Rothstein A. The topology of the major band 4.5 protein component of the human erythrocyte membrane: characterization of reactive cysteine residues. Biochim Biophys Acta. 1985 Sep 25;819(1):83–92. doi: 10.1016/0005-2736(85)90198-1. [DOI] [PubMed] [Google Scholar]
- Deziel M. R., Rothstein A. Proteolytic cleavages of cytochalasin B binding components of band 4.5 proteins of the human red blood cell membrane. Biochim Biophys Acta. 1984 Sep 19;776(1):10–20. doi: 10.1016/0005-2736(84)90245-1. [DOI] [PubMed] [Google Scholar]
- Deziel M., Pegg W., Mack E., Rothstein A., Klip A. Labelling of the human erythrocyte glucose transporter with 3H-labelled cytochalasin B occurs via protein photoactivation. Biochim Biophys Acta. 1984 May 30;772(3):403–406. doi: 10.1016/0005-2736(84)90157-3. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Gibbs A. F., Chapman D., Baldwin S. A. Proteolytic dissection as a probe of conformational changes in the human erythrocyte glucose transport protein. Biochem J. 1988 Dec 1;256(2):421–427. doi: 10.1042/bj2560421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem. 1984 Nov 1;142(2):421–436. doi: 10.1016/0003-2697(84)90486-x. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Griffin J. F., Rampal A. L., Jung C. Y. Inhibition of glucose transport in human erythrocytes by cytochalasins: A model based on diffraction studies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3759–3763. doi: 10.1073/pnas.79.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., Macpherson A. J. Assay, genetics, proteins, and reconstitution of proton-linked galactose, arabinose, and xylose transport systems of Escherichia coli. Methods Enzymol. 1986;125:387–429. doi: 10.1016/s0076-6879(86)25033-8. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Karim A. R., Karim B. Photolabeling of erythrocyte and adipocyte hexose transporters using a benzophenone derivative of bis(D-mannose). Biochim Biophys Acta. 1988 Dec 8;946(1):75–84. doi: 10.1016/0005-2736(88)90459-2. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Parkar B. A., Midgley P. J. Exofacial photoaffinity labelling of the human erythrocyte sugar transporter. Biochim Biophys Acta. 1986 Feb 13;855(1):115–126. doi: 10.1016/0005-2736(86)90195-1. [DOI] [PubMed] [Google Scholar]
- Holman G. D., Rees W. D. Photolabelling of the hexose transporter at external and internal sites: fragmentation patterns and evidence for a conformational change. Biochim Biophys Acta. 1987 Mar 12;897(3):395–405. doi: 10.1016/0005-2736(87)90437-8. [DOI] [PubMed] [Google Scholar]
- Horazdovsky B. F., Hogg R. W. High-affinity L-arabinose transport operon. Gene product expression and mRNAs. J Mol Biol. 1987 Sep 5;197(1):27–35. doi: 10.1016/0022-2836(87)90606-1. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ishii T., Tillotson L. G., Isselbacher K. J. Facilitated glucose transporter of human erythrocyte: proteolytic mapping of the [3H]cytochalasin B photoaffinity-labeled transporter polypeptide. Biochim Biophys Acta. 1985 Nov 8;832(1):14–21. doi: 10.1016/0167-4838(85)90169-4. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Habberfield A. D., Simpson I. A., Laurenza A., Seamon K. B. Activation of adenylate cyclase and inhibition of glucose transport in rat adipocytes by forskolin analogues: structural determinants for distinct sites of action. Mol Pharmacol. 1988 Apr;33(4):449–453. [PubMed] [Google Scholar]
- Karim A. R., Rees W. D., Holman G. D. Binding of cytochalasin B to trypsin and thermolysin fragments of the human erythrocyte hexose transporter. Biochim Biophys Acta. 1987 Sep 3;902(3):402–405. doi: 10.1016/0005-2736(87)90208-2. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D., Cohen A., Leung C. Y. Chemical and genetic comparison of the glucose and nucleoside transporters. Biochem Cell Biol. 1986 Nov;64(11):1170–1180. doi: 10.1139/o86-154. [DOI] [PubMed] [Google Scholar]
- Lienhard G. E., Crabb J. H., Ransome K. J. Endoglycosidase f cleaves the oligosaccharides from the glucose transporter of the human erythrocyte. Biochim Biophys Acta. 1984 Jan 25;769(2):404–410. doi: 10.1016/0005-2736(84)90324-9. [DOI] [PubMed] [Google Scholar]
- MacPherson A. J., Jones-Mortimer M. C., Henderson P. J. Identification of the AraE transport protein of Escherichia coli. Biochem J. 1981 Apr 15;196(1):269–283. doi: 10.1042/bj1960269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden M. C., Davis E. O., Baldwin S. A., Moore D. C., Henderson P. J. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987 Feb 12;325(6105):641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Parkar B. A., Midgley P. J., Holman G. D. Interaction of hydrophobic bis (D-mannose) derivatives with adipocyte and erythrocyte sugar transport systems. Biochim Biophys Acta. 1985 Mar 28;814(1):103–110. doi: 10.1016/0005-2736(85)90424-9. [DOI] [PubMed] [Google Scholar]
- Resek J. F., Ruoho A. E. Photoaffinity labeling the beta-adrenergic receptor with an iodoazido derivative of norepinephrine. J Biol Chem. 1988 Oct 5;263(28):14410–14416. [PubMed] [Google Scholar]
- Shanahan M. F. Characterization of cytochalasin B photoincorporation into human erythrocyte D-glucose transporter and F-actin. Biochemistry. 1983 May 24;22(11):2750–2756. doi: 10.1021/bi00280a024. [DOI] [PubMed] [Google Scholar]
- Shanahan M. F. Cytochalasin B. A natural photoaffinity ligand for labeling the human erythrocyte glucose transporter. J Biol Chem. 1982 Jul 10;257(13):7290–7293. [PubMed] [Google Scholar]
- Shanahan M. F., D'Artel-Ellis J. Orientation of the glucose transporter in the human erythrocyte membrane. Investigation by in situ proteolytic dissection. J Biol Chem. 1984 Nov 25;259(22):13878–13884. [PubMed] [Google Scholar]
- Shanahan M. F., Wadzinski B. E., Lowndes J. M., Ruoho A. E. Photoaffinity labeling of the human erythrocyte monosaccharide transporter with an aryl azide derivative of D-glucose. J Biol Chem. 1985 Sep 15;260(20):10897–10900. [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Szkutnicka K., Tschopp J. F., Andrews L., Cirillo V. P. Sequence and structure of the yeast galactose transporter. J Bacteriol. 1989 Aug;171(8):4486–4493. doi: 10.1128/jb.171.8.4486-4493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. F., Gagneja G. L. A model for the mode of action of cytochalasin B inhibition of D-glucose transport in the human erythrocyte. Can J Biochem. 1975 Oct;53(10):1078–1084. doi: 10.1139/o75-148. [DOI] [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Clark R. B., Ruoho A. E. Identification of the glucose transporter in mammalian cell membranes with a 125I-forskolin photoaffinity label. Biochem J. 1988 Nov 1;255(3):983–990. doi: 10.1042/bj2550983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadzinski B. E., Shanahan M. F., Ruoho A. E. Derivatization of the human erythrocyte glucose transporter using a novel forskolin photoaffinity label. J Biol Chem. 1987 Dec 25;262(36):17683–17689. [PubMed] [Google Scholar]
- Walmsley A. R. The dynamics of the glucose transporter. Trends Biochem Sci. 1988 Jun;13(6):226–231. doi: 10.1016/0968-0004(88)90089-8. [DOI] [PubMed] [Google Scholar]
- Weber T. M., Eichholz A. Characterization of a photosensitive glucose derivative. A photoaffinity reagent for the erythrocyte hexose transporter. Biochim Biophys Acta. 1985 Jan 25;812(2):503–511. doi: 10.1016/0005-2736(85)90325-6. [DOI] [PubMed] [Google Scholar]
- Wheeler T. J., Hinkle P. C. The glucose transporter of mammalian cells. Annu Rev Physiol. 1985;47:503–517. doi: 10.1146/annurev.ph.47.030185.002443. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Slaughter C., Ruoho A. E., Ross E. M. The catecholamine binding site of the beta-adrenergic receptor is formed by juxtaposed membrane-spanning domains. J Biol Chem. 1988 Jun 15;263(17):7925–7928. [PubMed] [Google Scholar]






