Abstract
Wolbachia bacteria encompass noteworthy reproductive manipulators of their arthropod hosts. which influence host reproduction to favour their own transmission, also exploiting toxin–antitoxin systems. Recently, multiple other bacterial symbionts of arthropods have been shown to display comparable manipulative capabilities. Here, we wonder whether such phenomena are truly restricted to arthropod hosts. We focused on protists, primary models for evolutionary investigations on eukaryotes due to their diversity and antiquity, but still overall under-investigated. After a thorough re-examination of the literature on bacterial–protist interactions with this question in mind, we conclude that such bacterial ‘addictive manipulators’ of protists do exist, are probably widespread, and have been overlooked until now as a consequence of the fact that investigations are commonly host-centred, thus ineffective to detect such behaviour. Additionally, we posit that toxin–antitoxin systems are crucial in these phenomena of addictive manipulation of protists, as a result of recurrent evolutionary repurposing. This indicates intriguing functional analogy and molecular homology with plasmid–bacterial interplays. Finally, we remark that multiple addictive manipulators are affiliated with specific bacterial lineages with ancient associations with diverse eukaryotes. This suggests a possible role of addictive manipulation of protists in paving the way to the evolution of bacteria associated with multicellular organisms.
Keywords: toxin–antitoxin systems, Wolbachia, intracellular bacteria, Rickettsiales, professional symbionts, protist
1. Overview and purposes
Multiple diverse bacteria live in association with a great variety of eukaryotic hosts [1–3]. Such symbiotic associations are widespread, exhibiting different shades of effects on the involved partners, ranging from mutualism to parasitism [4], with the same partnership varying depending on physiological states or external conditions [5,6]. Along with evolution, the functional properties of the symbiotic partners can be deeply influenced by the association [7].
A noteworthy and peculiar type of bacterial–host interaction is reproductive manipulation, exerted by some phylogenetically diverse bacteria (e.g. Wolbachia) on their arthropod hosts, with cytoplasmic incompatibility (CI) as the most distinctive instance [8,9]. As a result, the new host generation from an infected male cannot survive unless it receives the bacterium from the female (figure 1). This tight association might superficially resemble an obligatory mutualism. However, it is due to the ability of the bacterium to make the host ‘addicted’ and unable to get rid of the bacterium, rather than to the provision of benefits.
Figure 1.
Addictive manipulation mechanisms exerted by bacterial symbionts on their diverse eukaryotic hosts, involving molecular determinants linked to mobile genetic elements. In this way, the bacteria ensure their own proliferation by promoting their vertical transmission. Wolbachia (Rickettsiales) manipulates the reproduction of its vertebrate hosts by CI (and other mechanisms). The bacterium is vertically transmitted to the offspring only by the females. Gametes from infected males carry a prophage-linked toxin that kills the embryos, unless female gametes carry the bacterium with a cognate antitoxin, thus favouring the spread and maintenance of the bacterium in the host populations. Similarly, L. jeonii (Legionellales) manipulates the asexual life cycle of its unicellular eukaryotic hosts. When healthy amoebas become infected, they become unable to remove the bacteria. Most likely, a plasmid-encoded toxin by the bacteria epigenetically acts on host gene expression, a modification that persists after bacterial loss, and that can be rescued only in the presence of live bacteria. Caedimonas (Holosporales) and Caedibacter (Thiotrichales) counteract their loss by Paramecium hosts by an indirect mechanism. The bacteria produce a plasmid-encoded toxin, against which their hosts are protected by the cognate antitoxin. If a host loses the symbiont, it becomes sensitive to the toxin and will be killed when ingesting symbionts released by its, still-infected, sister cells.
A recent study explored the concept of ‘evolutionary addiction’ from the host perspective [10], proposing that, after prolonged associations with their microbiome, hosts may evolve dependence on the bacteria, thus becoming secondarily addicted (see box 1).
Box 1. Addictive symbiont–host interactions.
Addictive symbiont–host interactions imply that the host becomes damaged, up to potential death, if symbionts are lost, regardless of the direct benefits provided by the symbionts. As a consequence, the association is tightened, with potential advantages for the symbionts. The most thoroughly studied cases are those of reproductive manipulation exerted by Wolbachia, Spiroplasma and other bacteria on arthropods, through CI, male killing, feminization or parthenogenesis.
Additionally, the concept of evolutionary addiction was recently proposed, namely that coexistence with the symbionts will cause different evolutionary processes in the host, which would eventually result in dysregulation if the bacteria are removed [10]. Specifically, according to Hammer, ‘adaptive accommodation’ implies the irreversible accommodation of host regulatory mechanisms in the presence of bacteria, while ‘compensated trait loss’ implies that the redundancy of certain metabolic and functional features in host and symbionts may result in the loss of the respective genes in the host, which would need compensation by the symbionts.
On the other hand, in the case of reproductive manipulation, the addiction would depend directly on active properties exerted by the bacteria, specifically, in the experimentally validated cases of Wolbachia and Spiroplasma, by the action of toxins and antitoxins [9,11,12].
Here, we propose the concept of ‘addictive manipulation’, by generalizing the case of reproductive manipulation of arthropods to other eukaryotes, in particular protists. Under this condition, the hosts are addicted to bacterial symbionts as a result of some active property evolved and exerted by the symbionts themselves, without directly implying any evolutionary change in the hosts. As in the specific cases of reproductive manipulators of arthropods, addictive manipulation likely takes place thanks to molecular toxin–antitoxin systems and may consist of different phenomena depending on the physiology and ecology of the host and symbionts (see also box 2 ‘How to test addictive manipulation’).
Accordingly, host–symbionts interactions in cases of addictive manipulation expectedly result in complex interplays, which, to be fully delineated, should take into account several other features, such as the potential capability of symbionts to spread horizontally, and the interaction of host and/or professional symbionts with other organisms, including non-infected hosts (see the case of Wolbachia or Caedimonas/Caedibacter) [8,13].
At an evolutionary scale, we highlight the possibility that addictive manipulation could have had important consequences in the evolution of bacterial lineages with ancient and evolutionarily stable interactions with eukaryotic hosts (e.g. Rickettsiales, Legionellales, Holosporales, Chlamydiae).
Still, addiction may also be the consequence of active mechanisms exerted by the bacteria on their hosts, as in the case of CI. One could wonder whether such primary addictions are evolutionary oddities restricted to a few specific cases, or whether the phenomenon has wider evolutionary and ecological significance. Following this line of thought, here we explore the presence of addiction in host–bacterial interactions from the perspective of the bacteria, rather than sticking to a more ‘conventional’ host-centric approach. We focus on unicellular eukaryotic hosts (i.e. protists), which constitute the vast majority of eukaryotes including the most ancestral lineages [14,15], thus being fundamental for understanding the eukaryotic features and their evolution [16]. Bacterial–protist symbioses are widespread [1], but neglected, and in most cases their foundations still await to be understood. Given the distinctive and diverse physiology and ecology of protists [17–19], these associations only partly fit to ‘reference’ models of bacterial–host symbioses, chiefly nutritional mutualists of animal hosts [1].
Here, we reason on whether the origin and maintenance of some bacterial–protist associations could be explained by a process similar to the known cases of animals addicted to their bacterial symbionts, namely by an ‘addictive manipulation’ of host reproduction. Therefore, we examine the literature on bacterial–protist associations looking for indications of potential addictive phenomena and mechanisms. According to several lines of evidence, we propose that addictive manipulation (figure 1; box 1) is quite common, though not properly recognized, among bacterial–protist associations, possibly being fundamental in the evolution of many such interactions.
We will start by presenting the most relevant features of well-studied addictive manipulators in arthropods, exemplified by Wolbachia. Then, we will move to bacterial–protist symbioses, reasoning on the expected features of addictive manipulation in those associations, and why, in our view, available clues have not been properly recognized. Subsequently, we will focus on selected cases in which we found convincing signs of addictive manipulation, showing how their re-interpretation allowed us to draw an evolutionary framework that also accounts for possible underlying molecular mechanisms. We will then conclude with a general evolutionary perspective on addictive manipulation and its role in the evolution of bacterial lineages with evolutionarily conserved interactions with protists and other eukaryotes.
2. Wolbachia, a prototypical addictive manipulator
Reproductive manipulation is a quite well-known phenomenon in arthropod hosts, which can be made addictive by multiple diverse bacterial symbionts, including Rickettsiales (Wolbachia, Rickettsia and Mesenetia—formerly Mesenet [20]) [8,9,11,21–25], Mollicutes (Spiroplasma) [26], Cytophagales (Cardinium) [27] and Legionellales (Rickettsiella) [28].
Wolbachia is the most studied, and noteworthy enough to deserve the title of ‘master manipulator of invertebrate biology’ [8]. We will use this symbiont to delineate the major features of addictive manipulators. Wolbachia is widespread in insects and other arthropods [29,30], thanks to multiple strategies enhancing its vertical transmission through host generations, namely feminization, parthenogenesis, male killing and the intriguing CI [8]. CI makes crosses between infected males and non-infected females non-viable, thus favouring the fitness of infected females. In this way, since the symbionts’ vertical inheritance relies solely on transovarial transmission from the mother to the offspring, the bacteria massively increase their own fitness (figure 1). The effect of CI is so powerful that it is being successfully used for biocontrol of arthropod vectors of pathogens [31,32].
While reproductive manipulation has been known for a long time, its molecular mechanisms were elusive until recently [9,11,12,33]. A modification-rescue model had been proposed for CI [34], under which some bacterial-derived factor ‘poisons’ the male gametes, leading to the unsuccessful development of the zygote, and can be counteracted only by a rescue factor present in the infected female gametes. Two Wolbachia proteins responsible for these mechanisms were recently discovered [35,36] and shown to form a complex, which can act by toxin–antitoxin (or ‘toxin–antidote’) regulation [37] (figure 1). The toxic effect is probably dysregulation of ubiquitination [36,38,39], linked to defects in condensation of the male pronuclei [9,40]. Interestingly, the two involved genes are adjacent in the Wolbachia genome, within a putative phage-derived region, and their expression appears to be linked to prophage induction [35]. Several paralogues to these genes are present in different Wolbachia strains and may account for mechanisms of reproductive manipulation other than CI, host specificities, and/or competition between strains [12,35,41]. Among the very few homologs of these genes outside Wolbachia, notable are those found in Rickettsia and Spiroplasma [24]. Taken together, these data indicate a spread of CI-inducing factors by horizontal gene transfer (HGT), possibly driven by phages, suggesting that other symbionts could, by molecularly homologous mechanisms, be analogous ‘master manipulators’.
3. Addictive manipulation of unicellular eukaryotic hosts
Drawing an ideal parallel with the cases involving arthropod hosts listed above, one could wonder whether some bacterial symbionts associated with protists could exert addictive manipulation on their hosts, possibly exploiting analogous modification-rescue processes.
At first glance, it might seem surprising that, despite the diversity and abundance of protists and their bacterial symbionts, an actual addictive manipulation has never been clearly recognized and demonstrated. However, in our view, several aspects should be taken into account, in particular, the strong bias in the hosts investigated in most studies. Indeed, despite valuable past (e.g. [42–47]) and recent (e.g. [5,48–59]) investigations, bacterial–protist partnerships are still profoundly under-investigated compared with symbioses involving bacteria and multicellular hosts.
Moreover, studying such associations presents multiple inherent limitations, making any hint of addictive manipulation difficult to detect and likely disregarded. In metazoan hosts, vertical transmission is accomplished during sexual reproduction, allowing researchers a clear observation of the effects of potential addictive manipulation exerted by the symbionts (particularly, distortion of sex ratio in the progeny). On the other hand, unicellular eukaryotes most frequently (though not exclusively, see also box 2) reproduce asexually by cell division, which may nuance and completely ‘hide’ the effect of addiction, such as, plausibly, the death of daughter cells that did not receive the bacteria. Indeed, this is inherently hard to distinguish from a primary obligatory mutualism, in which the host is ‘simply’ dependent on the bacteria (see box 2 for potential proof-of-principle experiments).
Box 2. How to test addictive manipulation.
The inherent complexity of addictive manipulation hampers its proper identification in protists. Possible approaches to discern it could involve modelling bacterial–host interactions in cases of addictive manipulation, for instance by analogy with models of addiction of bacterial cells on plasmids [60], and then subjecting those models to experimental validation.
Herein, it seems appropriate to outline some simple general criteria as a starting ground, in particular by evaluating the effect of symbiont removal on the host. For this purpose, we assume that (i) the host is reproducing asexually, (ii) host survival, reproductive success and/or wellbeing can be measured (here collectively termed as ‘fitness’), (iii) a method for removing the addictive manipulator is available (e.g. antibiotics), and (iv) any addictive manipulation phenomenon is not 100% effective. The latter assumption seems reasonable based on the available knowledge on Wolbachia, Caedibacter/Caedimonas and L. jeonii, for which the addictive manipulation mechanisms are conditionally regulated (e.g. by prophage inductions) according to physiological states or external factors such as temperature [9,13,44]. Although this may represent a confounding factor, it can also be instrumental in discriminating an addictive manipulator from a necessary mutualist (see below).
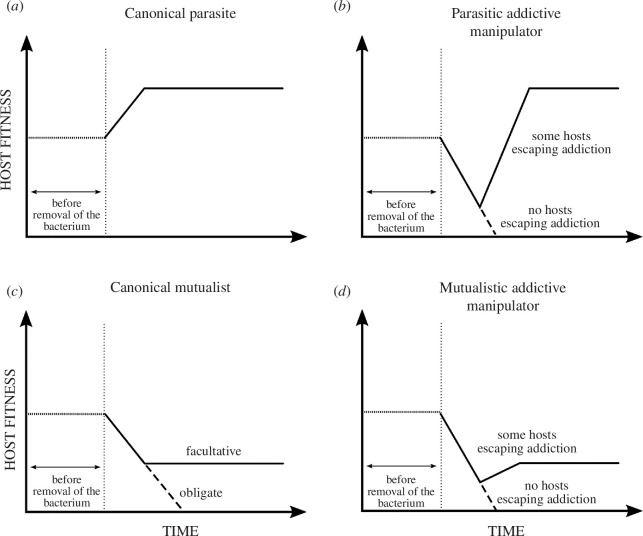
If an addictive manipulator is removed, we expect an initial reduction of host fitness, up to complete death, or followed by a subsequent recovery (by hosts escaping from non-100% effective addictive manipulation) (figure 2). The post-recovery fitness level would depend on whether the overall effect of the addictive manipulator is mutualistic or parasitic. Notably, the end result would be indistinguishable from canonical parasites or canonical mutualists, and, if taken alone, may mislead in the classification of the interaction. This seems to be the case of L. jeonii, originally interpreted as a necessary mutualist [61]. Rather, it is the temporal trajectory of the variation of fitness that matters, as the fitness ‘reduction–recovery’ process would be distinctive for an addictive manipulator (figure 2).
Inevitably, such an approach is prone to confounding factors and to detection limits (in particular relative to the speed of the process and the effect size). We put forward that identifying molecular determinants could complement such limits, not only demonstrating the mechanism for addiction manipulation of protists (or other asexual hosts) but also validating that it is actually taking place.
Additionally, it should be accounted for that several protist hosts have relatively common and discernible sexual processes, e.g. dictyosteliid amoebae and ciliates [62–64]. Thus, approaches more comparable to those traditionally employed to investigate addictive manipulators of insects could be attempted to investigate possible addictive manipulators of these protists (e.g. [13,42,64–66]).
Actually, while a number of bacterial–protist partnerships appear to be transient and unstable [1,67,68], several others have been stably maintained, even for decades [50,69,70], with targeted attempts to remove the bacteria frequently unsuccessful [48,71–73]. These data clearly indicate the presence of a ‘bond’ between those bacteria and their hosts, which in some cases could be assimilated to ‘true’ mutualisms [57,58]. However, multiple other cases display additional and differential features, which, we argue, are suggestive of ongoing addictive manipulation. Closely related bacteria, even belonging to the same species [69,70,74], are hosted by phylogenetically, physiologically and ecologically diverse hosts. For instance, the Rickettsiales bacterium Megaera (formerly, Megaira [75]) polyxenophila can be associated with heterotrophic protists such as ciliates, multiple photoautotrophic algae and even cnidarians [50,52,53,76]. Although, in principle, the bacteria may be able to provide universal mutualistic benefits to such host arrays, it seems meaningful to consider a potential involvement of addictive manipulation, which could enable tight associations to diverse hosts thanks to effector molecules with broad specificity on eukaryotic targets.
Moreover, protists that have been repeatedly found as hosts for stably associated bacteria (e.g. Paramecium aurelia, P. caudatum, Acanthamoeba) are also commonly found devoid of any bacterium [42,42,73,73]. This reminds us of Wolbachia present in multiple diverse arthropod species, with variable prevalence [29]. Eventually, many bacteria could be experimentally removed from their protist hosts by elaborate but potentially fluky approaches [77,78], with the hosts then surviving and often thriving [73,78]. This is sharply different from primary dependence on the bacteria, being instead reminiscent of addictive manipulators, which are not required by their hosts inherently.
Addictive manipulative mechanisms are also unlikely to be ‘all-or-nothing’ phenomena in every condition (figure 2; box 2). Even for Wolbachia, reproductive manipulation does not show full penetrance, being dependent on host genetic background [79] and age [80], as well as on external factors [81], so that in some hosts, it was initially completely overlooked [9].
Figure 2.
Comparisons of idealized fitness responses of a protist (or another asexually reproducing host) to the removal (dotted vertical line) of an addictive manipulator (b,d) in comparison to the removal of a canonical parasite (a) or a canonical mutualist (c). In turn, depending on other potential concomitant interactions, a manipulator may have overall detrimental or beneficial effects, respectively, behaving as a parasitic (b) or mutualistic (d) addictive manipulator.
Thus, the best indications for an ‘elusive’ trait such as addictive manipulation in protist hosts likely need comprehensive comparative investigations aimed at evidencing general trends, as herein.
4. Bacteria addictively manipulating protist hosts
Here, we highlight those cases showing, in our view, the most distinctive and convincing signs of addictive manipulation of protist hosts exerted by associated bacteria.
The first instance pertains to Legionella jeonii (initially termed ‘X-bacteria’ [44]), on which an interesting set of experiments was performed decades ago [82]. When introduced in symbiont-free Amoeba cells, it repeatedly produced harmful effects (reduced size, fragility, poor clonability, slower growth or even death) [43]. However, after some time, surviving subpopulations of amoebas became healthier and, surprisingly, dependent on the symbiont [83], so antibiotic treatments led not only to bacterial death, but also to the demise of the host [61]. In principle, these findings could be interpreted as the consequence of an experimentally induced mutualism (or an evolutionary addiction sensu Hammer [10]).
The observed effects were partly correlated with specific pairings of nucleus and cytoplasm (containing the bacteria), as experimental combinations of nuclei from infected cells with cytoplasms from non-infected ones were mostly unviable. However, such combinations survived in a minority of cases, thus not presenting an absolute ‘all-or-nothing’ outcome, as would be most probable in an ‘idealized’ obligatory mutualism.
Even more remarkably, the same series of effects were observed when L. jeonii was transferred to other Amoeba cells, which in turn eventually became dependent on the bacteria [83,84]. These data strongly indicate that the factor(s) leading to the non-breakability of the association are derived from L. jeonii. The mechanism of this interaction is unknown but was tentatively linked to a plasmid-encoded 29 kDa protein [44], which can influence host gene expression [85] after being translocated to the host cytoplasm and nucleus [86].
To summarize, the available data point to L. jeonii possessing the ability to manipulate its Amoeba host, making it addicted through context-dependent gene regulation involving plasmids, and resulting in host epigenetic mechanisms (figure 1).
Other noteworthy and long-time known cases are those of Caedibacter taeniospiralis (Thiotrichales) and Caedimonas varicaedens (Holosporales) [13,87], which, although phylogenetically unrelated, were originally grouped together in a single genus for their many shared traits [88]. These bacteria are typically intracellularly hosted by ciliate protists of the genus Paramecium, and are able to confer them a ‘killer trait’.
Under certain conditions such as starvation, part of the bacteria arrest their replication and produce R-bodies, i.e. large proteinaceous elements shaped as coiled ribbons [45]. Some bacteria are released extracellularly, and, if endocytosed by Paramecium cells lacking the symbiont, the acidification of the digestive vacuoles causes the unrolling of the R-bodies and the release of a still uncharacterized toxin [13]. This leads to Paramecium cell death by multiple alternative mechanisms, depending on the bacterial and host strain/species, namely hump killing, spin killing, vacuolization and paralysis [13,45]. These multifaceted lethal effects are reminiscent of the multiple reproductive manipulation phenomena by Wolbachia in arthropods. The Caedimonas/Caedibacter bacteria are assumed to produce an antitoxin that rescues the toxicity, thus protecting their natural hosts. R-bodies and possibly also toxin–antitoxin genes are encoded into plasmids that also bear phage genes [46,47], and the presence of R-bodies was associated with prophage induction [89].
The killer trait was proposed to provide a competitive advantage to the Paramecium hosts towards non-infected conspecifics, thus being indicative of mutualism [13]. In addition, we propose that it is an addictive manipulation phenomenon, in which the host that loses the symbionts is ‘punished’ indirectly, thanks to the probable close presence of ‘sister cells’ still bearing the bacteria (figure 1). One could say that Caedimonas/Caedibacter kills paramecia that have lost it pretty much as Wolbachia sterilizes females that do not have it. From an evolutionary perspective, competitive advantages would then represent an exaptation of a pre-existing control mechanism acting on the host cells, further strengthening the association.
Interestingly, in the past decades, several other bacteria were found to cause killer effects in protist hosts [90]. Among them, more recent molecular and phylogenetic characterizations revealed that Lyticum spp. are part of the Rickettsiales [65], which also encompass Wolbachia and other addictive manipulators of arthropods.
5. Mechanisms and evolution of addictive manipulation
The cases exposed above present common molecular traits, all involving modification/rescue mechanisms and mobile elements (plasmids and phages), which equate them to addictive manipulators of arthropods (figure 1).
Accordingly, we posit those modification/rescue mechanisms, mediated by toxin–antitoxin systems, could lie behind these and potentially many other cases of addictive manipulation of protist hosts. In the broadest sense [91], multiple types of molecules could be involved through various mechanisms, such as post-transcriptional and/or post-translational regulation. In bacteria, toxin–antitoxin systems are also involved in the addictive control exerted by plasmids [91,92]. Moreover, they were shown to be active on eukaryotic cells [93,94], and are thus plausible candidates for ‘exaptation’ towards addictive manipulation of eukaryotic host cells in general, as already hypothesized for some specific cases [13,95]. Multiple independent events of development/exaptation of molecular determinants of addictive manipulation could be envisioned in different bacterial symbionts of protists. Noteworthy is the Holosporales bacterium Bodocaedibacter, which expresses toxin and antitoxin genes, and whose suppression by antibiotics leads to the death of its host, the flagellate Bodo saltans, thus suggesting an addictive role and its determinants [48].
Under this framework, mobile elements, found in multiple bacterial symbionts of protists [45,96–98], could play a fundamental part, due to their well-recognized role in HGT [99], including specifically in protist-associated bacteria [51,100]. A single protist cell is frequently co-infected by different bacteria, which could easily exchange genes [101,102], thereby acquiring determinants for addictively manipulating their hosts. Accordingly, we can expect the presence of multiple alternative determinants in the same bacterium, with even significant variations between closely related bacteria. Such patterns could account for broad host ranges and their variation (which may be also explained by the molecular specificity of toxins towards targets in different hosts), as well as for competition among symbionts, such as in the case of Wolbachia [95]. Therefore, it seems highly intriguing the discovery of plasmid-encoded R-bodies, possibly linked with an addictive killer trait, in several protist-associated Holosporales bacteria other than Caedimonas [103].
Additionally, it would be alluring to investigate the impact of potential HGT events from addictively manipulating bacteria towards their protist hosts, similar to known cases of Wolbachia in insects [104,105]. Indeed, in principle, these events could provide the host with molecular determinants to modulate and counteract addiction.
6. Evolution of addictive manipulators
From the perspective of bacterial evolution, it is interesting that many of the bacteria with signs of addictive manipulation of different eukaryotes are phylogenetically akin. Particularly, it is remarkable to find multiple representatives of the Rickettsiales, the Legionellales and the Holosporales. Along with other independent lineages, these phylogenetically unrelated bacteria share some peculiar functional and evolutionary traits making them noteworthy for the study of bacterial–eukaryotic symbioses in general, which also led some authors to categorize them as ‘professional symbionts’ [1]. Their recurrent involvement in addictive manipulation suggests they should be examined further.
The representatives of such ‘professional symbionts’ live in association with eukaryotes, most likely since extremely ancient times (even over 1 bya) [55,106,107]. Each lineage collectively displays a broad host range, colonizing diverse protists, as well as multicellular organisms [48,52,55,56,108–119]. The most thoroughly investigated representatives of each lineage are arthropod-borne pathogens [120–123]. However, the majority are hosted by aquatic protists, which are considered the ancestral hosts, with multiple independent secondary adaptations to multicellular hosts [108,109,113,124].
Despite being unable to multiply in the absence of host cells (though with few possible exceptions [98,125,126]), ‘professional symbionts’ are not strictly host-confined. Indeed, along with vertical transmission, many of them can also perform horizontal transmission [127–130], even shifting between very different host species [109,131].
Consistently with their complex lifestyles, ‘professional symbionts’ bear rich repertoires of still largely uncharacterized molecular effectors [97,132–136], enabling them to actively modulate, and possibly even ‘control’ [1] those multifaceted interactions. In light of what is presented above, it seems intriguing to speculate that, among those molecular mechanisms, some capable of inducing addictive manipulation could be significant and widespread. Varied interactions with a wide array of eukaryotic hosts, as in the lineages of ‘professional symbionts’, would indeed be a plausible outcome for the descendants of hypothetical ancestral bacteria capable of addictive manipulation. Accordingly, addictive manipulation could have taken an active part in the evolution of these lineages, possibly even ‘determining’ it. Variations in the repertoire and/or specificity of toxin–antitoxin modules would allow us achieve such a breadth and evolutionary variability of host ranges, including in particular shifts from protist to multicellular hosts.
Addictive manipulation and other interactions might concur in the establishment and maintenance of tight bacterial–host associations, and might repeatedly supersede each other over evolutionary times. Such alternative interactions include more conventional mutualisms, as exemplified by some Wolbachia, which have become necessary for filarial nematodes [8,22] and some insects [137–141].
7. Concluding remarks
Through a targeted literature review and re-interpretation, here we propose a novel framework for the evolution and persistence of bacterial–protist associations, namely by addictive manipulation mechanisms enacted by many of those bacteria (box 1), comparable to the reproductive manipulation in arthropods [11,21,24,26]. This would result in the death of those hosts that have recently lost the symbionts, through toxic activity exerted by the bacteria under those specific circumstances, rather than due to some inherent inability of the hosts to cope with the lack of the symbionts.
Such addictive manipulators of protists or other asexual hosts would behave as selfish addictive elements (figure 2), with intriguing analogies with plasmid–bacteria interplay [60,142], especially when considering the repurposing of the same kind of molecular determinants (toxin–antitoxin systems) [13,37,44,95] and the probable involvement of mobile elements in spreading such determinants among eukaryote-associated bacteria. Notwithstanding significant differences in sexual processes between animals and protists, e.g. conjugation in ciliates [62], it seems also worthwhile to consider that addictive manipulators may influence the relative frequency of sexual and asexual reproduction in protists, analogous to Wolbachia in arthropods [8,143].
Considering the inherent difficulties in distinguishing addictive manipulation from other interactions among bacterial–protist associations, we posit that the herein-presented examples represent only the ‘tip of the iceberg’ of a widespread phenomenon. Thus, we underline the need for dedicated research to elucidate the diffusion, mechanisms, impact and evolutionary significance of addictive manipulation, in particular targeted experimental analyses (box 2).
Given the fundamental roles of protists in a broad range of ecosystems [17–19], addictive manipulation likely has deep ecological impacts as well. As exemplified by Wolbachia, addictive manipulators can provide fundamental insights into the eco-physiology and evolution of each host [8], which may become the basis for innovative applications [31,32].
It is an accepted notion that, due to their antiquity, diversity and diffusion, protists may act as ‘Trojan horses’ or ‘melting pots’ for the evolution of bacteria associated with multicellular hosts [102,144]. Thus, it seems thought-provoking to examine the evolutionary significance of the addictive manipulation of protists, in particular when considering the recurrent occurrence of addictive manipulators within lineages that encompass bacteria associated with both protists and multicellular organisms [98,109,124].
Acknowledgements
The authors would like to thank Giulio Petroni and Claudio Bandi for fruitful discussions on the development of this manuscript.
Contributor Information
Michele Castelli, Email: michele.castelli@unipv.it.
Tiago Nardi, Email: tiago.nardi01@universitadipavia.it; tiago.nardi@unimi.it.
Michele Giovannini, Email: giovanninimichele9@gmail.com.
Davide Sassera, Email: davide.sassera@unipv.it.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Supplementary material is available online [145].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
M.C.: conceptualization, data curation, formal analysis, investigation, methodology, supervision, writing—original draft; T.N.: data curation, methodology; M.G.: visualization; D.S.: conceptualization, resources, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
No funding has been received for this article.
References
- 1. Husnik F, Tashyreva D, Boscaro V, George EE, Lukeš J, Keeling PJ. 2021. Bacterial and archaeal symbioses with protists. Curr. Biol. 31 , R862–R877. ( 10.1016/j.cub.2021.05.049) [DOI] [PubMed] [Google Scholar]
- 2. Rosenblueth M, Martínez-Romero E. 2006. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe. Interact. 19 , 827–837. ( 10.1094/MPMI-19-0827) [DOI] [PubMed] [Google Scholar]
- 3. McFall-Ngai M, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110 , 3229–3236. ( 10.1073/pnas.1218525110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sapp J. 2004. The dynamics of symbiosis: an historical overview. Can. J. Bot. 82 , 1046–1056. ( 10.1139/b04-055) [DOI] [Google Scholar]
- 5. Herrera P, et al. 2020. Molecular causes of an evolutionary shift along the parasitism–mutualism continuum in a bacterial symbiont. Proc. Natl Acad. Sci. USA 117 , 21658–21666. ( 10.1073/pnas.2005536117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Regus JU, Gano KA, Hollowell AC, Sofish V, Sachs JL. 2015. Lotus hosts delimit the mutualism–parasitism continuum of Bradyrhizobium. J. Evol. Biol. 28 , 447–456. ( 10.1111/jeb.12579) [DOI] [PubMed] [Google Scholar]
- 7. Moran NA. 2007. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl Acad. Sci. USA 104 , (Suppl. 1) 8627–8633. ( 10.1073/pnas.0611659104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6 , 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 9. Shropshire JD, Leigh B, Bordenstein SR. 2020. Symbiont-mediated cytoplasmic incompatibility: what have we learned in 50 years? eLife 9 , e61989. ( 10.7554/eLife.61989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammer TJ. 2024. Why do hosts malfunction without microbes? Missing benefits versus evolutionary addiction. Trends Microbiol. 32 , 132–141. ( 10.1016/j.tim.2023.07.012) [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Zhang M, Hochstrasser M. 2020. The biochemistry of cytoplasmic incompatibility caused by endosymbiotic bacteria. Genes 11 , 852. ( 10.3390/genes11080852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harumoto T, Lemaitre B. 2018. Male-killing toxin in a bacterial symbiont of Drosophila. Nature 557 , 252–255. ( 10.1038/s41586-018-0086-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schrallhammer M, Schweikert M. 2009. The killer effect of Paramecium and its causative agents. In Endosymbionts in Paramecium, pp. 227–246. Berlin, Germany: Springer. ( 10.1007/978-3-540-92677-1_9) [DOI] [Google Scholar]
- 14. Adl SM, et al. 2019. Revisions to the classification, nomenclature, and diversity of eukaryotes. J. Eukaryot. Microbiol. 66 , 4–119. ( 10.1111/jeu.12691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keeling PJ, Burki F. 2019. Progress towards the tree of eukaryotes. Curr. Biol. 29 , R808–R817. ( 10.1016/j.cub.2019.07.031) [DOI] [PubMed] [Google Scholar]
- 16. O’Malley MA, Simpson AGB, Roger AJ. 2013. The other eukaryotes in light of evolutionary protistology. Biol. Philos. 28 , 299–330. ( 10.1007/s10539-012-9354-y) [DOI] [Google Scholar]
- 17. Caron DA, Countway PD, Jones AC, Kim DY, Schnetzer A. 2012. Marine protistan diversity. Ann. Rev. Mar. Sci. 4 , 467–493. ( 10.1146/annurev-marine-120709-142802) [DOI] [PubMed] [Google Scholar]
- 18. Geisen S, et al. 2018. Soil protists: a fertile frontier in soil biology research. FEMS Microbiol. Rev. 42 , 293–323. ( 10.1093/femsre/fuy006) [DOI] [PubMed] [Google Scholar]
- 19. Burki F, Sandin MM, Jamy M. 2021. Diversity and ecology of protists revealed by metabarcoding. Curr. Biol. 31 , R1267–R1280. ( 10.1016/j.cub.2021.07.066) [DOI] [PubMed] [Google Scholar]
- 20. Oren A. 2022. Candidatus list no. 4: lists of names of prokaryotic Candidatus taxa. Int. J. Syst. Evol. Microbiol. 72 , 005545. [DOI] [PubMed] [Google Scholar]
- 21. Hurst GDD, Frost CL. 2015. Reproductive parasitism: maternally inherited symbionts in a biparental world. Cold Spring Harb. Perspect. Biol. 7 , a017699. ( 10.1101/cshperspect.a017699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor MJ, Bandi C, Hoerauf A. 2005. Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasitol. 60 , 245–284. ( 10.1016/S0065-308X(05)60004-8) [DOI] [PubMed] [Google Scholar]
- 23. Perlman SJ, Hunter MS, Zchori-Fein E. 2006. The emerging diversity of Rickettsia. Proc. R. Soc. B 273 , 2097–2106. ( 10.1098/rspb.2006.3541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gillespie JJ, Driscoll TP, Verhoeve VI, Rahman MS, Macaluso KR, Azad AF. 2018. A tangled web: origins of reproductive parasitism. Genome Biol. Evol. 10 , 2292–2309. ( 10.1093/gbe/evy159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takano SI, Gotoh Y, Hayashi T. 2021. ‘Candidatus Mesenet longicola’: novel endosymbionts of Brontispa longissima that induce cytoplasmic incompatibility. Microb. Ecol. 82 , 512–522. ( 10.1007/s00248-021-01686-y) [DOI] [PubMed] [Google Scholar]
- 26. Pollmann M, Moore LD, Krimmer E, D’Alvise P, Hasselmann M, Perlman SJ, Ballinger MJ, Steidle JLM, Gottlieb Y. 2022. Highly transmissible cytoplasmic incompatibility by the extracellular insect symbiont Spiroplasma. iScience 25 , 104335. ( 10.1016/j.isci.2022.104335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen DT, Morrow JL, Spooner-Hart RN, Riegler M. 2017. Independent cytoplasmic incompatibility induced by Cardinium and Wolbachia maintains endosymbiont coinfections in haplodiploid thrips populations. Evolution 71 , 995–1008. ( 10.1111/evo.13197) [DOI] [PubMed] [Google Scholar]
- 28. Rosenwald LC, Sitvarin MI, White JA. 2020. Endosymbiotic Rickettsiella causes cytoplasmic incompatibility in a spider host. Proc. R. Soc. B. 287 , 20201107. ( 10.1098/rspb.2020.1107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. 2015. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B. 282 , 20150249. ( 10.1098/rspb.2015.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sazama EJ, Bosch MJ, Shouldis CS, Ouellette SP, Wesner JS. 2017. Incidence of Wolbachia in aquatic insects. Ecol. Evol. 7 , 1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoffmann AA, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 , 454–457. ( 10.1038/nature10356) [DOI] [PubMed] [Google Scholar]
- 32. Utarini A, et al. 2021. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 384 , 2177–2186. ( 10.1056/NEJMoa2030243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McNamara CJ, Ant TH, Harvey-Samuel T, White-Cooper H, Martinez J, Alphey L, Sinkins SP. 2024. Transgenic expression of cif genes from Wolbachia strain wAlbB recapitulates cytoplasmic incompatibility in Aedes aegypti. Nat. Commun. 15 , 869. ( 10.1038/s41467-024-45238-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Werren JH. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42 , 587–609. ( 10.1146/annurev.ento.42.1.587) [DOI] [PubMed] [Google Scholar]
- 35. LePage DP, et al. 2017. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543 , 243–247. ( 10.1038/nature21391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beckmann JF, Ronau JA, Hochstrasser M. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2 , 17007. ( 10.1038/nmicrobiol.2017.7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hochstrasser M. 2022. Cytoplasmic incompatibility: a Wolbachia toxin–antidote mechanism comes into view. Curr. Biol. 32 , R287–R289. ( 10.1016/j.cub.2022.02.014) [DOI] [PubMed] [Google Scholar]
- 38. Terretaz K, Horard B, Weill M, Loppin B, Landmann F. 2023. Functional analysis of Wolbachia Cid effectors unravels cooperative interactions to target host chromatin during replication. PLoS Pathog. 19 , e1011211. ( 10.1371/journal.ppat.1011211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harumoto T. 2023. Self-stabilization mechanism encoded by a bacterial toxin facilitates reproductive parasitism. Curr. Biol. 33 , 4021–4029.( 10.1016/j.cub.2023.08.032) [DOI] [PubMed] [Google Scholar]
- 40. Tram U, Ferree PM, Sullivan W. 2003. Identification of Wolbachia–host interacting factors through cytological analysis. Microbes Infect. 5 , 999–1011. ( 10.1016/s1286-4579(03)00192-8) [DOI] [PubMed] [Google Scholar]
- 41. Lindsey ARI, Rice DW, Bordenstein SR, Brooks AW, Bordenstein SR, Newton ILG. 2018. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in prophage WO of Wolbachia. Genome Biol. Evol. 10 , 434–451. ( 10.1093/gbe/evy012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fokin SI. 2012. Frequency and biodiversity of symbionts in representatives of the main classes of Ciliophora. Eur. J. Protistol. 48 , 138–148. ( 10.1016/j.ejop.2011.12.001) [DOI] [PubMed] [Google Scholar]
- 43. Jeon KW, Lorch IJ. 1967. Unusual intra-cellular bacterial infection in large, free-living amoebae. Exp. Cell Res. 48 , 236–240. ( 10.1016/0014-4827(67)90313-8) [DOI] [PubMed] [Google Scholar]
- 44. Jeon KW. 1987. Change of cellular ‘pathogens’ into required cell components. Ann. NY Acad. Sci. 503 , 359–371. ( 10.1111/j.1749-6632.1987.tb40622.x) [DOI] [PubMed] [Google Scholar]
- 45. Pond FR, Gibson I, Lalucat J, Quackenbush RL. 1989. R-body-producing bacteria. Microbiol. Rev. 53 , 25–67. ( 10.1128/mr.53.1.25-67.1989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quackenbush RL, Burbach JA. 1983. Cloning and expression of DNA sequences associated with the killer trait of Paramecium tetraurelia stock 47. Proc. Natl Acad. Sci. USA 80 , 250–254. ( 10.1073/pnas.80.1.250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeblick J, Kusch J. 2005. Sequence, transcription activity, and evolutionary origin of the R-body coding plasmid pKAP298 from the intracellular parasitic bacterium Caedibacter taeniospiralis. J. Mol. Evol. 60 , 164–173. ( 10.1007/s00239-004-0002-2) [DOI] [PubMed] [Google Scholar]
- 48. Midha S, Rigden DJ, Siozios S, Hurst GDD, Jackson AP. 2021. Bodo saltans (Kinetoplastida) is dependent on a novel Paracaedibacter-like endosymbiont that possesses multiple putative toxin–antitoxin systems. ISME J. 15 , 1680–1694. ( 10.1038/s41396-020-00879-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Castelli M, et al. 2019. Deianiraea, an extracellular bacterium associated with the ciliate Paramecium, suggests an alternative scenario for the evolution of Rickettsiales. ISME J. 13 , 2280–2294. ( 10.1038/s41396-019-0433-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lanzoni O, Sabaneyeva E, Modeo L, Castelli M, Lebedeva N, Verni F, Schrallhammer M, Potekhin A, Petroni G. 2019. Diversity and environmental distribution of the cosmopolitan endosymbiont ‘Candidatus Megaira’ Sci. Rep. 9 , 1179. ( 10.1038/s41598-018-37629-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castelli M, Lanzoni O, Nardi T, Lometto S, Modeo L, Potekhin A, Sassera D, Petroni G. 2021. ‘Candidatus Sarmatiella mevalonica’ endosymbiont of the ciliate Paramecium provides insights on evolutionary plasticity among Rickettsiales. Environ. Microbiol. 23 , 1684–1701. ( 10.1111/1462-2920.15396) [DOI] [PubMed] [Google Scholar]
- 52. Davison HR, Hurst GDD, Siozios S. 2023. ‘Candidatus Megaira’ are diverse symbionts of algae and ciliates with the potential for defensive symbiosis. Microb. Genom. 9 , mgen000950. ( 10.1099/mgen.0.000950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hess S. 2017. Description of Hyalodiscus flabellus sp. nov. (Vampyrellida, Rhizaria) and identification of its bacterial endosymbiont, ‘Candidatus Megaira polyxenophila’ (Rickettsiales, Alphaproteobacteria). Protist 168 , 109–133. ( 10.1016/j.protis.2016.11.003) [DOI] [PubMed] [Google Scholar]
- 54. Arthofer P, Delafont V, Willemsen A, Panhölzl F, Horn M. 2022. Defensive symbiosis against giant viruses in amoebae. Proc. Natl Acad. Sci. USA 119 , e2205856119. ( 10.1073/pnas.2205856119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dharamshi JE, Köstlbacher S, Schön ME, Collingro A, Ettema TJG, Horn M. 2023. Gene gain facilitated endosymbiotic evolution of Chlamydiae. Nat. Microbiol. 8 , 40–54. ( 10.1038/s41564-022-01284-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paight C, Hunter ES, Lane CE. 2022. Codependence of individuals in the Nephromyces species swarm requires heterospecific bacterial endosymbionts. Curr. Biol. 32 , 2948–2955.( 10.1016/j.cub.2022.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boscaro V, Syberg-Olsen MJ, Irwin NAT, George EE, Vannini C, Husnik F, Keeling PJ. 2022. All essential endosymbionts of the ciliate Euplotes are cyclically replaced. Curr. Biol. 32 , R826–R827. ( 10.1016/j.cub.2022.06.052) [DOI] [PubMed] [Google Scholar]
- 58. Boscaro V, Husnik F, Vannini C, Keeling PJ. 2019. Symbionts of the ciliate Euplotes: diversity, patterns and potential as models for bacteria–eukaryote endosymbioses. Proc. R. Soc. B 286 , 20190693. ( 10.1098/rspb.2019.0693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maita C, et al. 2018. Amoebal endosymbiont Neochlamydia protects host amoebae against Legionella pneumophila infection by preventing Legionella entry. Microbes Infect. 20 , 236–244. ( 10.1016/j.micinf.2017.12.012) [DOI] [PubMed] [Google Scholar]
- 60. Rankin DJ, Turner LA, Heinemann JA, Brown SP. 2012. The coevolution of toxin and antitoxin genes drives the dynamics of bacterial addiction complexes and intragenomic conflict. Proc. R. Soc. B 279 , 3706–3715. ( 10.1098/rspb.2012.0942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jeon KW, Hah JC. 1977. Effect of chloramphenicol on bacterial endosymbiotes in a strain of Amoeba proteus. J. Protozool. 24 , 289–293. ( 10.1111/j.1550-7408.1977.tb00979.x) [DOI] [PubMed] [Google Scholar]
- 62. Miyake A. 1974. Cell interaction in conjugation of ciliates. Curr. Top. Microbiol. Immunol. 64 , 49–77. ( 10.1007/978-3-642-65848-8_2) [DOI] [PubMed] [Google Scholar]
- 63. Flowers JM, Li SI, Stathos A, Saxer G, Ostrowski EA, Queller DC, Strassmann JE, Purugganan MD. 2010. Variation, sex, and social cooperation: molecular population genetics of the social amoeba Dictyostelium discoideum. PLoS Genet. 6 , e1001013. ( 10.1371/journal.pgen.1001013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mather RV, Larsen TJ, Brock DA, Queller DC, Strassmann JE. 2023. Paraburkholderia symbionts isolated from dictyostelium discoideum induce bacterial carriage in other dictyostelium species. Proc. R. Soc. B 290 , 20230977. ( 10.1098/rspb.2023.0977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boscaro V, et al. 2013. Rediscovering the genus Lyticum, multiflagellated symbionts of the order Rickettsiales. Sci. Rep. 3 , 3305. ( 10.1038/srep03305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Noh S, Peck RF, Larson ER, Covitz RM, Chen A, Roy P, Hamilton MC, Dettmann RA. 2024. Facultative symbiont virulence determines horizontal transmission rate without host specificity in Dictyostelium discoideum social amoebas. Evol. Lett. 8 , 437–447. ( 10.1093/evlett/qrae001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gast RJ, Sanders RW, Caron DA. 2009. Ecological strategies of protists and their symbiotic relationships with prokaryotic microbes. Trends Microbiol. 17 , 563–569. ( 10.1016/j.tim.2009.09.001) [DOI] [PubMed] [Google Scholar]
- 68. Nowack ECM, Melkonian M. 2010. Endosymbiotic associations within protists. Phil. Trans. R. Soc. B 365 , 699–712. ( 10.1098/rstb.2009.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Potekhin A, Schweikert M, Nekrasova I, Vitali V, Schwarzer S, Anikina A, Kaltz O, Petroni G, Schrallhammer M. 2018. Complex life cycle, broad host range and adaptation strategy of the intranuclear Paramecium symbiont Preeria caryophila comb. nov. FEMS Microbiol. Ecol. 94 . ( 10.1093/femsec/fiy076) [DOI] [PubMed] [Google Scholar]
- 70. Schweikert M, Meyer B. 2001. Characterization of intracellular bacteria in the freshwater dinoflagellate Peridinium cinctum. Protoplasma 217 , 177–184. ( 10.1007/BF01283398) [DOI] [PubMed] [Google Scholar]
- 71. Mironov T, Sabaneyeva E. 2020. A robust symbiotic relationship between the ciliate Paramecium multimicronucleatum and the bacterium ‘Ca. Trichorickettsia mobilis’. Front. Microbiol. 11 , 603335. ( 10.3389/fmicb.2020.603335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mironov T, Yakovlev A, Sabaneyeva E. 2022. Together forever: inseparable partners of the symbiotic system Paramecium multimicronucleatum/Ca. Trichorickettsia mobilis. Symbiosis 87 , 19–30. ( 10.1007/s13199-022-00854-z) [DOI] [Google Scholar]
- 73. Flemming FE, Grosser K, Schrallhammer M. 2021. Natural shifts in endosymbionts’ occurrence and relative frequency in their ciliate host population. Front. Microbiol. 12 , 791615. ( 10.3389/fmicb.2021.791615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Senra MVX, Dias RJP, Castelli M, Silva-Neto ID, Verni F, Soares CAG, Petroni G. 2016. A house for two—double bacterial infection in Euplotes woodruffi Sq1 (Ciliophora, Euplotia) sampled in Southeastern Brazil. Microb. Ecol. 71 , 505–517. ( 10.1007/s00248-015-0668-6) [DOI] [PubMed] [Google Scholar]
- 75. Oren A, Garrity GM, Parker CT, Chuvochina M, Trujillo ME. 2020. Lists of names of prokaryotic Candidatus taxa. Int. J. Syst. Evol. Microbiol. 70 , 3956–4042. ( 10.1099/ijsem.0.003789) [DOI] [PubMed] [Google Scholar]
- 76. Schrallhammer M, Ferrantini F, Vannini C, Galati S, Schweikert M, Görtz HD, Verni F, Petroni G. 2013. ‘Candidatus Megaira polyxenophila’ gen. nov., sp. nov.: considerations on evolutionary history, host range and shift of early divergent rickettsiae.. PLoS One 8 , e72581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bella C, Koehler L, Grosser K, Berendonk TU, Petroni G, Schrallhammer M. 2016. Fitness impact of obligate intranuclear bacterial symbionts depends on host growth phase. Front. Microbiol. 7 , 2084. ( 10.3389/fmicb.2016.02084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pasqualetti C, Szokoli F, Rindi L, Petroni G, Schrallhammer M. 2020. The obligate symbiont ‘Candidatus Megaira polyxenophila’ has variable effects on the growth of different host species. Front. Microbiol. 11 , 1425. ( 10.3389/fmicb.2020.01425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walker T, et al. 2011. The wmel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476 , 450–453. ( 10.1038/nature10355) [DOI] [PubMed] [Google Scholar]
- 80. Layton EM, On J, Perlmutter JI, Bordenstein SR, Shropshire JD. 2019. Paternal grandmother age affects the strength of Wolbachia-induced cytoplasmic incompatibility in Drosophila melanogaster. MBio 10 . ( 10.1128/mBio.01879-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ross PA, Ritchie SA, Axford JK, Hoffmann AA. 2019. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl. Trop. Dis. 13 , e0007357. ( 10.1371/journal.pntd.0007357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Park M, Yun ST, Kim MS, Chun J, Ahn TI. 2004. Phylogenetic characterization of Legionella-like endosymbiotic X-bacteria in Amoeba proteus: a proposal for ‘Candidatus Legionella jeonii’ sp. nov. Environ. Microbiol. 6 , 1252–1263. ( 10.1111/j.1462-2920.2004.00659.x) [DOI] [PubMed] [Google Scholar]
- 83. Jeon KW. 1972. Development of cellular dependence on infective organisms: micrurgical studies in amoebas. Science 176 , 1122–1123. ( 10.1126/science.176.4039.1122) [DOI] [PubMed] [Google Scholar]
- 84. Jeon KW, Ahn TI. 1978. Temperature sensitivity: a cell character determined by obligate endosymbionts in amoebas. Science 202 , 635–637. ( 10.1126/science.202.4368.635) [DOI] [PubMed] [Google Scholar]
- 85. Jeon TJ, Jeon KW. 2004. Gene switching in Amoeba proteus caused by endosymbiotic bacteria. J. Cell. Sci. 117 , 535–543. ( 10.1242/jcs.00894) [DOI] [PubMed] [Google Scholar]
- 86. Pak JW, Jeon KW. 1997. A symbiont-produced protein and bacterial symbiosis in Amoeba proteus. J. Eukaryot. Microbiol. 44 , 614–619. ( 10.1111/j.1550-7408.1997.tb05968.x) [DOI] [PubMed] [Google Scholar]
- 87. Kusch J, Görtz HD. 2006. Towards an understanding of the killer trait: Caedibacter endocytobionts in Paramecium. Prog. Mol. Subcell. Biol. 41 , 61–76. ( 10.1007/3-540-28221-1_4) [DOI] [PubMed] [Google Scholar]
- 88. Schrallhammer M, Castelli M, Petroni G. 2018. Phylogenetic relationships among endosymbiotic R-body producer: bacteria providing their host the killer trait. Syst. Appl. Microbiol. 41 , 213–220. ( 10.1016/j.syapm.2018.01.005) [DOI] [PubMed] [Google Scholar]
- 89. Preer LB, Rudman BM, Preer JR, Jurand A. 1974. Induction of R bodies by ultraviolet light in killer paramecia. J. Gen. Microbiol. 80 , 209–215. ( 10.1099/00221287-80-1-209) [DOI] [Google Scholar]
- 90. Görtz HD, Fokin SI. 2009. Diversity of endosymbiotic bacteria in Paramecium . In Endosymbionts in Paramecium, pp. 131–160. Berlin, Germany: Springer. ( 10.1007/978-3-540-92677-1_6) [DOI] [Google Scholar]
- 91. Jurėnas D, Fraikin N, Goormaghtigh F, Van Melderen L. 2022. Biology and evolution of bacterial toxin–antitoxin systems. Nat. Rev. Microbiol. 20 , 335–350. ( 10.1038/s41579-021-00661-1) [DOI] [PubMed] [Google Scholar]
- 92. Harms A, Brodersen DE, Mitarai N, Gerdes K. 2018. Toxins, targets, and triggers: an overview of toxin–antitoxin biology. Mol. Cell 70 , 768–784. ( 10.1016/j.molcel.2018.01.003) [DOI] [PubMed] [Google Scholar]
- 93. Yeo CC, Abu Bakar F, Chan WT, Espinosa M, Harikrishna JA. 2016. Heterologous expression of toxins from bacterial toxin–antitoxin systems in eukaryotic cells: strategies and applications. Toxins 8 , 49. ( 10.3390/toxins8020049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. You S, et al. 2023. A toxin–antidote system contributes to interspecific reproductive isolation in rice. Nat. Commun. 14 , 7528. ( 10.1038/s41467-023-43015-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, Merçot H, Weill M, Sicard M, Charlat S. 2019. The toxin–antidote model of cytoplasmic incompatibility: genetics and evolutionary implications. Trends Genet. 35 , 175–185. ( 10.1016/j.tig.2018.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang Z, Wu M. 2015. An integrated phylogenomic approach toward pinpointing the origin of mitochondria. Sci. Rep. 5 , 7949. ( 10.1038/srep07949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. George EE, Husnik F, Tashyreva D, Prokopchuk G, Horák A, Kwong WK, Lukeš J, Keeling PJ. 2020. Highly reduced genomes of protist endosymbionts show evolutionary convergence. Curr. Biol. 30 , 925–933.( 10.1016/j.cub.2019.12.070) [DOI] [PubMed] [Google Scholar]
- 98. Castelli M, Nardi T, Gammuto L, Bellinzona G, Sabaneyeva E, Potekhin A, Serra V, Petroni G, Sassera D. 2024. Host association and intracellularity evolved multiple times independently in the Rickettsiales. Nat. Commun. 15 , 1093. ( 10.1038/s41467-024-45351-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Haudiquet M, de Sousa JM, Touchon M, Rocha EPC. 2022. Selfish, promiscuous and sometimes useful: how mobile genetic elements drive horizontal gene transfer in microbial populations. Phil. Trans. R. Soc. B 377 , 20210234. ( 10.1098/rstb.2021.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. George EE, Tashyreva D, Kwong WK, Okamoto N, Horák A, Husnik F, Lukeš J, Keeling PJ. 2022. Gene transfer agents in bacterial endosymbionts of microbial eukaryotes. Genome Biol. Evol. 14 , evac099. ( 10.1093/gbe/evac099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gomez-Valero L, Buchrieser C. 2019. Intracellular parasitism, the driving force of evolution of Legionella pneumophila and the genus Legionella. Microbes Infect. 21 , 230–236. ( 10.1016/j.micinf.2019.06.012) [DOI] [PubMed] [Google Scholar]
- 102. Wang Z, Wu M. 2017. Comparative genomic analysis of Acanthamoeba endosymbionts highlights the role of amoebae as a ‘melting pot’ shaping the Rickettsiales evolution. Genome Biol. Evol. 9 , 3214–3224. ( 10.1093/gbe/evx246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Giovannini M, Petroni G, Castelli M. 2024. Novel evolutionary insights on the interactions of the Holosporales (Alphaproteobacteria) with eukaryotic hosts from comparative genomics. Environ. Microbiol. 26 , e16562. ( 10.1111/1462-2920.16562) [DOI] [PubMed] [Google Scholar]
- 104. Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T. 2002. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl Acad. Sci. USA 99 , 14280–14285. ( 10.1073/pnas.222228199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dunning Hotopp JC, et al. 2007. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317 , 1753–1756. ( 10.1126/science.1142490) [DOI] [PubMed] [Google Scholar]
- 106. Wang S, Luo H. 2021. Dating Alphaproteobacteria evolution with eukaryotic fossils. Nat. Commun. 12 , 3324. ( 10.1038/s41467-021-23645-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hugoson E, Guliaev A, Ammunét T, Guy L. 2022. Host adaptation in Legionellales is 1.9 Ga, coincident with eukaryogenesis. Mol. Biol. Evol. 39 , msac037. ( 10.1093/molbev/msac037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Castelli M, Sassera D, Petroni G. 2016. Biodiversity of ‘non-model’ Rickettsiales and their association with aquatic organisms. In Rickettsiales, pp. 59–91. Cham, Switzerland: Springer International Publishing. ( 10.1007/978-3-319-46859-4_3) [DOI] [Google Scholar]
- 109. Duron O, Doublet P, Vavre F, Bouchon D. 2018. The importance of revisiting Legionellales diversity. Trends Parasitol. 34 , 1027–1037. ( 10.1016/j.pt.2018.09.008) [DOI] [PubMed] [Google Scholar]
- 110. Muñoz-Gómez SA, Hess S, Burger G, Lang BF, Susko E, Slamovits CH, Roger AJ. 2019. An updated phylogeny of the Alphaproteobacteria reveals that the parasitic Rickettsiales and Holosporales have independent origins. eLife 8 . ( 10.7554/eLife.42535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Carrier TJ, Leigh BA, Deaker DJ, Devens HR, Wray GA, Bordenstein SR, Byrne M, Reitzel AM. 2021. Microbiome reduction and endosymbiont gain from a switch in sea urchin life history. Proc. Natl Acad. Sci. USA 118 . ( 10.1073/pnas.2022023118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Guidetti R, Vecchi M, Ferrari A, Newton ILG, Cesari M, Rebecchi L. 2020. Further insights in the Tardigrada microbiome: phylogenetic position and prevalence of infection of four new Alphaproteobacteria putative endosymbionts. Zool. J. Linn. Soc. 188 , 925–937. ( 10.1093/zoolinnean/zlz128) [DOI] [Google Scholar]
- 113. Szokoli F, Castelli M, Sabaneyeva E, Schrallhammer M, Krenek S, Doak TG, Berendonk TU, Petroni G. 2016. Disentangling the taxonomy of Rickettsiales and description of two novel symbionts (‘Candidatus Bealeia paramacronuclearis' and 'Candidatus Fokinia cryptica') sharing the cytoplasm of the ciliate protist Paramecium biaurelia. Appl. Environ. Microbiol. 82 , 7236–7247. ( 10.1128/AEM.02284-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Köstlbacher S, Collingro A, Halter T, Schulz F, Jungbluth SP, Horn M. 2021. Pangenomics reveals alternative environmental lifestyles among chlamydiae. Nat. Commun. 12 , 4021. ( 10.1038/s41467-021-24294-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Potekhin A, Nekrasova I, Flemming FE. 2021. In shadow of Holospora—the continuous quest for new Holosporaceae members. Protistology 15 , 127–141. ( 10.21685/1680-0826-2021-15-3-3) [DOI] [Google Scholar]
- 116. Gruber-Vodicka HR, Leisch N, Kleiner M, Hinzke T, Liebeke M, McFall-Ngai M, Hadfield MG, Dubilier N. 2019. Two intracellular and cell type-specific bacterial symbionts in the placozoan Trichoplax H2. Nat. Microbiol. 4 , 1465–1474. ( 10.1038/s41564-019-0475-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Dittmer J, Bredon M, Moumen B, Raimond M, Grève P, Bouchon D. 2023. The terrestrial isopod symbiont ‘Candidatus Hepatincola porcellionum’ is a potential nutrient scavenger related to Holosporales symbionts of protists. ISME Commun. 3 , 18. ( 10.1038/s43705-023-00224-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Halter T, Köstlbacher S, Collingro A, Sixt BS, Tönshoff ER, Hendrickx F, Kostanjšek R, Horn M. 2022. Ecology and evolution of chlamydial symbionts of arthropods. ISME Commun. 2 , 45. ( 10.1038/s43705-022-00124-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Galindo LJ, Torruella G, Moreira D, Eglit Y, Simpson AGB, Völcker E, Clauß S, López-García P. 2019. Combined cultivation and single-cell approaches to the phylogenomics of nucleariid amoebae, close relatives of fungi. Phil. Trans. R. Soc. B 374 , 20190094. ( 10.1098/rstb.2019.0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chauhan D, Shames SR. 2021. Pathogenicity and virulence of Legionella: intracellular replication and host response. Virulence 12 , 1122–1144. ( 10.1080/21505594.2021.1903199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Renvoisé A, Merhej V, Georgiades K, Raoult D. 2011. Intracellular Rickettsiales: insights into manipulators of eukaryotic cells. Trends Mol. Med. 17 , 573–583. ( 10.1016/j.molmed.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 122. Elwell C, Mirrashidi K, Engel J. 2016. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 14 , 385–400. ( 10.1038/nrmicro.2016.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. van Schaik EJ, Chen C, Mertens K, Weber MM, Samuel JE. 2013. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat. Rev. Microbiol. 11 , 561–573. ( 10.1038/nrmicro3049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dharamshi JE, Tamarit D, Eme L, Stairs CW, Martijn J, Homa F, Jørgensen SL, Spang A, Ettema TJG. 2020. Marine sediments illuminate chlamydiae diversity and evolution. Curr. Biol. 30 , 1032–1048.( 10.1016/j.cub.2020.02.016) [DOI] [PubMed] [Google Scholar]
- 125. Schön ME, Martijn J, Vosseberg J, Köstlbacher S, Ettema TJG. 2022. The evolutionary origin of host association in the Rickettsiales. Nat. Microbiol. 7 , 1189–1199. ( 10.1038/s41564-022-01169-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Singh S, Eldin C, Kowalczewska M, Raoult D. 2013. Axenic culture of fastidious and intracellular bacteria. Trends Microbiol. 21 , 92–99. ( 10.1016/j.tim.2012.10.007) [DOI] [PubMed] [Google Scholar]
- 127. Rizzoli A, et al. 2014. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front. Public Health 2 , 251. ( 10.3389/fpubh.2014.00251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing SA. 2010. The natural history of Anaplasma marginale. Vet. Parasitol. 167 , 95–107. ( 10.1016/j.vetpar.2009.09.012) [DOI] [PubMed] [Google Scholar]
- 129. Huigens ME, de Almeida RP, Boons PAH, Luck RF, Stouthamer R. 2004. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc. R. Soc. B. 271 , 509–515. ( 10.1098/rspb.2003.2640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dantas-Torres F, Chomel BB, Otranto D. 2012. Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 28 , 437–446. ( 10.1016/j.pt.2012.07.003) [DOI] [PubMed] [Google Scholar]
- 131. Modeo L, et al. 2020. ‘Ca. Trichorickettsia mobilis’, a Rickettsiales bacterium, can be transiently transferred from the unicellular eukaryote paramecium to the planarian Dugesia japonica. PeerJ 8 , e8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gillespie JJ, Kaur SJ, Rahman MS, Rennoll-Bankert K, Sears KT, Beier-Sexton M, Azad AF. 2015. Secretome of obligate intracellular Rickettsia. FEMS Microbiol. Rev. 39 , 47–80. ( 10.1111/1574-6976.12084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Meir A, Macé K, Lukoyanova N, Chetrit D, Hospenthal MK, Redzej A, Roy C, Waksman G. 2020. Mechanism of effector capture and delivery by the type IV secretion system from Legionella pneumophila. Nat. Commun. 11 , 2864. ( 10.1038/s41467-020-16681-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Betts-Hampikian HJ, Fields KA. 2010. The chlamydial type III secretion mechanism: revealing cracks in a tough nut. Front. Microbiol. 1 , 114. ( 10.3389/fmicb.2010.00114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Merhej V, Royer-Carenzi M, Pontarotti P, Raoult D. 2009. Massive comparative genomic analysis reveals convergent evolution of specialized bacteria. Biol. Direct 4 , 13. ( 10.1186/1745-6150-4-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gillespie JJ, et al. 2016. The Rickettsia type IV secretion system: unrealized complexity mired by gene family expansion. Pathog. Dis. 74 , ftw058. ( 10.1093/femspd/ftw058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. 2010. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA 107 , 769–774. ( 10.1073/pnas.0911476107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jaenike J, Stahlhut JK, Boelio LM, Unckless RL. 2010. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol. Ecol. 19 , 414–425. ( 10.1111/j.1365-294X.2009.04448.x) [DOI] [PubMed] [Google Scholar]
- 139. Mahmood S, Nováková E, Martinů J, Sychra O, Hypša V. 2023. Supergroup F Wolbachia with extremely reduced genome: transition to obligate insect symbionts. Microbiome 11 , 22. ( 10.1186/s40168-023-01462-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl Acad. Sci. USA 98 , 6247–6252. ( 10.1073/pnas.101304298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kremer N, Voronin D, Charif D, Mavingui P, Mollereau B, Vavre F. 2009. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 5 , e1000630. ( 10.1371/journal.ppat.1000630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rodríguez-Beltrán J, DelaFuente J, León-Sampedro R, MacLean RC, San Millán Á. 2021. Beyond horizontal gene transfer: the role of plasmids in bacterial evolution. Nat. Rev. Microbiol. 19 , 347–359. ( 10.1038/s41579-020-00497-1) [DOI] [PubMed] [Google Scholar]
- 143. Wedell N. 2020. Selfish genes and sexual selection: the impact of genomic parasites on host reproduction. J. Zool. 311 , 1–12. ( 10.1111/jzo.12780) [DOI] [Google Scholar]
- 144. Barker J, Brown MR. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140 , 1253–1259. ( 10.1099/00221287-140-6-1253) [DOI] [PubMed] [Google Scholar]
- 145. Castelli M, Nardi T, Giovannini M, Sassera D. 2024. Data from: Addictive manipulation: a perspective on the role of reproductive parasitism in the evolution of bacteria-eukaryote symbioses. Figshare. ( 10.6084/m9.figshare.c.7449491) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary material is available online [145].