Abstract
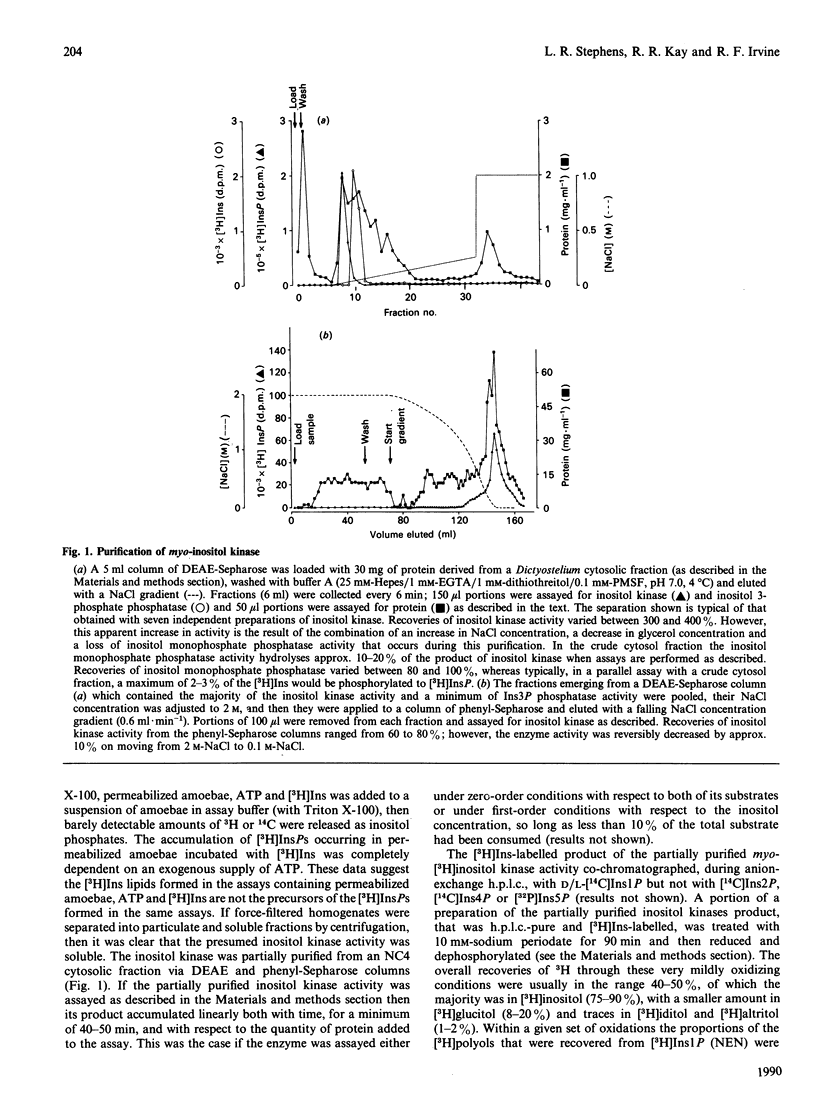
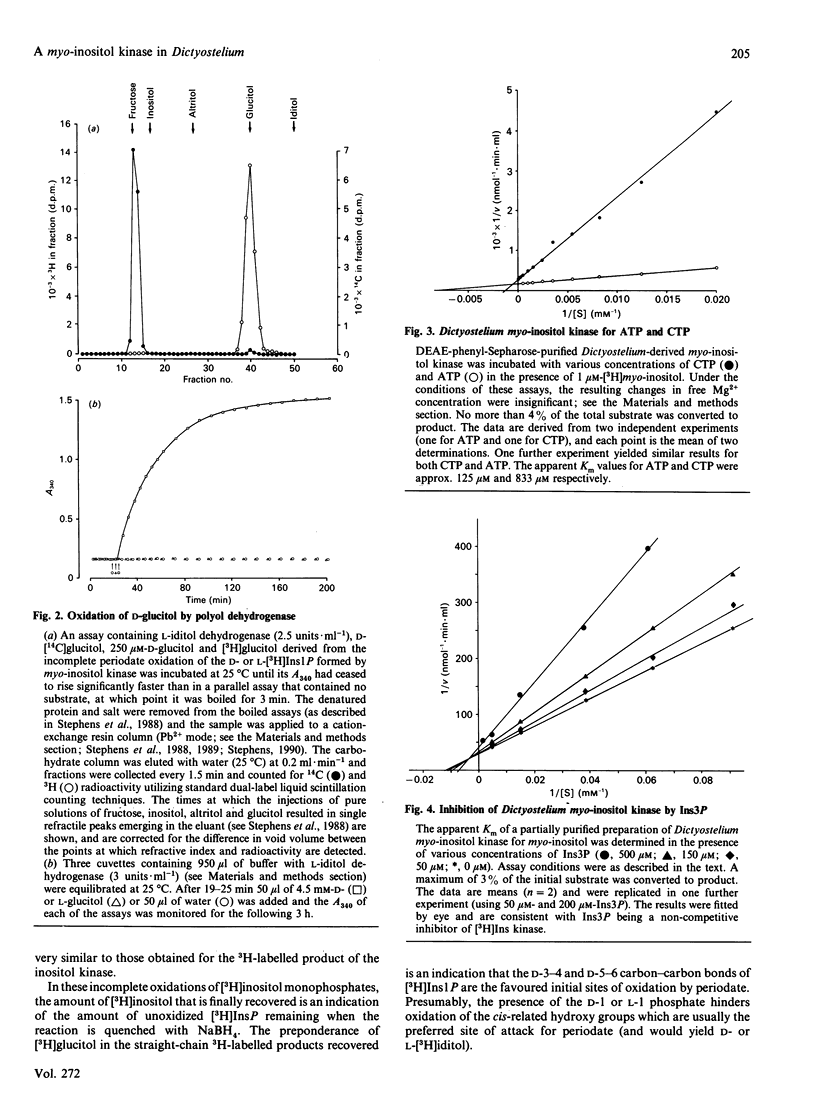
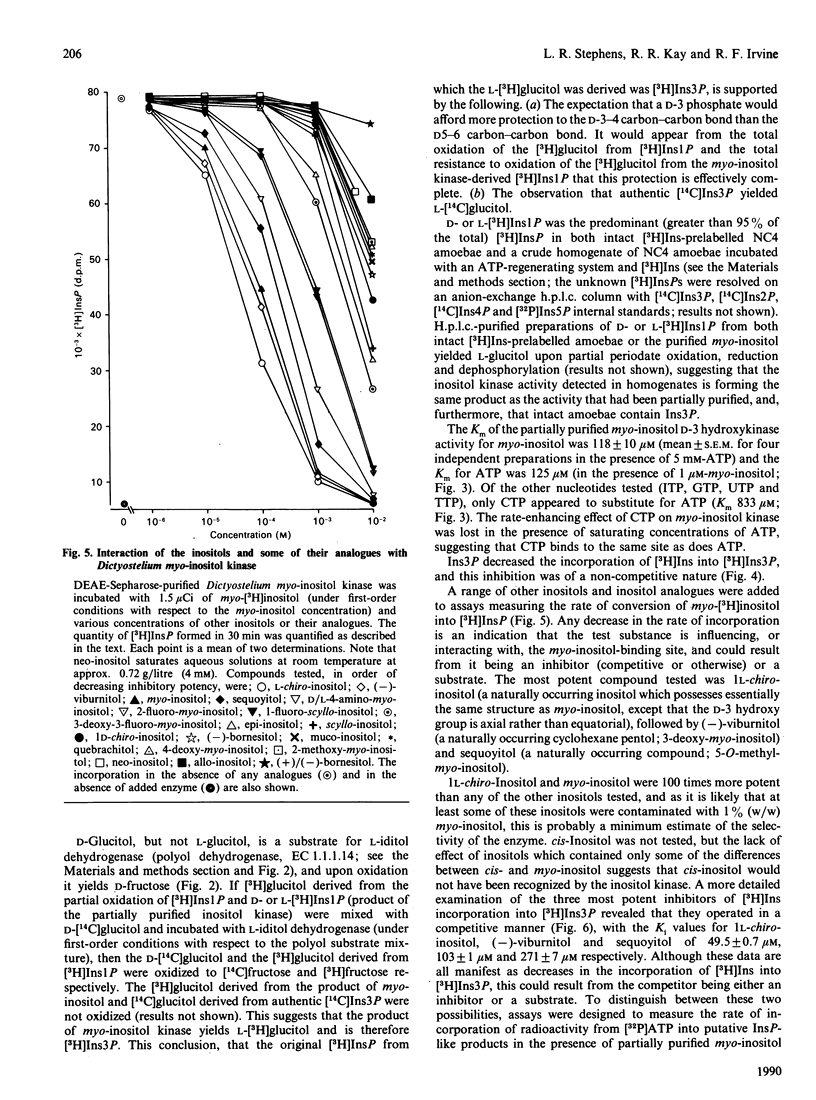
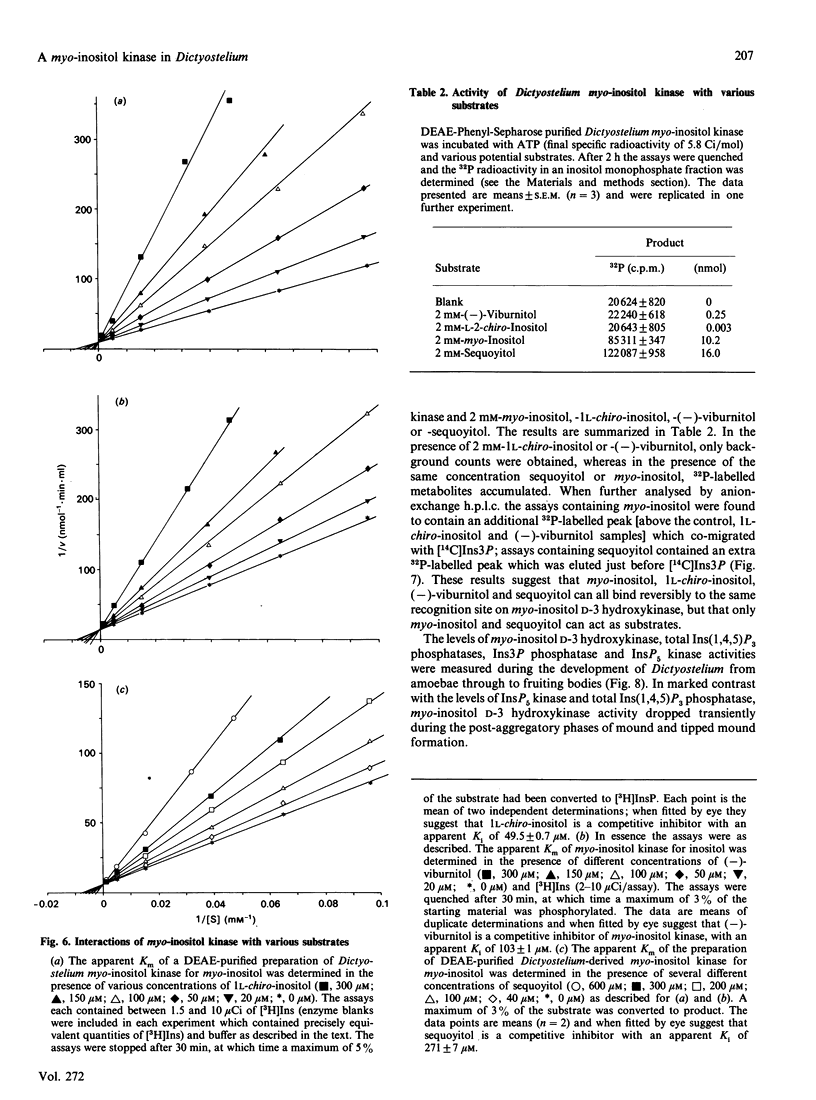
A soluble ATP-dependent enzyme which phosphorylates myo-inositol has been characterized in Dictyostelium. The myo-inositol kinase activity was partially purified from amoebae by chromatography on DEAE-Sepharose and phenyl-Sepharose columns. The product of both the partially purified activity and of a crude cytosolic fraction was myo-inositol 3-phosphate. The partially purified preparations of myo-inositol kinase (a) possessed a Km for myo-inositol of 120 microM (in the presence of 5 mM-ATP) and for ATP of 125 microM (in the presence of 1 microM-myo-inositol), (b) did not recognize allo-, epi-, muco-, neo-, scyllo-, 1 D-chiro or 1 L-chiro-inositol as substrates, (c) were competitively inhibited by three naturally occurring analogues of myo-inositol: 1 L-chiro-inositol (Ki 49.5 +/- 0.7 microM: the structural equivalent of myo-inositol, except that the D-3 hydroxy moiety is axial), D-3-deoxy-myo-inositol [Ki 103 +/- 1 microM: (-)-viburnitol], and sequoyitol (Ki 271 +/- 7 microM; unlike 1 L-chiro-inositol and D-3-deoxy-myo-inositol, this was a substrate for the kinase), and finally (d) were apparently non-competitively inhibited by myo-inositol 3-phosphate. The product of myo-inositol kinase could be detected in intact amoebae and was a substrate for the first in a series of inositol polyphosphate kinases present in Dictyostelium which ultimately yield myo-inositol hexakisphosphate. The activity of myo-inositol D-3-hydroxykinase in Dictyostelium lysates showed evidence of developmental regulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGRANOFF B. W., BRADLEY R. M., BRADY R. O. The enzymatic synthesis of inositol phosphatide. J Biol Chem. 1958 Nov;233(5):1077–1083. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bollmann O., Strother S., Hoffmann-Ostenhof O. The enzymes involved in the synthesis of phytic acid in Lemna gibba (studies on the biosynthesis of cyclitols, XL.(1)). Mol Cell Biochem. 1980 May 7;30(3):171–175. doi: 10.1007/BF00230171. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dietz M., Albersheim P. The enzymic phosphorylation of myo-inositol. Biochem Biophys Res Commun. 1965 May 18;19(5):598–603. doi: 10.1016/0006-291x(65)90381-5. [DOI] [PubMed] [Google Scholar]
- English P. D., Dietz M., Albersheim P. Myoinositol kinase: partial purification and identification of product. Science. 1966 Jan 14;151(3707):198–199. doi: 10.1126/science.151.3707.198. [DOI] [PubMed] [Google Scholar]
- Kay R. R. Gene expression in Dictyostelium discoidium: mutually antagonistic roles of cyclic-AMP and ammonia. J Embryol Exp Morphol. 1979 Aug;52:171–182. [PubMed] [Google Scholar]
- Kay R. R., Trevan D. J. Dictyostelium amoebae can differentiate into spores without cell-to-cell contact. J Embryol Exp Morphol. 1981 Apr;62:369–378. [PubMed] [Google Scholar]
- Loewus M. W., Sasaki K., Leavitt A. L., Munsell L., Sherman W. R., Loewus F. A. Enantiomeric Form of myo-Inositol-1-Phosphate Produced by myo-Inositol-1-Phosphate Synthase and myo-Inositol Kinase in Higher Plants. Plant Physiol. 1982 Dec;70(6):1661–1663. doi: 10.1104/pp.70.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari E., Hoffmann-Ostenhof O. Untersuchungen über die Biosynthese der Cyclite. XXI. Uber ein Enzymsystem, das myo-inosit zu Phytinsäure phosphorylieren kann. Hoppe Seylers Z Physiol Chem. 1968 Dec;349(12):1797–1799. [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Barker C. J., Downes C. P. Synthesis of myo-inositol 1,3,4,5,6-pentakisphosphate from inositol phosphates generated by receptor activation. Biochem J. 1988 Aug 1;253(3):721–733. doi: 10.1042/bj2530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Irvine R. F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990 Aug 9;346(6284):580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Downes C. P. Metabolic and structural evidence for the existence of a third species of polyphosphoinositide in cells: D-phosphatidyl-myo-inositol 3-phosphate. Biochem J. 1989 Apr 1;259(1):267–276. doi: 10.1042/bj2590267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J., De Vries M. J., Penning L. C., Roovers E., Van der Kaay J., Erneux C., Van Lookeren Campagne M. M. Chemoattractant and guanosine 5'-[gamma-thio]triphosphate induce the accumulation of inositol 1,4,5-trisphosphate in Dictyostelium cells that are labelled with [3H]inositol by electroporation. Biochem J. 1989 Mar 1;258(2):577–586. doi: 10.1042/bj2580577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]


