Abstract
CyaA, the adenylate cyclase toxin from Bordetella pertussis, can deliver its N-terminal catalytic domain into the cytosol of a large number of eukaryotic cells and particularly into professional antigen-presenting cells. We have previously identified within the primary structure of CyaA several permissive sites at which insertion of peptides does not alter the ability of the toxin to enter cells. This property has been exploited to design recombinant CyaA toxoids capable of delivering major histocompatibility complex (MHC) class I-restricted CD8+ T-cell epitopes into antigen-presenting cells and to induce specific CD8+ cytotoxic T-lymphocyte (CTL) responses in vivo. Here we have explored the capacity of the CyaA vector carrying several different CD8+ T-cell epitopes to prime multiple CTL responses. The model vaccine consisted of a polyepitope made of three CTL epitopes from lymphocytic choriomeningitis virus (LCMV), the V3 region of human immunodeficiency virus gp120, and chicken ovalbumin, inserted at three different sites of the catalytic domain of genetically detoxified CyaA. Each of these epitopes was processed on delivery by CyaA and presented in vitro to specific T-cell hybridomas. Immunization of mice by CyaA toxoids carrying the polyepitope lead to the induction of specific CTL responses for each of the three epitopes, as well as to protection against a lethal viral challenge. Moreover, mice primed against the vector by mock CyaA or a recombinant toxoid were still able to develop strong CTL responses after subsequent immunization with a recombinant CyaA carrying a foreign CD8+ CTL epitope. These results highlight the potency of the adenylate cyclase vector for induction of protective CTL responses with multiple specificity and/or broad MHC restriction.
CD8+ cytotoxic T lymphocytes (CTL) are recognized as important mediators of protective immunity against many viruses (22), tumors (25), intracellular bacteria (23), and parasites (24, 29). Vaccination strategies aimed at generating CTL responses in vivo have been investigated for several years, and recombinant viruses (39), bacteria (15, 28, 34), and DNA (7, 42) have proven to be excellent inducers of CTL. However, the inherent safety concerns linked to these strategies of CTL induction may limit their future application in humans. Vaccination with synthetic peptides corresponding to CTL epitopes also appears to lead to a protective CTL-mediated immunity against tumors or viruses (1, 35). Peptide-based vaccines, however, require adjuvants that are often incompatible with human vaccination. Moreover, as shown in the model of the adenovirus type 5 E1A-tumor system, immunization with tumor-specific peptides may lead to a diminished rather than a protective immune response (40).
An attractive approach to the design of CTL-inducing vaccines is the delivery of peptide epitopes by nonreplicative protein vectors, such as bacterial toxins reaching the cytosol of antigen-presenting cells (3, 6, 10, 13). Among these, the adenylate cyclase (AC) toxin of Bordetella pertussis (CyaA or ACT) has been gaining attention over the past few years. CyaA has a unique mechanism of cell entry, which consists of direct translocation of the catalytic domain (AC) of CyaA across the plasma membrane of target cells. We have previously demonstrated that presentation of a recombinant CyaA to CD8+ T cells does not require endocytosis of CyaA and is mediated by the classical major histocompatibility complex (MHC) class I pathway (16). It was further shown that recombinant CyaA toxins carrying a single CD8+ T-cell epitope, from the nucleoprotein of the lymphocytic choriomeningitis virus (LCMV) or from the V3 region of the human immunodeficiency virus (HIV) glycoprotein, remained cell invasive and were able to prime protective MHC class I-restricted cytotoxic T-cell responses (13, 31). More recently, protective and therapeutic antitumor immunity against melanoma cells expressing chicken ovalbumin have been induced by a detoxified CyaA harboring a CTL epitope derived from chicken ovalbumin (12).
Since different MHC molecules of various haplotypes generally bind different peptides, a vaccine based on a single CTL epitope would be effective for only a small percentage of an outbred population. To prevent mutant escape and to overcome the MHC haplotypic diversity of the human population, a practical vaccine would therefore have to deliver multiple epitopes. In the present study, we investigated the capacity of CyaA to deliver simultaneously three defined immunodominant CTL epitopes from LCMV, HIV type 1 (HIV-1), and chicken ovalbumin. We show that on delivery by a single recombinant CyaA, all three epitopes are efficiently processed, are presented to CD8+ T cells, and induce epitope-specific cytotoxic responses in vivo against each epitope. Moreover, the immunity induced by these polyepitope constructs protected against a subsequent lethal challenge by LCMV. This demonstrates that immunodominant epitopes derived from different pathogens or antigens can be delivered by a single CyaA molecule without loss of immunogenicity.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (H-2b) and BALB/c (H-2d) mice were obtained from CER Janvier (Le Gesnet St-Isle, France). They were used at 6 to 12 weeks of age.
Synthetic peptides.
All peptides were synthetized by Neosystem (Strasbourg, France). Peptide p118–132 corresponds to the H-2Ld T-cell epitope of LCMV nucleoprotein (1). Peptide p257–264 corresponds to the H-2Kb T-cell epitope encompassing OVA residues 257 to 264 recognized by the B3Z CD8+ T-cell hybridoma (5). Peptide p316–327 corresponds to the H-2Kd immunodominant CTL epitope from the V3 region of gp120 of the 18IIIB HIV (Lai) isolate (35).
Cells.
Target cells for CTL lysis assays were DBA/2 mouse mastocytoma P815 (H-2d) and mouse thymoma EL4 cells (American Type Culture Collection, Manassas, Va). B3Z (21), a CD8+ T-cell hybridoma specific for the OVA 257–264 peptide (SIINFEKL) in the context of Kb, was a generous gift from N. Shastri (University of California, Berkeley, Calif.). These cells were cultured in complete medium (CM) consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 50 μM 2-mercaptoethanol, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. B3Z was maintained in CM containing 1 mg of G418 per ml and 400 μg of hygromycin B per ml.
Production of specific LCMV–CD8+ T-cell hybridoma.
Female BALB/c mice were injected subcutaneously at the base of the tail with 0.1 ml of incomplete Freund adjuvant emulsion containing 100 μg of LCMV peptide p118–126 (RPQASGVYM). On the two following days, the mice were depleted of CD4+ T cells with 300 μg of CD4-specific rat anti-mouse monoclonal antibody (GK 1–5), semipurified from ascitic fluids as previously described (11). One week later, the mice were killed and the inguinal lymph nodes and spleens were removed aseptically. A single-cell suspension was prepared in CM and cultured in the presence of an equal number of irradiated syngeneic splenocytes and 10 μg of immunizing peptide per ml. Four days later, viable lymphocytes were isolated by fractionation with Lympholyte (Cedarlane, Ontario, Canada) and fused with CD8-transfected BW5147 myeloma (kindly provided by Mireille Viguier, Institut Cochin, Paris, France) in a ratio of 1:1 by using 0.5 ml of polyethylene glycol 1500 (50%; Boehringer GmbH, Mannheim, Germany). The cell suspension was brought to a final volume of 40 ml with RPMI 1640 supplemented with 20% fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM glutamine, and antibiotics.
After the suspension was incubated for 4 h at 37°C, feeder HAT-sensitive A20 cells were added to a final concentration of 105/ml. Then the cells were plated onto 96-well flat-bottom microtiter plates at 100 μl/well; 16 h later, 20 μl of HAT 6× (Boehringer) was added to each well. Hybridomas appeared 7 to 15 days after fusion and were assayed for peptide-specific reactivity with 1 μg of the immunizing peptide per ml and 5 × 104 P815 cells as antigen-presenting cells (APC), and 24-h supernatants were analyzed for their interleukin-2 (IL-2) content.
From over 20 hybridomas specific for LCMV p118–126, LC 3A10, an Ld-restricted CD8+ T-cell hybridoma, was selected.
Preparation of recombinant adenylate cyclase toxins carrying the V3-OVA-LCMV polyepitope.
Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.) was used throughout this work for DNA manipulation and for expression of CyaA. Bacteria were grown at 37°C in Luria-Bertani medium supplemented with 150 μg of ampicillin per ml. pT7CACT1 is a construct with enhanced expression of the cyaC and cyaA genes in E. coli under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lacZp promoter, which was derived from pCACT3 (4). The sequence encoding the polyepitope was inserted in frame at three different sites into the gene for CyaA (cyaA), between codons 107 and 108, codons 232 and 233, and codons 335 and 336. For this purpose, three plasmids derived from pT7CACT1 were used, each with a unique BsrGI restriction site engineered at the respective position (27). This allowed a two-step insertion of two pairs of annealed synthetic oligonucleotides, that on assembly yielded the sequence 5′-GTA CGT ATT CAA CGT GGA CCC GGG CGT GCA TTT GTT ACA ATA CGT CCG CAA GCT TCT GGT GTT TAC ATG GGT AAC CTG ACC GCT CAG GCT TCA ATA ATT AAT TTT GAA AAG CTC for the coding strand and 5′-GTA CGA GCT TTT CAA AAT TAA TTA TTG AAG CCT GAG CGG TCA GGT TAC CCA TGT AAA CAC CAG AAG CTT GCG GAC GTA TTG TAA CAA ATG CAC GCC CGG GTC CAC GTT GAA TAC for the noncoding strand. This oligonucleotide was designed (i) to introduce a unique HindIII restriction site for rapid identification of insertion mutants, (ii) to stop CyaA synthesis when inserted in the inverted orientation, and (iii) to destroy the original BsrGI insertion site ligation. The oligonucleotide encoded the following CTL epitopes in the respective order: (i) the RIQRGPGRAFVTI peptide corresponding to an H-2d T-cell epitope from the V3 loop of gp160 of the 18IIIB HIV-1 (Lai) isolate; (ii) the RPQASGVYMGNLTAQ peptide corresponding to the H-2d T-cell epitope from the LCMV nucleoprotein, and (iii) the Kb-restricted T, CD8+ epitope SIINFEKL corresponding to residues 257 to 264 from OVA. The orientation and exact sequence of all inserted oligonucleotides were verified by DNA sequencing. After characterization of the cell-invasive AC activity of the generated CyaA-polypeptide fusions, the constructs were detoxified by ablating their catalytic activity. For this purpose, the individual plasmids were partially digested by EcoRV, and the linearized molecules were purified and ligated with the BamHI synthetic linker 5′-GGATCC, which introduced a dipeptide GlyPhe insert between residues 188 and 189, thereby disrupting the ATP binding site of CyaA (32). The resulting proteins were free of any detectable AC activity. Details of the construction of the plasmids will be provided on request.
The recombinant CyaA proteins were produced on IPTG induction (1 mM) of 500-ml exponential cultures of E. coli XL1-Blue transformed with the respective plasmid constructs. The CyaA proteins were extracted from insoluble cell debris after sonication with 8 M urea–50 mM Tris-HCl (pH 8.0)–0.2 mM CaCl2 and purified as described previously (32). AC activities and cell-invasive and hemolytic activities of the CyaA constructs were measured as previously described (32).
Antigen presentation assay.
The stimulation of the LC3A10 and B3Z T-cell hybridomas (105 cells/well) was monitored by measuring the release of IL-2 into the supernatants of 24-h cultures in 96-well plates in the presence of antigens and of either BALB/c or C57BL/6 splenocytes (3 × 105 cells/well), respectively. The antigen concentrations used in each experiment are indicated in the figure legends. After 24 h, the supernatants were harvested and frozen for at least 2 h at −70°C. Then 104 cells of the CTLL cell line, which proliferates specifically in response to IL-2, were cultured per well with 100 μl of supernatant in a total volume of 0.2 ml. At 2 days later, [3H]thymidine (NEN Life Sciences Products, Boston, Mass.) was added, and after a further 18 h of growth, the cells were harvested with an automated cell harvester (Skatron, Lier, Norway). Incorporated [3H]thymidine was detected by scintillation counting. In all experiments, each point was measured at least in duplicate. Results are expressed as Δcpm (cpm in the presence of CyaA − cpm in the absence of CyaA).
Cytotoxicity assay.
BALB/c (H-2d) and C57BL/6 (H-2b) mice were immunized intraperitoneally (i.p.) on days 0 and 14 with 50 μg of purified AC toxoids in phosphate-buffered saline or mixed with 1 mg of aluminum hydroxide. After 7 to 10 days, the spleens were removed and 2.5 × 107 cells were cultured in CM in the presence of 1 μg of the relevant peptide per ml and 2.5 × 107 irradiated (3,000 rads) syngeneic naive spleen cells. After 5 days, the effector cells were harvested and cultured in duplicate with 104 target cells at the indicated effector-to-target ratio in a final volume of 200 μl per well. Target cells, P815 and EL4 (4 × 106), were sensitized during 51Cr labeling with a 50 μM concentration of the appropriate peptide for 1 h at 37°C and were washed prior to use. In each assay, target cells incubated in the absence of peptide were used as a control for nonspecific lysis. After 4 h at 37°C, 50 μl of cell-free supernatant was collected from each well and counted in a MicroBeta Trilux Liquid Scintillation Counter (Wallac, Turku, Finland).
The amount of spontaneously released 51Cr was determined by incubating target cells in medium alone. The total amount of incorporated 51Cr was determined by cell permeabilization with 10% Triton X-100, and the percent specific release was calculated as follows: percent lysis = 100 × (sample cpm − spontaneous cpm release)/(total cpm − spontaneous cpm release).
Limiting-dilution analysis.
The frequencies of LCMV-specific effector CTL cells present in culture after in vitro stimulation were determined by limiting-dilution analysis (LDA), as previously described (19). Briefly, between 40,000 and 60,000 cells from a 5-day in vitro stimulation culture were assayed for cytotoxicity on 51Cr-labeled P815 target cells (104) pulsed with 50 μM p118–132 peptide. Each dilution was tested in 24 replicate wells, and supernatants were counted for radioactivity after 5 h of incubation. A well was considered positive if the amount of released 51Cr exceeded by 3 standard deviations the mean of 51Cr release in wells containing target cells alone. Effector cell frequencies were calculated as previously described (36).
The number of LCMV-specific CTL precursors cells present in immunized mice was determined as follows. Microcultures were performed under LDA conditions with 50 to 106 splenocytes from immunized mice in 24 replicate wells. Each microculture contained 104 syngeneic irradiated naive spleen cells and 1 μg of p118–132 peptide per ml. At 3 days later, 10% rat concanavalin A supernatant was added to each microculture as a source of IL-2. On day 10, the microculture in each well was split and assayed for cytotoxicity on 51Cr-labeled P815 target cells (104) sensitized with 50 μM p118–132 peptide or not sensitized. Frequencies were determined as mentioned above.
Single IFN-γ-producing cell enzyme-linked-immunospot assay for secreting cells.
Multiscreen filtration plates (96 wells; Millipore, Molshein, France) were coated with 4 μg of rat anti-mouse gamma interferon (IFN-γ) antibody (clone R4-6A2; PharMingen, San Diego, Calif.) per ml overnight at room temperature. Then the plates were washed and blocked with RPMI supplemented with 10% fetal calf serum. Serial twofold dilutions of spleen cells from immunized mice were added to the wells along with 5 × 105 γ-irradiated (3,000 rads) syngeneic feeder cells and 10 U of recombinant murine IL-2 (PharMingen) per ml. The cells were incubated for 36 h with or without p118–132 peptide at 1 μg/ml. After extensive washes, the plates were revealed by incubation with 4 μg of biotinylated rat anti-mouse IFN-γ antibody (clone XMG 1.2; PharMingen) per ml followed by incubation with streptavidin-alkaline phosphatase (PharMingen). Finally, spots were revealed using 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (Sigma, St. Louis, Mo.) as the substrate. The number of IFN-γ-producing cells was determined by counting the number of spot-forming cells (SFC) in each well, and the results were expressed as the total number of SFC per spleen (26).
Virus protection experiment.
BALB/c mice were immunized on days 0 and 21 by the i.p. route either with 50 μg of wild-type CyaA mixed with 1 mg of alum, with the LCMV strain Arm/53b (105 foci), with 50 μg of CyaA224LCMV-E5, or with 50 μg of the recombinant CyaA carrying multiple epitopes mixed with 1 mg of alum. One week later, the mice were challenged intracerebrally with 101.7 foci of LCMV and their survival was monitored for 21 days.
RESULTS
Insertion of several foreign CTL epitopes at three different positions of the AC domain does not affect the cell-invasive activity of CyaA.
It was important to determine whether the AC vector can simultaneously deliver several CTL epitopes into the MHC class I pathway. Therefore, three CyaA toxins were constructed, carrying at different permissive sites of the catalytic AC domain (27) a 36-residue polyepitope made of three CTL epitopes from LCMV, HIV-1 (Lai), or chicken OVA. Compared to intact CyaA in Table 1, the capacity of the CyaA constructs to invade target cells was not affected by insertion of the polyepitope at positions 108 (CyaA108MEP) and 233 (CyaA233MEP) of the catalytic domain. The third protein (CyaA336MEP) lost the marker AC enzyme activity on insertion of the peptide at position 336, and its cell-invasive capacity could not be measured. However, the results presented below strongly suggest that it was also fully cell invasive. CyaA can therefore accommodate rather long foreign peptides at all three insertion sites without losing the capacity to penetrate cells.
TABLE 1.
Characteristics of the CyaA constructs
| CyaA protein | Insertion point and flanking sequencesa | Invasive activityb (% by wt) | Hemolytic activityb (% by wt) |
|---|---|---|---|
| CyaA (wild type) | None | 100 | 100 |
| CyaA108MEP | SSLAHG107VR-V3-LCMV-OVA-VH108HTAVDL | 100 | 100 |
| CyaA233MEP | SEATGG232VR-V3-LCMV-OVA-VH233LDRERI | 100 | 100 |
| CyaA336MEP | LKEYIG335VR-V3-LCMV-OVA-VH336QQRGEG | NDc | 100 |
The underlined V3-LCMV-OVA polyepitope insert consisted of the sequence IQRGPGRAFVTIRPQSGVYMGNLTAQASIINFEKL. The flanking residues are indicated in bold type.
The cell-invasive and hemolytic activities of the toxins were determined prior to ablation of the enzymatic AC activity of the proteins by disruption of the ATP-binding site of CyaA with a GlyPhe dipeptide inserted between residues 188 and 189 of the catalytic domain.
ND, not determined (due to the lack of enzymatic activity of the protein with a multiepitope insert at that site).
CyaA toxoids can simultaneously deliver three CD8+ T-cell epitopes to the MHC class I pathway and elicit MHC-restricted and antigen-specific stimulation of T-cell hybridomas.
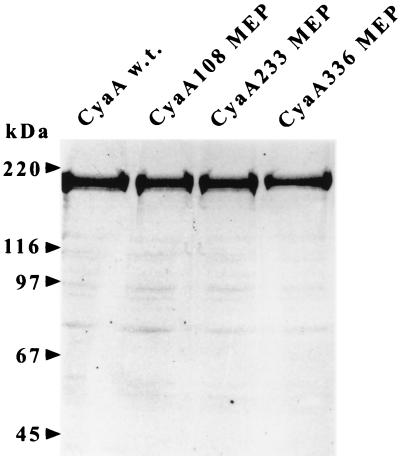
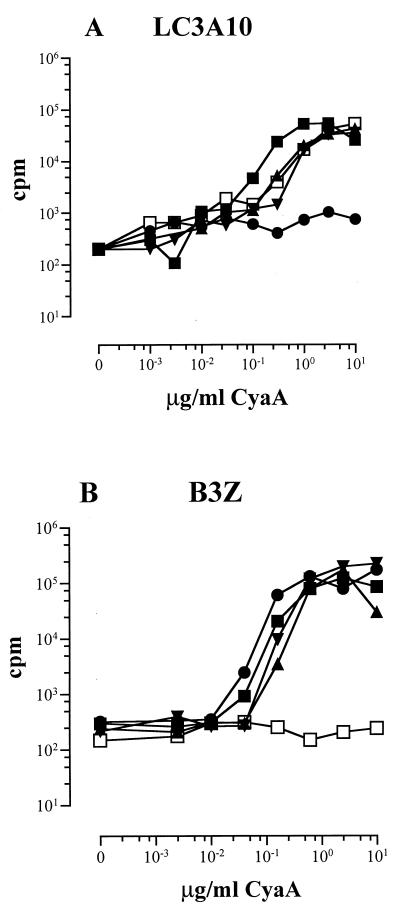
We next analyzed the efficiency of the polyepitope CyaA delivering the different CTL epitopes to the MHC class I pathway of APCs. To allow cellular in vitro assays, the CyaA constructs were genetically detoxified by ablation of the cytotoxic AC enzyme activity (27) and the resulting CyaA toxoids (labeled by an “-E5”) were purified close to homogeneity (Fig. 1). The capacity of these toxoids to deliver CTL epitopes to the MHC class I pathway was determined by measuring their presentation to a specific MHC class I-restricted T-cell hybridoma. BALB/c (H-2d) or C57BL/6 (H-2b) splenocytes were incubated with various concentrations of the toxoids and hybridomas specific for the LCMV (hybridoma LC3A10) or OVA (hybridoma B3Z) CD8+ T-cell epitopes. T-cell hybridoma stimulation was assessed by measuring the amount of IL-2 secreted into culture supernatants. As shown in Fig. 2, both the LCMV and OVA-specific T-cell hybridomas responded to the peptides processed from all three CyaA toxoids in a concentration-dependent and epitope-specific manner. This demonstrates that the polyepitopes inserted at three different permissive sites of CyaA were appropriately delivered into APC, processed into individual epitopes, and presented on the cell surface by the respective H-2d and H-2b class I molecules in a form recognized by the specific T-cell hybridoma. It should be noted that the three epitopes inserted at all three sites of CyaA were processed and presented with essentially the same efficiency. Moreover, all three CyaAs bearing the polyepitope exhibited the same efficiency in delivering the individual CTL epitopes as did the CyaA224OVA-E5 and CyaA224LCMV-E5 toxoids carrying each a single epitope. Therefore, it could be concluded that the processing and presentation of the three different epitopes was not affected by their combination into a single polyepitope or by the insertion site and that the toxoids with the polyepitope at different sites were all efficiently reaching the MHC class I pathway.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified CyaA constructs carrying multiple epitopes. The individual constructs were expressed in E. coli XL-1, extracted with 8 M urea, and purified close to homogeneity by a combination of DEAE-Sepharose and phenyl-Sepharose chromatography, as described in Materials and Methods. Two micrograms of each purified protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 7.5% acrylamide gel and visualized by Coomassie blue staining.
FIG. 2.
Presentation of detoxified CyaAs carrying multiple epitopes to anti-LCMV LC3A10 and anti-OVA B3Z class I-restricted T-cell hybridomas. APCs (splenocytes from BALB/c [A] or C57BL/6 mice [B]) were incubated in the presence of various concentrations of the CyaA toxoids, harboring either OVA (●) or LCMV (□) epitope at site 224 or the three epitopes (MEP) at different sites (108 [■], 233 [▴], or 336 [▾]) and were cocultured, respectively, with the anti-LCMV CD8+ hybridoma LC3A10 (A) or the anti-OVA CD8+ hybridoma B3Z (B). IL-2 secretion by the stimulated hybridoma was determined by the CTL proliferation assay. Results are expressed in cpm of incorporated [3H]thymidine (cpm in the presence of CyaA − cpm in the absence of CyaA). Data represent the mean values of duplicate samples (standard error of the mean, <10%) and are representative of two (A) and three (B) experiments.
Immunization with CyaA toxoids bearing multiple epitopes induces polyspecific CTL responses.
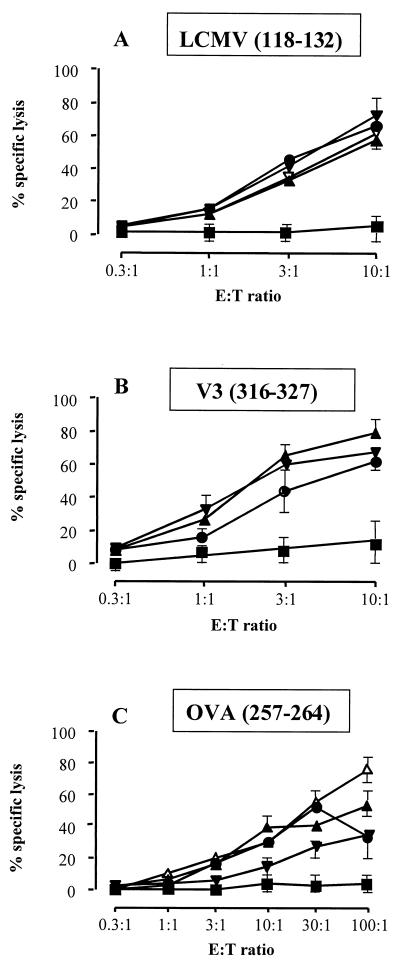
To test whether CTL responses could be induced against all three CD8+ T-cell epitopes delivered by a single CyaA, BALB/c and C57BL/6 mice were immunized with the polyepitope CyaA toxoids with or without alum. Splenocytes were harvested and stimulated in vitro with the indicated peptide. Five days later, a 51Cr release assay was performed to determine their ability to lyse target cells sensitized with the corresponding peptides. As shown in Fig. 3, immunization of BALB/c mice with all three polyepitope CyaAs mixed with alum induced strong and specific CTL responses to both LCMV p118–132 and HIV V3 p316–327 epitopes. The CTL activities induced by all three constructs were quite similar and high (i.e., 60 to 70% specific lysis at an effector-to-target ratio of 10:1). Moreover, the polyepitope CyaA toxoids exhibited the same efficiency in inducing LCMV-specific CTL response in mice as did the CyaA carrying a single LCMV epitope. Immunization with the polyepitope toxoids also induced CTLs specific to OVA (p257–264) in C57BL/6 mice (Fig. 3C). As expected, in vitro-stimulated control splenocytes from mice injected with the control CyaA yielded no specific target cell lysis (Fig. 3). Taken together, these results demonstrate that immunization with the polyepitope CyaA toxoids induced specific and MHC-restricted CTL responses to each of the three CD8+ T-cell epitopes.
FIG. 3.
CTL induction by the detoxified CyaAs bearing multiple epitopes. BALB/c (A and B) and C57BL/6 (C) mice (n = 3 for each group) were immunized i.p. on days 0 and 14 with 50 μg of CyaA toxoids carrying the polyepitope at different sites (108 [●], 233 [▴], or 336 [▾]) or with either the LCMV (▿) or OVA (▵) epitope at site 224 or with control detoxified CyaA (CyaA w.t.-E5) (■) mixed with alum. Seven days later, the animals were sacrificed, the splenocytes were restimulated in vitro for 5 days with 1 μg of the LCMV (A), V3 (B), or OVA (C) peptide per ml in the presence of irradiated syngeneic splenocytes, and used as effectors against unsensitized target cells (P815 for panels A and B and EL4 for panel C) or against target cells sensitized with the same peptide as used for in vitro stimulation. Target lysis was evaluated by 51Cr release. Lysis of unsensitized target cells was less than 10% and is not shown. (A and B) LCMV (p118–132)-specific CTL (A) and V3 (p316–327)-specific CTL (B) responses in the same BALB/c mice injected with the detoxified recombinant CyaAs. (C) OVA (p257–264)-specific CTL response in C57BL/6 mice injected with the detoxified recombinant CyaAs. CTL responses of control mice injected with the wild-type CyaA-E5 are shown to illustrate the specificity of the responses. The data represent the mean percentage of the specific lysis values from duplicate samples and standard error of the mean and are representative of four experiments. E:T ratio, effector-to-target-cell ratio.
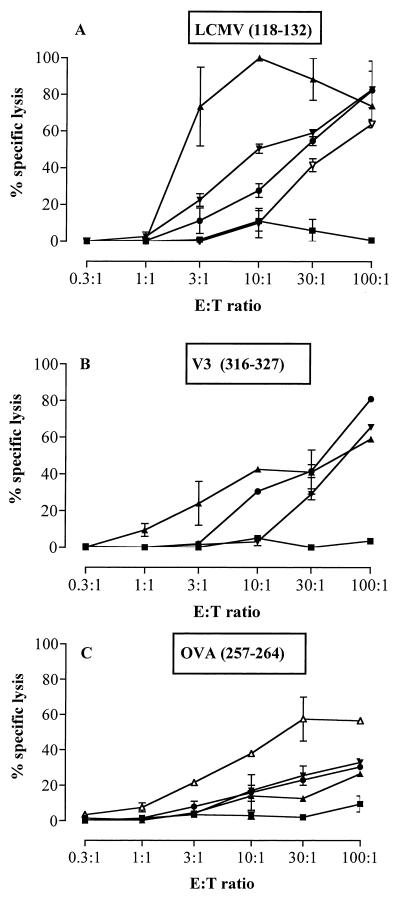
When injected without alum, all three polyepitope toxoids also induced good specific cytotoxic responses to LCMV, V3, and OVA epitopes, as shown in Fig. 4. However, an enhancement of CTL responses to LCMV and V3 epitopes was observed when CyaA toxoids carrying multiepitope were injected with alum, and this observation is consistent with an earlier study (8). These results show that alum is not strictly required to induce good CTL responses as previously described (8) but allows optimal CTL induction by these recombinant molecules. Therefore, the following experiments were carried out in the presence of alum.
FIG. 4.
Immunization of mice with detoxified CyaA toxoids bearing multiple epitopes in the absence of adjuvant induces high specific CTL responses. BALB/c (A and B) and C57BL/6 (C) mice (n = 3 for each group) were immunized i.p. on days 0 and 14 with 50 μg of CyaA toxoids carrying the polyepitope at different sites (108 [●], 233, [▴], or 336) [▾] or with either the LCMV (▿) or OVA (▵) epitope at site 224 or with control detoxified CyaA (CyaA wt-E5) (■) in PBS. Seven days later, animals were sacrificed, the splenocytes were restimulated in vitro for 5 days with 1 μg of the LCMV (A), V3 (B), or OVA (C) peptide per ml in the presence of irradiated syngeneic splenocytes and used as effectors against unsensitized target cells (P815 for panels A and B and EL4 for panel C) or against target cells sensitized with the same peptide as used for in vitro stimulation. Target lysis was evaluated by 51Cr release. Lysis of unsensitized target cells was less than 10% and is not shown. (A and B) LCMV (p118–132)-specific CTL (A) and V3 (p316–327)-specific CTL (B) responses in the same BALB/c mice injected with the detoxified recombinant CyaA toxoids. (C) OVA (p257–264)-specific CTL response in C57BL/6 mice injected with the detoxified recombinant CyaAs. CTL responses of control mice injected with the wild-type CyaA-E5 are shown to illustrate the specificity of the responses. The data represent the mean percentage of the specific lysis values from duplicate samples and standard error of the mean and are representative of two experiments. E:T ratio, effector-to-target-cell ratio.
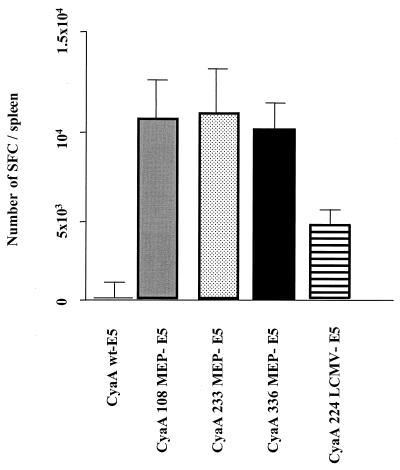
To estimate ex vivo the frequencies of LCMV-specific splenocytes in mice immunized with the three recombinant CyaAs, enzyme-linked immunospot (26) and LDA assays were performed (36). First, the number of cells producing IFN-γ in response to in vitro stimulation with the LCMV peptide was quantified in spleen cell preparations from mice immunized with the recombinant CyaA toxoids. As shown in Fig. 5, the T-cell frequencies were remarkably high and in the same range for each group of mice immunized with three different polyepitope toxoids. A very small number of IFN-γ-producing splenocytes was obtained from mice immunized with the wild-type CyaA serving as mock control (Fig. 5). Moreover, in all cases the response was epitope specific and the spleen cells from these mice did not produce IFN-γ in the absence of stimulation by the LCMV peptide (data not shown).
FIG. 5.
Detection of LCMV-specific IFNγ-producing cells after immunization with the CyaA toxoids carrying multiple epitopes. BALB/c mice (n = 6 for each group) were immunized i.p. on days 0 and 14 with 50 μg of detoxified CyaAs carrying either the polyepitope at different positions (108, 233, or 336), the LCMV epitope alone at position 224, or control CyaA toxoid, mixed with alum. On day 21, spleen cells isolated from immunized mice were cultured in vitro for 36 h without stimulation (i.e., no peptide) or with 1 μg of the LCMV peptide per ml in the presence of syngeneic irradiated splenocytes and 10 U of recombinant IL-2 per ml. The data are expressed as the number of SFC per spleen and represent the mean and standard error of the mean obtained with six mice in three independent experiments.
The frequencies of LCMV-specific lytic CTL precursors and of effector CTL induced by the polyepitope CyaA toxoids were further determined by LDA, both directly ex vivo and after 5 days of in vitro restimulation. As shown in Table 2, the frequencies of LCMV-specific CTL precursors induced after immunization with the three toxoids were rather similar. Moreover, they were very close to those induced by the CyaA224LCMV-E5 protein carrying a single LCMV epitope. Similarly, the effector CTL frequencies observed after in vitro culture were very comparable. Taken together, these results, confirm that the processing and the in vivo immunogenicity of each individual epitope within the polyepitope were not affected by its size, the presence of other flanking epitopes, or the insertion site in the CyaA vector.
TABLE 2.
Comparison of the frequencies of ex vivo LCMV-specific precursor CTL and of effector CTL after in vitro stimulation of spleens from mice immunized with the CyaA carrying multiple epitopes
| In vivo immunizationa | Ex vivo CTL precursor frequencyb (no. of expts) | Effector CTL frequency after in vitro culturec (no. of expts) |
|---|---|---|
| CyaA wt-E5 | NDd (3) | ND (3) |
| CyaA108MEP-E5 | 1/126,100 ± 1/39,120 (3) | 1/4,667 ± 1/1,128 (3) |
| CyaA233MEP-E5 | 1/257,800 ± 1/179,800 (3) | 1/3,495 ± 1/928 (3) |
| CyaA336MEP-E5 | 1/152,900 ± 1/33,870 (3) | 1/9,073 ± 1/4,952 (3) |
| CyaA224LCMV-E5 | 1/282,000 ± 1/169,300 (3) | 1/2,793 ± 1/177 (3) |
BALB/c mice (two per group) were immunized i.p. on days 0 and 14 with 50 μg of recombinant CyaA toxoids mixed with 1 mg of alum. Control mice were immunized under the same conditions with detoxified wild-type CyaA.
LCMV-specific precursor CTL frequencies were determined 7 days after the last injection by LDA, as described in Materials and Methods. Results are expressed as the mean ± standard error of the mean of the frequencies obtained in the different experiments.
LCMV-specific effector CTL frequencies were determined after the 5-day in vitro stimulation by LDA as described in Materials and Methods. Results are expressed as the mean ± standard error of the mean of the frequencies obtained in the different experiments.
ND, not detectable.
Immunization with CyaA toxoids carrying multiple epitopes protects mice against a lethal LCMV challenge.
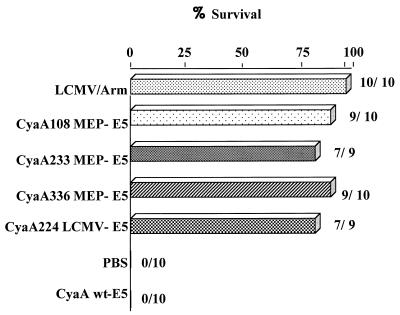
It was then important to examine the capacity of CTL generated by polyepitope CyaA toxoids to protect mice against a viral challenge. As expected, control mice immunized with mock CyaA or PBS developed a fatal choriomeningitis within 8 days after intracerebral inoculation of the virus (Fig. 6). In contrast, high protection rates against a lethal intracerebral LCMV challenge (90, 78, and 90% survival) were observed after immunization with the CyaA108MEP-E5, CyaA233MEP-E5, or CyaA336MEP-E5 protein. Morever, the induced protection was fully comparable to the response induced by the CyaA224LCMV-E5 toxoid carrying the LCMV epitope alone (78% survival) and was quite close to the full protection induced by a transient LCMV infection after i.p. administration of the virus. It should be noticed that all surviving mice completely cleared LCMV (data not shown). Therefore, these results demonstrate that immunization with polyepitope CyaA toxoids induces biologically significant CTL responses capable of protecting mice against LCMV.
FIG. 6.
Immunization with the detoxified CyaA toxoids carrying multiple epitopes induces full protection against a lethal LCMV challenge. On days 0 and 14, BALB/c mice were immunized with 50 μg of wild-type CyaA E5 (mock control, CyaA wt-E5) or the recombinant CyaA carrying the LCMV epitope alone (CyaA224LCMV-E5) or the polyepitope at different positions (CyaA108MEP-E5, CyaA233MEP-E5, or CyaA336MEP-E5), mixed with 1 mg of alum. Control groups received either PBS or 105 foci of LCMV i.p. On day 21, the mice were challenged intracerebrally with 101.7 foci of LCMV. Mortality was monitored for 21 days. The percentage of mice that survived and the number of surviving mice out of the total number of challenged mice are shown for each group. The data represent the cumulative results of two experiments.
Priming of mice with the mock CyaA vector or a detoxified recombinant CyaA does not interfere with the subsequent induction of epitope-specific CTL responses by CyaA carrying a heterologous epitope.
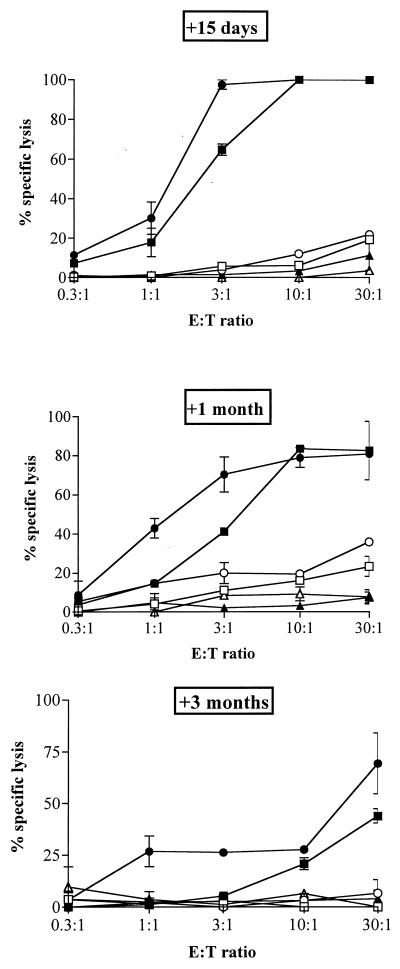
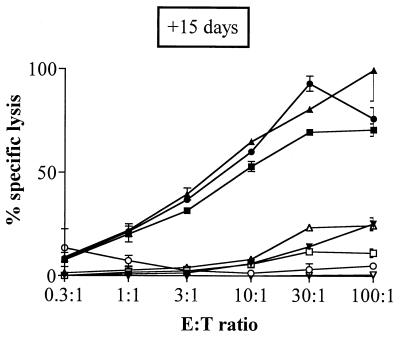
The antigen delivery capacity of a practical vector should not be inhibited by priming with the vector molecule itself, such as priming against CyaA on a natural Bordetella infection, or after repeated administration of the CyaA-derived toxoids. Therefore, we first examined whether priming of mice with the mock CyaA vector has an effect on the subsequent response to recombinant CyaA carrying an LCMV epitope. Mice received two i.p. injections of a dose as high as 50 μg of wild-type CyaA prior to i.p. immunizations with CyaA224LCMV. From previous experiments, it is known that such immunization protocols typically induce very high levels of CyaA-specific antibodies with titers above 1:125,000 (unpublished results). Nevertheless, as shown in Fig. 7, mice which received wild-type CyaA, 15 days, 1 month, or 3 months before immunization with the recombinant CyaA224LCMV still developed strong CTL responses to the LCMV epitope. These responses were, indeed, fully comparable to the responses of unprimed mice. In addition, comparable responses were obtained in mice which were injected with CyaA wt-E5 or CyaA224OVA-E5 15 days prior immunization with CyaA224LCMV-E5 (Fig. 8). Therefore, priming with the wild-type CyaA, the CyaA toxoid, or a recombinant detoxified CyaA has no inhibitory effect on the subsequent CTL response induced by a recombinant CyaA carrying another CTL epitope.
FIG. 7.
Effect of prior priming with the mock CyaA vector on the induction of CTL responses by a recombinant CyaA carrying a CD8+ T-cell epitope. BALB/c mice were primed with PBS (□, ■) or 50 μg of wild-type CyaA (○, ●, ▵, ▴) mixed with 1 mg of alum by i.p. injection on days 0 and 14. After 15 days, 1 month, or 3 months, all mice were immunized twice i.p. with 50 μg of CyaA224LCMV (□, ■, ○, ●) or wild-type CyaA (▵, ▴), mixed with 1 mg of alum, at a 3-week interval. At 7 days after the last injection, spleen cells were stimulated in vitro with the p118–132 peptide in the presence of syngeneic spleen cells. The cytotoxic activity of these effector cells was measured on 51Cr-labeled P815 target cells pulsed with the same peptide (solid symbols) or incubated with medium alone (open symbols). The data represent the mean percentage of the specific lysis values from duplicate samples and standard error of the mean and are representative of three experiments. E:T ratio, effector-to-target-cell ratio.
FIG. 8.
Effect of prior priming with a detoxified recombinant CyaA on the induction of CTL responses by a recombinant CyaA toxoid carrying another CTL epitope. BALB/c mice were primed with either PBS (○, ●), 50 μg of detoxified wild-type CyaA (CyaA wt-E5) (□, ■, ▿, ▾), or 50 μg of detoxified CyaA224OVA-E5 mixed with 1 mg of alum (▵, ▴) by i.p. injection on days 0 and 14. After 15 days, all mice were immunized twice i.p. with 50 μg of CyaA224LCMV-E5 (□, ■, ▵, ▴, ○, ●) or CyaA wt-E5 (▿, ▾), mixed with 1 mg of alum, at a 3-week interval. Seven days after the last injection, spleen cells were stimulated in vitro with the p118–132 peptide in the presence of syngeneic spleen cells. The cytotoxic activity of these effector cells was measured on 51Cr-labeled P815 target cells pulsed with the same peptide (solid symbols) or incubated with medium alone (open symbols). The data represent the mean percentage of the specific lysis values from duplicate samples and standard error of the mean and are representative of three experiments. E:T ratio, effector-to-target-cell ratio.
DISCUSSION
Induction of CTL responses directed against multiple epitopes appears to be crucial for the development of efficient recombinant vaccines against many important diseases. Several approaches have been tested recently, involving the use of polyepitope constructs and different vaccine vehicles, such as viral vectors or naked DNA (2, 14, 37–39, 41). Mouse and macaque immunization studies using polyepitope immunogens established the validity of this approach, and an HIV vaccine based on such a strategy entered a phase I clinical trial in 2000 (17). Our previous results demonstrated that detoxified CyaA is a promising nonreplicative vector for efficient activation of CTL responses (12, 13). The objective of this study was therefore to evaluate the feasibility of delivering multiple CTL epitopes by the detoxified CyaA vectors. The data reported here clearly show that AC toxoids, carrying three different model epitopes, were clearly capable of reaching the MHC class I pathway and inducing in vivo MHC-restricted CTL responses specific for each of the three inserted epitopes. In addition, strong and protective anti-LCMV responses were obtained in mice immunized with these polyepitope CyaA constructs, illustrating that the induced CTL responses were fully functional in vivo.
This study thus established that insertion of a foreign 35-amino-acid polypeptide into three different sites of the catalytic domain of CyaA did not affect its capacity to target and penetrate APCs. Indeed, we have previously demonstrated that the permissive site at residue 224 of the catalytic domain of CyaA can accommodate up to four copies of the CD8+ T-cell LCMV epitope, corresponding to a 76-residue heterologous polypeptide insert (8, 33). In that case, however, both the specific AC activity and the cell invasiveness of the toxin progressively decreased with increasing numbers of inserted copies of the LCMV epitope. The construct carrying four copies of this epitope exhibited about 50% of the activities of wild-type CyaA (8, 33). In the present study, no decrease of cell invasiveness was observed for the two CyaA constructs with polyepitope inserts at positions 108 and 233, which exhibited AC activity and could be characterized. Given the identical efficiency of epitope delivery for presentation, which depends strictly on cell invasiveness, it could be concluded that the third toxoid (CyaA336MEP) was most probably also fully cell invasive. This difference in specific cell invasiveness of toxoids presented here and of the previous constructs with inserts at position 224 could be due to the characteristics of the inserted polypeptide, to the sites used for polypeptide insertion, or to a combination of the two. Indeed, the local electrostatic charge of the insert was shown to be crucial when located at site 224 (20). Introduction of a net negative charge higher than −1 at this position completely blocked translocation of the AC domain of CyaA across target membranes. However, this does not seem to be the case for introduction of negative charges at positions 108, 233, and 336 of the AC domain (P. Sebo, unpublished data). Therefore, it appears that the difficulty of delivery by CyaA of negatively charged but immunologically relevant epitopes could be overcome by inserting these peptides into the permissive sites newly characterized here.
We recently analyzed in vitro the presentation of the OVA CD8+ T-cell epitope inserted at 10 different permissive sites of CyaA along the toxin molecule (27). While all six constructs bearing the OVA epitope within the noninvasive part of the molecule failed to deliver the epitope to the MHC class I molecules, all four toxoids with inserts in different sites of the AC domain (including positions 108, 233, and 336) efficiently delivered the epitope into the cytosolic pathway. However, these results were based on in vitro stimulation of a specific CD8+ T-cell hybridoma; the capacity of the hybrid toxins to stimulate CTL responses was not evaluated in vivo. The present study provides clear evidence that these three sites are also fully permissive for the insertion of multiple heterologous peptides and can be used for constructing CyaA toxoids directing in vivo CTL activation. It also shows that the position of the polyepitope within the N-terminal domain of the CyaA does not affect the induction of specific CTLs, as demonstrated here by the similar frequency and magnitude of the LCMV-specific CTL responses induced after immunization with the three polyepitope toxoids.
Flanking sequences are also important for the efficient processing of CD8+ T-cell epitopes (9), but there are now many examples of presentation of epitopes regardless of their context (38). The finding reported here, i.e., that individual CTL epitopes can be processed from the polyepitope when linked together without intervening sequences, shows that the environment of the CTL epitopes did not interfere with the processing events. Hence, the present study opens the way toward rational design of a new generation of polyvalent hybrid toxoids carrying strings of epitopes inserted into different sites along the catalytic domain of CyaA and with specifically designed properties and increased versatility.
The development of CyaA carrying protective antigens therefore represents a promising option in the development of efficient vaccines against various pathogens. However, immune reponses induced by vaccination against pertussis or natural infections by Bordetella, which appear to be much more common in vaccinated populations than was previously appreciated (18), might interfere with the immunogenicity of future CyaA vaccines. Therefore, the effect of sequential immunizations by this delivery system was analyzed. Our results demonstrate that previous immunization with the CyaA toxin or the CyaA toxoid carrying a CTL epitope does not prevent secondary immunization directed toward another CTL epitope carried by CyaA. This suggests that preexisting immunity against the CyaA toxin does not prevent its presentation by MHC class I molecules. Thus, the immunity against the carrier toxin does not limit the use of this delivery system as a potential vaccine, in comparison with recombinant life vectors (30).
Besides these attractive properties, the adenylate cyclase of B. pertussis appears to exhibit several additional advantages for vaccine use. Indeed, we have recently established that the recombinant CyaA carrying the OVA epitope can specifically target myeloid dendritic cells by means of its specific interaction with the CD11b integrin at the cell surface and can deliver the OVA epitope to the cytosolic antigen-processing pathway for efficient MHC class I presentation in vivo (P. Guermonprez et al., submitted for publication). As a result, efficient and specific CTL responses can be induced in vivo independently on CD4+ helper T cells (notably via CD40) (11; Guermonprez et al., submitted). All these observations indicate that CyaA has a strong potential for use in preventive or therapeutic treatments of cancers as well as infectious diseases.
ACKNOWLEDGMENTS
This work was supported by Agence Nationale de Recherche sur le SIDA (ANRS), by grant QLK2-CT-1999-00556 from the 5th FP of EU, and grants 310/95/0432 from the Grant Agency and ME167 and VS96149 of the Ministry of Education, Youth, and Sports of the Czech Republic.
We thank Gilles Dadaglio and Mohammed El Azami El-Idrissi for critical reading of the manuscript.
REFERENCES
- 1.Aichele P, Hengartner H, Zinkernagel R M, Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An L-L, Whitton L. A multivalent minigene vaccine, containing B-cell, cytotoxic T-lymphocyte, and Th epitopes from several microbes, induces appropriate responses in vivo and confers protection against more than one pathogen. J Virol. 1997;71:2292–2302. doi: 10.1128/jvi.71.3.2292-2302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballard J D, Collier R J, Starnbach M N. Anthrax toxin-mediated delivery of a cytotoxic T-lymphocyte epitope in vivo. Proc Natl Acad Sci USA. 1996;93:12531–12534. doi: 10.1073/pnas.93.22.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betsou F, Sebo P, Guiso N. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun. 1993;61:3583–3589. doi: 10.1128/iai.61.9.3583-3589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone F R, Bevan M J. Induction of ovalbumin specific cytotoxic T cells by in vivo peptide immunization. J Exp Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbonetti N H, Irish T J, Chen C H, O'Connell C B, Hadley G A, McNamara U, Tuskan R G, Lewis G K. Intracellular delivery of a cytolytic T-lymphocyte epitope peptide by pertussis toxin to major histocompatibility complex class I without involvement of the cytosolic class I antigen processing pathway. Infect Immun. 1999;67:602–607. doi: 10.1128/iai.67.2.602-607.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condon C, Watkins S C, Celluzzi C, Thompson K, Falo L D J. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 8.Dadaglio G, Moukrin Z, Lo-Man R, Sheshko V, Sebo P, Leclerc C. Induction of a polarized Th1 response by insertion of multiple copies of a viral T-cell epitope into adenylate cyclase of Bordetella pertussis. Infect Immun. 2000;68:3867–3872. doi: 10.1128/iai.68.7.3867-3872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Val M, Schlicht H, Rupert T, Reddehase M J, Koszinowski U H. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighbouring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-y. [DOI] [PubMed] [Google Scholar]
- 10.Donelly J J, Ulmer J B, Hawe L A, Friedmann A, Shi X P, Leander K R, Shiwer J W, Oliff A I, Martinez D, Montgomery D, Liu M A. Targeted delivery of peptides epitopes to class I major histocompatibility molecules of a modified Pseudomonas exotoxin. Proc Natl Acad Sci USA. 1993;90:3530–3534. doi: 10.1073/pnas.90.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991;147:4069–4073. [PubMed] [Google Scholar]
- 12.Fayolle C, Ladant D, Karimova G, Ullmann A, Leclerc C. Therapy of murine tumors with recombinant Bordetella pertussis adenylate cyclase toxins carrying a cytotoxic T-cell epitope. J Immunol. 1999;162:4157–4162. [PubMed] [Google Scholar]
- 13.Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ epitopes. J Immunol. 1996;156:4697–4706. [PubMed] [Google Scholar]
- 14.Gilbert S, Plebanski M, Harris S, Allsopp C, Thomas R, Layton G, Hill A. A protein particle vaccine containing multiple malaria epitopes. Nat Biotechnol. 1997;15:1280–1284. doi: 10.1038/nbt1197-1280. [DOI] [PubMed] [Google Scholar]
- 15.Goossens P L, Milon G, Cossart P, Saron M F. Attenuated Listeria monocytogenes as a live vector for induction of CD8+ T cells in vivo: a study with the nucleoprotein of the lymphocytic choriomeningitis virus. Int Immunol. 1995;7:797–805. doi: 10.1093/intimm/7.5.797. [DOI] [PubMed] [Google Scholar]
- 16.Guermonprez P, Ladant D, Karimova G, Ullmann A, Leclerc C. Direct delivery of the Bordetella pertussis adenylate cyclase toxin to the MHC class I antigen presentation pathway. J Immunol. 1999;162:1910–1916. [PubMed] [Google Scholar]
- 17.Hanke T, McMichael A J. Design and construction of an experimental HIV-1 vaccine for a year-2000 clinical trial in Kenya. Nat Med. 2000;6:951–955. doi: 10.1038/79626. [DOI] [PubMed] [Google Scholar]
- 18.He Q, Viljanen M K, Arvilommi H, Aittanen B, Mertsola J. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA. 1998;280:635–637. doi: 10.1001/jama.280.7.635. [DOI] [PubMed] [Google Scholar]
- 19.Hoffenbach A, Langlade-Demoyen P, Dadaglio G, Vilmer E, Michel F, Mayaud C, Autran B, Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989;142:452–462. [PubMed] [Google Scholar]
- 20.Karimova G, Fayolle C, Gmira S, Ullmann A, Leclerc C, Ladant D. Charge-dependent translocation of Bordetella pertussis adenylate cyclase toxin into eukaryotic cells: implication for the in vivo delivery of CD8+ T cell epitopes into antigen-presenting cells. Proc Natl Acad Sci USA. 1998;95:12532–12537. doi: 10.1073/pnas.95.21.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karttunen J, Anderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T cell antigens. Proc Natl Acad Sci USA. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kast W M, Roux L, Curren J, Blom H, Voordouw A, Meloen R, Kolakofsky D, Melief C. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci USA. 1991;88:2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 24.Khusmith S, Sedegah M, Hoffman S L. Complete protection against Plasmodium yoelii by adoptive transfert of a CD8+ cytotoxic T-cell clone recognizing sporozoite surface protein 2. Infect Immun. 1994;62:2979–2983. doi: 10.1128/iai.62.7.2979-2983.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melief C J. Tumor eradication by adoptive transfert of cytotoxic T lymphocytes. Adv Cancer Res. 1992;58:143–175. doi: 10.1016/s0065-230x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 26.Miyahira Y, Murata K, Rodriguez D R R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen-specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 27.Osicka R, Osickova A, Basar T, Guermonprez P, Rojas M, Leclerc C, Sebo P. Delivery of CD8+ T cell epitopes into major histocompatibility complex class I antigen presentation pathway by Bordetella pertussis adenylate cyclase: delineation of cell invasive structures and permissive insertion sites. Infect Immun. 1999;68:247–256. doi: 10.1128/iai.68.1.247-256.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Z-K, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y A. A recombinant Listeria monocytogenes vaccine expressing a model tumor antigen protects mice against lethal tumor challenge and causes regression of established tumors. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 29.Romero P, Maryanski J L, Cordey A-S, Corradin G, Nussenzweig R S, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg S A, Zhai Y, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Seipp C A, Einhorn J H, Roberts B, White D E. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding Mart-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saron M F, Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from choriomeningitis virus. Proc Natl Acad Sci USA. 1997;94:3314–3319. doi: 10.1073/pnas.94.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebo P, Fayolle C, d'Andria O, Ladant D, Leclerc C, Ullmann A. Cell-invasive activity of epitope-tagged adenylate cyclase of Bordetella pertussis allows in vitro presentation of a foreign epitope to CD8+ cytotoxic T cells. Infect Immun. 1995;63:3851–3857. doi: 10.1128/iai.63.10.3851-3857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebo P, Moukrin Z, Kalhous M, Schaft N, Dadaglio G, Sheshko V, Fayolle C, Leclerc C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying multiple copies of a viral CD8+ T-cell epitope. FEMS Immunol Med Microbiol. 1999;26:167–173. doi: 10.1111/j.1574-695X.1999.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 34.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi H, Takeshita T, Morein B, Putney S, Germain R N, Bersofsky J A. Induction of CD8+ cytotoxic cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990;344:873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- 36.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:1614–1619. [PubMed] [Google Scholar]
- 37.Thomson S, Sherrit M, Medveczky J, Elliott S, Moss D, Fernando G, Brown L, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–1723. [PubMed] [Google Scholar]
- 38.Thomson S A, Khanna R, Gardner J, Burrows S R, Coupar B, Moss D J, Suhrbier A. Minimal epitopes expressed in a recombinant polyepitope protein are processed and presented to CD8+ cytotoxic T cells: implications for vaccine design. Proc Natl Acad Sci USA. 1995;92:5845–5849. doi: 10.1073/pnas.92.13.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toes R E M, Hoeben R C, Van der Voort E I H, Ressing M E, Van der Eb A J, Melief C J M, Offringa R. Protective anti-tumor immunity induced by vaccination with recombinant adenoviruses encoding multiple tumor-associated cytotoxic T lymphocyte epitopes in a string-of-beads fashion. Proc Natl Acad Sci USA. 1997;94:14660–14665. doi: 10.1073/pnas.94.26.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toes R E M, Offringa R, Blom R J J, Ressing M E, Melief C J M, Kast W M. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci USA. 1996;93:7855–7860. doi: 10.1073/pnas.93.15.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitton J L, Sheng N, Oldstone M B A, McKee T A. A “string-of-beads” vaccine, comprising linked minigenes confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokohama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]