Abstract
This study aimed to explore the factors influencing thyroid nodules (TNs) in individuals with type 2 diabetes mellitus (T2DM) and evaluate the consistency between different American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) grades and Bethesda scores. A total of 642 T2DM patients were divided into the TN group (245) and the control group (397) based on the presence or absence of TNs. TN patients were further categorized into ACR TI-RADS classification (TR) 1–4 subgroups and TR5 subgroups. Diabetes-related clinical and biochemical parameters were collected, and differences were analyzed using univariate analysis. Logistic regression analysis was utilized to pinpoint independent influencing factors for TN occurrence and different TN classifications. Consequently, age, body mass index (BMI), fasting plasma glucose level (FBGL), low-density lipoprotein cholesterol (LDL-C), diabetic progression, and family history of TNs emerged as independent risk factors for TN development in T2DM patients. Additionally, glycosylated hemoglobin (HbA1c), nodule diameter, and family history of TNs were identified as independent risk factors for TR5 TN development in T2DM patients. All TR1–2 nodules had a Bethesda score of 2 and all showed benign pathological findings. In 97.10% of cases (67/69), nodules classified as TR3 exhibited a Bethesda score of 2, with all pathological results indicating benign findings, aligning with the Bethesda score. In addition, the concordance between TR4 nodules and Bethesda score was only 78.57% (88/112). In conclusion, TNs and their malignancy in T2DM patients are significantly linked to blood glucose and lipid metabolism indices. TR3 classification in T2DM patients poses a low malignancy risk, suggesting caution when conducting fine needle aspiration cytology (FNAC) testing.
Keywords: Type 2 diabetes mellitus (T2DM), thyroid nodules (TNs), American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS) grading, Bethesda score, logistic regression analysis
Introduction
The thyroid gland stands out as a pivotal player in the human endocrine system [1]. The thyroid gland is mainly regulated by the hypothalamus–pituitary gland, and its synthesized and secreted thyroid hormones play a vital role in human growth, development, and the regulation of glucose and lipid metabolism, among other functions [1]. Thyroid nodules (TNs), common maladies of the endocrine system, denote isolated anomalies characterized by localized abnormal growth of thyroid cells distinctly separated from surrounding tissue [2]. The occurrence and development of TNs are mainly caused by genetic predisposition, environmental factors, abnormal iodine intake, dietary habits, and other influences [3]. In the absence of prior imaging techniques, TNs can only be detected by palpation by experienced clinicians with an incidence between 4% and 7%, with a considerable rate of underdiagnosis [4]. Typically, TNs have no obvious clinical manifestations, appearing solely as palpable masses moving within the anterior cervical region upon swallowing [5]. However, as nodules expand, they may cause neck enlargement and compression symptoms like dysphagia and dyspnea [5]. With the development of imaging techniques, the diagnostic efficacy of TNs has markedly improved. The detection rate via color ultrasonography ranges from 20% to 76%, with malignancies accounting for 7% to 15% [6]. As per 2022 statistics from the Chinese Cancer Center, thyroid cancer ranked seventh among various cancers, and the prevalence was significantly increased compared with that of five years ago [7]. Therefore, regular ultrasonography for TN detection and preventative measures against malignant TNs are imperative.
Epidemiological investigations have unveiled a close association between type 2 diabetes mellitus (T2DM) and TN occurrence [8]. Studies have shown that the risk of TNs in patients with T2DM is 1.78-fold higher than that in healthy individuals [9]. Nonetheless, the underlying pathogenesis of T2DM and TNs remains elusive. Presently, insulin resistance (IR) is a widely accepted mechanism by many researchers. Clinical studies reveal significantly higher IR levels in TN patients compared to non-TN patients, with a notable correlation between IR and TNs [10]. Further studies indicate that the size of TNs increases with elevated IR index (HOMA-IR) in patients with T2DM [11]. Moreover, findings from Blanc et al. [12] suggest that higher glycosylated hemoglobin A1c (HbA1c) levels may serve as a risk factor for TN formation and tissue growth in elderly patients with metabolic syndrome, correlating with altered thyroid morphology. Given the intricate relationship between diabetes mellitus and TNs, the influencing factors for TNs in the diabetic population are unclear.
Presently, the optimal utilization of ultrasound to discern clinically significant thyroid cancer remains a focal point of recent research. The American College of Radiology Thyroid Imaging Reporting and Data System (ACR TI-RADS), proposed by the American College of Radiology (ACR), represents the latest risk stratification criteria. ACR TI-RADS primarily assigns relative scores based on TN composition, echogenicity, morphology, margins, and echogenic foci, thereby enhancing the diagnostic precision of TNs [13]. Additionally, Fine Needle Aspiration Cytology (FNAC) serves as the primary diagnostic tool to differentiate benign from malignant TNs, with cytopathological diagnoses classified by the Bethesda Reporting System. However, literature reports indicate that approximately 20% of FNAC results are inconclusive due to unsatisfactory specimens [14]. Currently, the gold standard for diagnosing benign and malignant TNs remains pathological biopsy following surgical resection. Moreover, FNAC represents an invasive procedure, and given that most nodules are benign and do not necessitate FNAC, unnecessary procedures should be minimized. To address this, we analyzed factors influencing TNs across different ACR TI-RADS risk stratifications. Concurrently, this study aimed to assess the consistency between different ACR TI-RADS classification (TR) nodules and Bethesda scores in diabetic patients, thereby facilitating more accurate diagnosis of benign and malignant TNs and reducing unnecessary FNAC procedures.
Materials and methods
Study subjects
According to the inclusion and exclusion criteria, 642 patients with T2DM, aged 29–81 years, were selected from the General Practice Health Management Center of Zhejiang Provincial People’s Hospital from June 2020 to June 2023. The patients’ diagnoses of T2DM were consistent with the Chinese guidelines [15]. Inclusion criteria included: (1) meeting the diagnostic criteria of T2DM; (2) undergoing thyroid color ultrasonography; (3) completing FNAC for nodules ranked 3–5 in the ACR TI-RADS system, and obtaining cytopathological diagnosis and classification by Bethesda reporting system; (4) all nodules with FNAC were found to have histopathological results; and (5) patients were required to sign informed consent. Exclusion criteria included: (1) patients with a history of thyroid surgery; (2) patients with thyroid metastasis; (3) patients with a history of neck radiation; (4) patients with other malignant tumors; and (5) patients with severe heart, liver, and kidney dysfunction.
Clinical data
Demographic and medical information, including age, sex, duration of diabetes, and family history of TNs, was collected for all participants. Height and weight were also measured to calculate body mass index (BMI). Blood samples were taken in the morning after a 10-h fast to determine levels of fasting plasma glucose (FBGL, mmol/L), fasting insulin (FINS, mIU/L), glycosylated hemoglobin (HbA1c, %), triglycerides (TG, mmol/L), total cholesterol (TC, mmol/L), high-density lipoprotein cholesterol (HDL-C, mmol/L), and low-density lipoprotein cholesterol (LDL-C, mmol/L). IR index (HOMA-IR) was calculated from FBGL and FINS. HOMA-IR ═ (FBGL×FINS)/22.5 [16].
Thyroid examination
All patients underwent thyroid color ultrasonography using a 7.5 MHz probe and the HS-2000 color Doppler ultrasound machine (Honda Electronics Co., Ltd.). The procedure was performed while the patients were in a supine position with their anterior cervical region fully visible. All ultrasound examinations were performed by a senior physician with more than five years of experience in ultrasound diagnosis of the thyroid gland. If a TN was detected, its size, boundary, location, echo, morphology, and presence of calcification were noted. The nodules were then classified using the ACR TI-RADS system [13]. ACR TI-RADS risk stratification interpretations were evaluated individually by two experienced senior physicians in a double-blind manner. In cases of disagreement, a third senior physician at the rank of deputy director or higher was consulted. The final conclusion was reached through joint consultation. All TNs were measured in a three-dimensional manner and the largest diameter was recorded to assess TN size regardless of the number of nodules [17].
Thyroid fine needle aspiration cytology
The patient was instructed to take a supine position and place his shoulder and neck high so that it was in an extended position to fully expose the anterior cervical region. The anterior cervical area is routinely disinfected. After local anesthesia, a 22 G needle was punctured into the TN under ultrasound guidance and the needle core was removed. Remove a few tumor cells by suction and collect cell debris by rapid lifting and inserting back and forth under negative pressure. After the operation, the puncture point was pressed for hemostasis. According to the classification criteria of thyroid cytopathology, the Bethesda score was divided into six categories: 1 as not diagnostic value or dissatisfactory, 2 as benign, 3 as atypical hyperplasia of unclear significance or follicular hyperplasia of uncertain significance, 4 as a follicular tumor or suspicious follicular tumor, 5 as suspicious malignant, and 6 as malignant [18]. Cytological aspirate samples with a Bethesda score of 1, indicating no value or unsatisfactory results, were not included in the study. Samples with a score of 3 or 4 were not included because they could not be confidently classified as either benign or malignant.
Ethical statement
Informed consent has been obtained from the guardians of all patients for publication.
Statistical analysis
The data was analyzed using SPSS 27.0 statistical software. Measurement data with a normal distribution were expressed as mean ± SD, while non-normal distribution data were presented as median (interquartile range). The independent sample t-test was used to compare normal distribution measurement data, and the Kolmogorov–Smirnov test was used for non-normal distribution data. Categorical data were compared using a χ2 test, and logistic regression was used for multivariate analysis of different groups. A P value of less than 0.05 was considered statistically significant.
Results
Analysis of influencing factors of TNs in diabetic patients
This study included 642 patients with diabetes who underwent thyroid color ultrasonography, with 245 patients ultimately screened for TNs. Table 1 shows the analysis of risk factors for TNs in diabetic patients. Compared to controls, patients with TNs were significantly older (P < 0.001), but there was no significant difference in sex distribution (P > 0.05). In addition, BMI was significantly higher in patients with TNs than in controls (P < 0.001). The levels of FBGL, FINS, HOMA-IR, and HbA1c were significantly higher in patients with thyroid nodules compared to controls (all P < 0.01). In terms of lipid metabolism, the levels of TG, TC, and LDL-C in patients with TNs were significantly higher than those in the control group (all P < 0.05), while HDL-C levels were significantly lower than those in the control group (P < 0.01). Additionally, patients with diabetes for ten years or more had a significantly higher incidence of TNs (P < 0.001), and there was a higher proportion of patients with TNs who had a family history compared to controls (P < 0.001).
Table 1.
Univariate analysis of thyroid nodules in diabetic patients
| Variable | Thyroid nodule (n ═ 245) | Control (n ═ 397) | t/Z/X2 | P | |
|---|---|---|---|---|---|
| Age (years) | 56.00 (51.00, 61.00) | 47.00 (43.00, 52.00) | 5.344 | <0.001 | |
| Sex | Male | 118 | 171 | 1.586 | 0.208 |
| Female | 127 | 226 | |||
| BMI (kg/m2) | 26.42 ± 2.53 | 24.96 ± 2.83 | 6.771 | <0.001 | |
| FBGL (mmol/L) | 9.24 (8.27, 10.07) | 8.31 (7.60, 9.30) | 3.468 | <0.001 | |
| FINS (mIU/L) | 17.70 (12.66, 20.38) | 14.48 (11.73, 16.98) | 3.993 | <0.001 | |
| HOMA-IR | 7.13 (5.14, 8.56) | 5.31 (4.32, 6.50) | 4.841 | <0.001 | |
| HbA1c (%) | 7.61 (6.83, 8.48) | 7.54 (6.33, 8.99) | 1.886 | 0.002 | |
| TG (mmol/L) | 2.04 (1.56, 2.56) | 1.96 (1.70, 2.23) | 2.388 | <0.001 | |
| TC (mmol/L) | 3.97 (3.01, 4.82) | 3.86 (3.28, 4.50) | 1.615 | 0.011 | |
| HDL-C (mmol/L) | 0.97 ± 0.16 | 1.01 ± 0.26 | −2.916 | 0.004 | |
| LDL-C (mmol/L) | 2.76 ± 0.43 | 2.67 ± 0.48 | 2.409 | 0.016 | |
| Diabetic progression | <10 yrs | 79 | 355 | 226.132 | <0.001 |
| ≥10 yrs | 166 | 42 | |||
| Family history of thyroid nodules | Yes | 55 | 23 | 39.376 | <0.001 |
| No | 190 | 374 |
Data are represented as counts, mean ± SD or median (IQR). BMI: Body mass index; HbA1c: Hemoglobin A1c; FBGL: Fasting plasma glucose; FINS: Fasting insulin; TG: Triglycerides; TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol.
Logistic regression analysis of influencing factors of TNs in diabetic patients
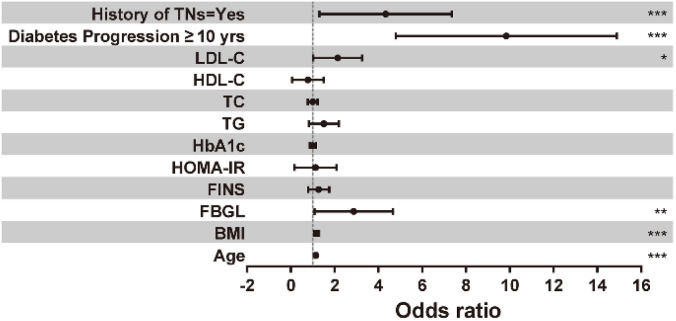
The 12 candidate variables with P < 0.05 in the univariate were included in the multivariate logistic regression analysis and the results are shown in Table 2 and Figure 1. Age (OR ═ 1.149) and BMI (OR ═ 1.173) were independent risk factors for TNs in diabetic patients (P < 0.001). Specifically, for every 1-unit increase in age or BMI, there was a 14.9% and 17.3% increase in the risk of developing TNs in diabetic patients, respectively. While FINS, HOMA-IR, and HbA1c levels were included in the multivariate logistic regression analysis as metabolic indices of diabetes mellitus, their impact on the risk of TNs was not statistically significant (all P > 0.05). This indicates that these factors were not independent risk factors for the development of TNs. However, higher FBGL levels were identified as an independent risk factor (OR ═ 2.504, P < 0.01), with each 1-unit increase corresponding to a 1.504-fold increase in the risk of developing TNs in diabetic patients. Among the indices of lipid metabolism, an elevated LDL-C level was also found to be an independent risk factor for the development of TNs in diabetic patients (OR ═ 1.951, P < 0.05). For every 1-unit increase in LDL-C, there was a 95.1% increase in the risk of developing TNs. Additionally, having a diabetes course of ten years or longer or a family history of TNs were associated with 7.979 and 2.628 times higher likelihood, respectively, of developing TNs compared to diabetic patients with a diabetes course of less than ten years or no family history of TNs.
Table 2.
Analysis of independent risk factors for thyroid nodules in diabetic patients
| Variable | B | SE | WaldX2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.139 | 0.017 | 64.382 | <0.001 | 1.149 | (1.111–1.189) |
| BMI | 0.160 | 0.048 | 11.143 | <0.001 | 1.173 | (1.068–1.289) |
| FBGL | 0.918 | 0.335 | 7.527 | 0.006 | 2.504 | (1.300–4.824) |
| FINS | 0.196 | 0.196 | 1.006 | 0.316 | 1.217 | (0.829–1.785) |
| HOMA-IR | −0.152 | 0.477 | 0.101 | 0.750 | 0.859 | (0.337–2.189) |
| HbA1c | 0.000 | 0.070 | 0.000 | 1.000 | 1.000 | (0.871–1.148) |
| TG | 0.343 | 0.238 | 2.083 | 0.149 | 1.410 | (0.884–2.247) |
| TC | −0.018 | 0.116 | 0.024 | 0.876 | 0.982 | (0.782–1.233) |
| HDL-C | −0.572 | 0.527 | 1.175 | 0.278 | 0.565 | (0.201–1.587) |
| LDL-C | 0.668 | 0.275 | 5.925 | 0.015 | 1.951 | (1.139–3.342) |
| Diabetes progression ≥ 10 yrs | 2.195 | 0.271 | 65.741 | <0.001 | 8.979 | (5.282–15.263) |
| Family history of thyroid nodules ═ Yes | 1.289 | 0.380 | 11.522 | <0.001 | 3.628 | (1.724–7.635) |
BMI: Body mass index; HbA1c: Hemoglobin A1c; FBGL: Fasting plasma glucose; FINS: Fasting insulin; TG: Triglycerides; TC: Total cholesterol; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol.
Figure 1.
Logistic regression analysis of influencing factors of thyroid nodules in diabetic patients. TNs: Thyroid nodules.
Analysis of influencing factors of TNs with different ACR TR in diabetic patients
Further risk determination was performed for all patients with TNs by the ACR TI-RADS risk stratification system, and the influencing factors for ACR TI-RADS ═ 5 (TR5) nodules were analyzed. As shown in Table 3, there were no statistically significant differences in age, sex distribution, and BMI levels between TR5 and TR1–4 TNs (all P > 0.05). The levels of FBGL, FINS, HOMA-IR, and HbA1c in patients with TR5 TNs were significantly higher than those in patients with TR1–4 (all P < 0.05). In terms of lipid metabolism, LDL-C levels in patients with TR5 TNs were significantly higher than those in TR1–4 groups (P < 0.05). However, TG, TC, and HDL-C levels were not statistically different between patients with TR5 and TR1–4 TNs (all P > 0.05). Furthermore, patients with TR5 TNs had larger nodule diameters than those with TR1–4 nodules (P < 0.001). The proportion of patients with a family history of TNs was also significantly higher in the TR5 group compared to the TR1–4 group (P < 0.05).
Table 3.
Univariate analysis of thyroid nodules by ACR TI-RADS classification in diabetic patients
| Variable | ACR TI-RADS ═ 1∼4(n ═ 211) | ACR TI-RADS ═ 5 (n ═ 34) | t/Z/X2 | P | |
|---|---|---|---|---|---|
| Age (years) | 56.00 (51.00, 61.00) | 57.50 (50.00, 61.00) | 0.536 | 0.936 | |
| Sex | Male | 103 | 15 | 0.259 | 0.611 |
| Female | 108 | 19 | |||
| BMI (kg/m2) | 26.16 ± 2.42 | 27.03 ± 2.37 | 1.949 | 0.052 | |
| FBGL (mmol/L) | 9.28 (8.41, 10.23) | 9.81 (9.36, 10.37) | 2.031 | <0.001 | |
| FINS (mIU/L) | 17.24 (11.80, 20.18) | 19.51 (16.07, 22.72) | 1.455 | 0.029 | |
| HOMA-IR | 7.05 (5.09, 8.39) | 8.44 (7.03, 9.59) | 1.763 | 0.004 | |
| HbA1c (%) | 7.53 (6.76, 8.37) | 7.95 (7.53, 9.09) | 1.450 | 0.030 | |
| TG (mmol/L) | 2.00 (1.52, 2.56) | 2.07 (1.67, 2.57) | 0.728 | 0.664 | |
| TC (mmol/L) | 3.89 (2.97, 4.71) | 4.61 (3.30, 5.14) | 1.300 | 0.068 | |
| HDL-C (mmol/L) | 0.96 ± 0.16 | 0.99 ± 0.16 | 1.010 | 0.314 | |
| LDL-C (mmol/L) | 2.73 ± 0.42 | 2.94 ± 0.42 | 2.692 | 0.008 | |
| Nodal diameter | 4.58 (2.30, 6.46) | 6.04 (4.76, 6.71) | 2.000 | <0.001 | |
| Diabetic progression | <10 yrs | 96 | 11 | 2.057 | 0.152 |
| ≥10 yrs | 115 | 23 | |||
| Family history of thyroid nodules | Yes | 42 | 13 | 5.651 | 0.017 |
| No | 169 | 21 |
Data are represented as counts, mean ± SD or median (IQR). ACR: American College of Radiology; FBGL: Fasting plasma glucose; FINS: Fasting insulin; BMI: Body mass index; HbA1c: Hemoglobin A1c; TG: Triglycerides; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol.
Logistic regression analysis of influencing factors of TNs with different ACR TR in diabetic patients
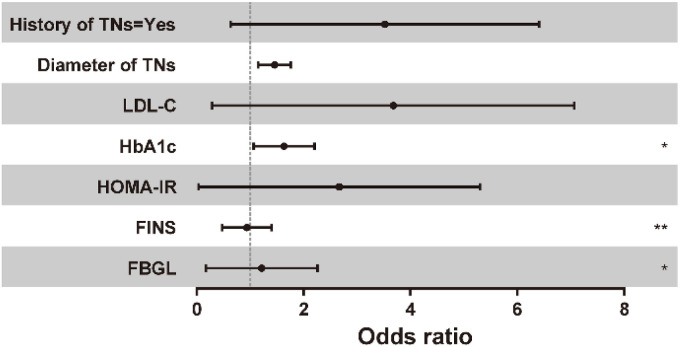
Seven candidate variables with P < 0.05 in the univariate were included in the multivariate Logistic regression analysis and the results are shown in Table 4 and Figure 2. When FBGL, FINS, and HOMA-IR levels were included in multivariate logistic regression analysis, the significant effect on the risk of TR5 TNs disappeared (all P > 0.05), suggesting that these factors were not independent risk factors for the development of TR5 thyroid. In addition, HbA1c was an independent risk factor for the development of TR5 TNs (OR ═ 1.566, P < 0.05). When HbA1c was increased by 1, diabetic patients had an independent 56.6% increased risk of developing TR5 TNs. There was no significant independent effect of LDL-C on the occurrence of TR5 TNs (P > 0.05). In addition, the nodule diameter was an independent risk factor for the development of TR5 TNs (OR ═ 1.433, P < 0.01). This indicates that diabetic patients with a family history of TNs have a 1.729-fold increased risk compared to those without a family history.
Table 4.
Analysis of independent risk factors of thyroid nodules by ACR TI-RADS classification in diabetic patients
| Variable | B | SE | Wald X2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| FBGL | −0.088 | 0.487 | 0.033 | 0.856 | 0.916 | (0.353–2.376) |
| FINS | −0.149 | 0.260 | 0.330 | 0.566 | 0.861 | (0.518–1.433) |
| HOMA-IR | 0.590 | 0.580 | 1.033 | 0.309 | 1.803 | (0.578–5.622) |
| HbA1c | 0.448 | 0.182 | 6.084 | 0.014 | 1.566 | (1.096–2.235) |
| LDL-C | 0.969 | 0.531 | 3.336 | 0.068 | 2.637 | (0.932–7.462) |
| Nodal diameter | 0.360 | 0.108 | 11.049 | 0.001 | 1.433 | (1.159–1.772) |
| Family history of thyroid nodules | 1.004 | 0.460 | 4.765 | 0.029 | 2.729 | (1.108–6.722) |
ACR: American College of Radiology; FBGL: Fasting plasma glucose; FINS: Fasting insulin; LDL-C: Low-density lipoprotein cholesterol.
Figure 2.
Logistic regression analysis of influencing factors of thyroid nodules with different ACR TI-RADS classification. ACR TI-RADS: American College of Radiology Thyroid Imaging Reporting and Data System.
Consistency analysis of TNs with different ACR TR and Bethesda score in patients with diabetes mellitus
All 236 study participants underwent FNAC and results were analyzed concordantly using the ACR TI-RADS and Bethesda scoring systems. Table 5 displays the results for 211 patients. 28 nodules unable to be adequately classified as benign or malignant (Bethesda 1 ═ 4, Bethesda 3 ═ 10, Bethesda 4 ═ 11) were excluded from the analysis. Among the remaining nodules classified as TR3, only 2 had a Bethesda score of 5, while the others (n ═ 67) were classified as Bethesda score 2. Of these TR3 nodules, 97.10% (67/69) were pathologically confirmed as benign, demonstrating 97.10% agreement with the Bethesda scoring system. Out of the 112 TR4 nodules, 15 were classified as Bethesda score 5 and 9 as Bethesda score 6. Of these, 88 were confirmed as benign, indicating that 78.57% (88/112) did not support the ACR TI-RADS recommendation. However, it should be noted that five nodules with a Bethesda score of 5 showed negative pathological findings. For TR5 nodules, which were recommended for FNAC, 12 had a Bethesda score of 5 and 18 had a Bethesda score of 6. All TR5 nodules were confirmed as malignant, supporting the ACR TI-RADS recommendation.
Table 5.
Consistency analysis of thyroid nodules and Bethesda scores by ACR TI-RADS classification
| Bethesda score | ACR TI-RADS classification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||
| + | − | + | − | + | − | + | − | + | − | |
| 2 | 0 | 2 | 0 | 4 | 0 | 67 | 0 | 88 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 2 | 0 | 10 | 5 | 12 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 18 | 0 |
| X2 | 185.003 | |||||||||
| P | <0.001 | |||||||||
ACR: American College of Radiology; ACR TI-RADS: American College of Radiology Thyroid Imaging Reporting and Data System.
Discussion
The thyroid gland is one of the target tissues that is affected by metabolic disorders [19]. Among endocrine and metabolic system issues, T2DM and thyroid disease stand out as the most prevalent [20]. Diabetes mellitus has been shown to be closely related to the development of TNs, and there is a positive correlation between blood glucose and the formation of TNs [21]. IR, characterized by hyperglycemia and high insulin levels, serves as the primary trigger in the onset of most type 2 diabetes cases. Excessive insulin can bind to insulin-like growth factor binding proteins, thereby increasing levels of free insulin-like growth factor 1 (IGF-1) in the blood [22]. IGF-1 and its receptors have been shown to be expressed in follicular and C cells of the thyroid gland and involved in cell regulation and proliferation [23]. One of the target tissues for the disturbance of glucose metabolism is the thyroid gland, and the statement that IR and hyperinsulinemia are associated with the development of TNs has been confirmed by Ayturk et al. [24]. In addition, Auchincloss et al. [25] reported that insulin receptors are expressed in both thyroid cells and insulin and that increased insulin levels may reduce production of IGF-1 binding proteins, leading to elevated levels of free IGF-1, which subsequently stimulates protein and DNA synthesis and promotes mitosis in thyroid cells. Belfiore [26] found that insulin itself is a pro-cytokine that can induce the growth, differentiation, and proliferation of thyroid cells and stimulate TN formation. In the present study, FBGL, FINS, HOMA-IR, HbA1c levels, and diabetes progression were significantly higher in patients with T2DM and TNs than in the non-nodular group. In addition, the results of logistic regression analysis showed that both FBGL level and diabetes progression were independent risk factors for the development of TNs. Currently, the exact mechanism linking disturbances in glucose metabolism to TNs remains unclear. However, we hypothesize that insulin not only acts as a glucose-lowering hormone but also promotes vascular endothelial cell proliferation. Prolonged exposure to high glucose levels in poorly controlled type 2 diabetes patients can lead to IR and mild inflammatory responses in cells and tissues, thereby promoting angiogenesis in TNs.
Currently, due to the widespread use of high-frequency ultrasound in TN diagnosis, there has been a significant increase in nodule detection rates. The ACR TI-RADS grading system serves as a method for assessing the risk of thyroid malignancy based on ultrasonographic features. It is generally accepted that TR1–3 nodules can be considered benign nodules, while TR4–5 should be highly suspected of malignancy [27]. However, recent studies have revealed that the optimal diagnostic threshold on the ROC curve for distinguishing between benign and malignant TNs is > TR4, indicating that TR5 nodules are highly suspicious for malignancy. Therefore, we categorized all patients with TNs into TR1–4 and TR5 groups to further explore the factors influencing the malignancy of TNs. The results of this study showed that FBGL, FINS, HOMA-IR, and HbA1c levels were significantly higher in patients with TR5 TNs than in patients with TR1–4. Furthermore, logistic regression analysis identified HbA1c levels as an independent risk factor for elevated TN grade in patients with T2DM. This may be attributed to impaired glucose tolerance leading to IR, subsequently triggering a chronic inflammatory response and increasing TN grade. Additionally, Yildirim et al. [28] discovered that IR poses a risk factor for papillary thyroid cancer, indicating a significant link between IR and malignant thyroid lesions, which aligns with our findings.
In this study, no statistically significant difference was observed in the distribution of sexes between TNs and controls, nor between the different ACR TI-RADS groups. This finding contrasts with the research by Xu et al., which suggested a higher likelihood of TNs in women [29]. This inconsistency could be attributed to the relatively small sample size of our study and the specific selection of diabetic patients. It is known that cellular hyperplasia and fibrosis in the thyroid gland increase with age, contributing to the formation of TNs [6]. However, it has also been suggested that the increased incidence of TNs in the elderly is largely attributable to more extensive thyroid ultrasonography in the elderly population [30]. The results of this study indicate that age independently acts as a risk factor for TNs in diabetic patients, with the risk increasing by 14.9% for each additional year of age. This finding is in line with Guth et al.’s [30] research, which demonstrated a higher prevalence of TNs among the elderly, with nearly 80% incidence in individuals aged ≥ 60 years. However, it has been shown that the incidence of malignant TNs decreases with age [31]. A study of thyroid fine-needle aspiration revealed that the prevalence of thyroid cancer was 17% to 23% in the 20–49-year-old group, compared to only 13% in patients aged 70 years (±14 years), suggesting a reduced prevalence of malignant nodules in older patients [31]. Interestingly, our study found no statistically significant difference in age between patients with TR5 and those with TR1–4 TNs, suggesting that age may not play a significant role in the degree of thyroid malignancy.
In 2015, Bétry et al. found that individuals with higher BMI can stimulate abnormal proliferation and differentiation of individual thyroid tissues by inducing local systemic metabolic disturbances, causing abnormalities in the hypothalamic–pituitary thyroid axis. Similarly, Sari et al. [32] discovered that higher BMI and body fat content were associated with elevated concentrations of thyroid-stimulating hormone (TSH) and larger thyroid gland volumes. Similarly, Kitahara et al. [33] discovered that higher BMI and body fat content were associated with elevated concentrations of TSH and larger thyroid gland volumes. In addition, Kitahara et al. conducted a prospective study of 434,953 men and 413,3979 women in the United States and demonstrated a significant positive association between BMI and thyroid cancer risk. However, in our study, BMI levels did not differ statistically between TR5 and TR1–4 nodules, possibly due to the small sample size. Furthermore, our study demonstrated that levels of TG, TC, and LDL-C were notably higher in diabetic patients with TNs compared to controls, with LDL-C levels being identified as independent risk factors for TN development in diabetic patients. Lee and Wang [34] have shown a close relationship between TC, LDL-C, and TSH levels in patients with hyperthyroidism, suggesting that lipid metabolism influences TSH levels, thereby influencing TNs. The current “hypothalamic–pituitary–thyroid–adipose tissue balance” hypothesis may explain the mechanism by which lipids affect TNs [34]. Elevated blood lipids, which can induce leptin resistance, play a role in regulating the expression of thyrotropin-releasing hormone genes [34]. Clinically, dyslipidemia usually coexists with thyroid disorders, and both hypersecretion and hypothyroid hormone can cause dyslipidemia [35]. Hypersecretion of thyroid hormones enhances metabolism, leading to decreased lipid levels, while decreased thyroid hormone secretion slows metabolism, resulting in elevated lipid levels. Additionally, excessive fat deposition in individuals with hyperlipidemia can increase the autoinflammatory response and promote the overexpression of inflammatory factors, such as IL-6 and MCP-1, potentially contributing to TN formation [36].
At present, the treatment of TNs primarily falls into two categories. Benign nodules are typically managed through observation, with surgery or radiofrequency ablation performed electively if compression symptoms arise [37]. Conversely, if nodules exhibit malignant characteristics, surgical intervention is usually warranted [37]. Therefore, distinguishing between benign and malignant TNs remains a pivotal clinical concern [38]. At present, FNAC is the most effective method of differentiating between benign and malignant TNs [39]. However, given that the majority of nodules are benign, and FNAC is an invasive procedure, not all nodules necessitate FNAC [40]. Hence, there is a need for an effective, noninvasive approach to identify which nodules require FNAC. ACR TI-RADS offers a set of simple, easy, and standardized classification criteria, effectively mitigating diagnostic bias arising from varying levels of ultrasound expertise among practitioners. Hoang et al. [41] utilized ACR TR to assess the diagnostic accuracy of TNs and found notable improvement in diagnostic precision. The results of this study show that 97.10% of TR3 nodules in diabetic patients with TNs do not recommend further FNAC examination as their pathological findings were benign, contradicting the ACR TI-RADS recommendation of FNAC for TR3–TR5 nodules. Additionally, nodule diameter emerged as an independent risk factor for the development of TR5 nodules (OR ═ 1.433, P < 0.01). Consequently, nodal malignancy risk increased by 86.6% when the nodule diameter reached 3 cm. Thus, we propose that TR3 nodules ≥ 3 cm in diameter should be considered for further FNAC examination.
Furthermore, among 112 TR4 nodules, 88 were benign, indicating that 78.57% (88/112) of these nodules did not align with the ACR TI-RADS recommendation. Hence, we suggest that the determination of TR4 nodules for further FNAC examination should be based on additional parameters, such as nodule size, morphology, calcification, etc. Moreover, all TR5 nodules were malignant, affirming the ACR TI-RADS recommendation.
There are several limitations to this study: (1) It relies on a single data source, lacks a multicenter control analysis, and the sample size is small, potentially introducing bias and limiting the generalizability of the results to other clinical settings and patient groups; (2) Due to space constraints, factors, such as patient lifestyle, iodine status, occupational exposure, nodule characteristics, and other related environmental factors were not included in the analysis; and (3) The distinction between benign and malignant subgroups within TR4 nodules remains unexplored and warrants further investigation.
Conclusion
In summary, TNs in patients with T2DM are linked to age, BMI, and metabolic factors like blood sugar and lipid levels. The risk of TN malignancy is also associated with blood sugar and lipid metabolism indicators. Patients with T2DM generally exhibit a low risk of malignancy within the TR3 classification of TNs, thus further FNAC testing is typically not recommended. However, nodules classified as TR3 with a diameter of 3 cm or more may need further FNAC assessment. For nodules classified as TR4–TR5, additional FNAC should be considered on an individual basis. When using the ACR TI-RADS risk assessment system, it may be necessary to clarify the nodule category, scoring criteria, etc., to accurately diagnose and distinguish between benign and malignant nodules, and guide clinical management more effectively.
Footnotes
Conflicts of interest: Authors declare no conflicts of interest.
Funding: This work was supported by Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang Province: Based on the combination of sorafenib and polyethylene glycol resveratrol to improve the synergistic therapeutic effect of renal cell carcinoma (024ZL279), and General scientific research project of Zhejiang Provincial Department of Education: Study on the mechanism of ultrasound-targeted damage technology regulating myocardial fibroblasts in myocardial infarction (Y202352011), as well as Education Department project number Y202044779: Disulfiram/copper inhibits Nrf2 pathway to activate ferroptosis and reverse drug resistance of AML cells.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Ha EJ, Baek JH, Na DG. Risk stratification of thyroid nodules on ultrasonography: current status and perspectives. Thyroid. 2017;27(12):1463–8. doi: 10.1089/thy.2016.0654. https://doi.org/10.1089/thy.2016.0654. [DOI] [PubMed] [Google Scholar]
- 2.Walsh JP. Managing thyroid disease in general practice. Med J Aust. 2016;205(4):179–84. doi: 10.5694/mja16.00545. https://doi.org/10.5694/mja16.00545. [DOI] [PubMed] [Google Scholar]
- 3.Kobaly K, Kim CS, Mandel SJ. Contemporary management of thyroid nodules. Annu Rev Med. 2022;73:517–28. doi: 10.1146/annurev-med-042220-015032. https://doi.org/10.1146/annurev-med-042220-015032. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Nie F, Wang Y, Yang D, Dong T, Liu T, et al. Differential diagnosis of thyroid nodules by the Demetics ultrasound-assisted diagnosis system and contrast-enhanced ultrasound combined with thyroid image reporting and data systems. Clin Endocrinol (Oxf) 2022;97(1):116–23. doi: 10.1111/cen.14741. https://doi.org/10.1111/cen.14741. [DOI] [PubMed] [Google Scholar]
- 5.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–53. doi: 10.1038/nrendo.2016.110. https://doi.org/10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. https://doi.org/10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5):584–90. doi: 10.1097/CM9.0000000000002108. https://doi.org/10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buscemi S, Massenti FM, Vasto S, Galvano F, Buscemi C, Corleo D, et al. Association of obesity and diabetes with thyroid nodules. Endocrine. 2018;60(2):339–47. doi: 10.1007/s12020-017-1394-2. https://doi.org/10.1007/s12020-017-1394-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HM, Feng QW, Niu YX, Su Q, Wang X. Thyroid nodules in type 2 diabetes mellitus. Curr Med Sci. 2019;39(4):576–81. doi: 10.1007/s11596-019-2076-5. https://doi.org/10.1007/s11596-019-2076-5. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Niu Y, Zhang W, Li X, Lin N, Yang Z, et al. OPG is associated with thyroid nodule development in type 2 diabetes. J Clin Lab Anal. 2022;36(9):e24615. doi: 10.1002/jcla.24615. https://doi.org/10.1002/jcla.24615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y, Yan T, Wang G, Chen Y, Zhu Y, Jiang Z, et al. Correlation between insulin resistance and thyroid nodule in type 2 diabetes mellitus. Int J Endocrinol. 2017;2017:1617458. doi: 10.1155/2017/1617458. https://doi.org/10.1155/2017/1617458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanc E, Ponce C, Brodschi D, Nepote A, Barreto A, Schnitman M, et al. Association between worse metabolic control and increased thyroid volume and nodular disease in elderly adults with metabolic syndrome. Metab Syndr Relat Disord. 2015;13(5):221–6. doi: 10.1089/met.2014.0158. https://doi.org/10.1089/met.2014.0158. [DOI] [PubMed] [Google Scholar]
- 13.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14(5):587–95. doi: 10.1016/j.jacr.2017.01.046. https://doi.org/10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Misiakos EP, Margari N, Meristoudis C, Machairas N, Schizas D, Petropoulos K, et al. Cytopathologic diagnosis of fine needle aspiration biopsies of thyroid nodules. World J Clin Cases. 2016;4(2):38–48. doi: 10.12998/wjcc.v4.i2.38. https://doi.org/10.12998/wjcc.v4.i2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. doi: 10.1002/dmrr.3158. https://doi.org/10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 16.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. https://doi.org/10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Anil C, Kut A, Atesagaoglu B, Nar A, Tutuncu NB, Gursoy A. Metformin decreases thyroid volume and nodule size in subjects with insulin resistance: a preliminary study. Med Princ Pract. 2016;25(3):233–6. doi: 10.1159/000442821. https://doi.org/10.1159/000442821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Philippe Vielh PV, VanderLaan PA. The 2023 Bethesda system for reporting thyroid cytopathology. Thyroid. 2023;33(9):1039–44. doi: 10.1089/thy.2023.0141. https://doi.org/10.1089/thy.2023.0141. [DOI] [PubMed] [Google Scholar]
- 19.Cerit L. Thyroid disorders might be related to metabolic syndrome components. Clin Nutr. 2017;36(2):610. doi: 10.1016/j.clnu.2017.01.019. https://doi.org/10.1016/j.clnu.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Feng S, Zhang Z, Xu S, Mao X, Feng Y, Zhu Y, et al. The prevalence of thyroid nodules and their association with metabolic syndrome risk factors in a moderate iodine intake area. Metab Syndr Relat Disord. 2017;15(2):93–7. doi: 10.1089/met.2016.0077. https://doi.org/10.1089/met.2016.0077. [DOI] [PubMed] [Google Scholar]
- 21.Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther. 2019;10(6):2035–44. doi: 10.1007/s13300-019-00700-4. https://doi.org/10.1007/s13300-019-00700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitale G, Barbieri M, Kamenetskaya M, Paolisso G. GH/IGF-I/insulin system in centenarians. Mech Ageing Dev. 2017;165(Pt B):107–14. doi: 10.1016/j.mad.2016.12.001. https://doi.org/10.1016/j.mad.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Sheng X, Miao T, Yao K, Yao D. Effect of insulin on thyroid cell proliferation, tumor cell migration, and potentially related mechanisms. Endocr Res. 2019;44(1–2):55–70. doi: 10.1080/07435800.2018.1522641. https://doi.org/10.1080/07435800.2018.1522641. [DOI] [PubMed] [Google Scholar]
- 24.Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB, et al. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur J Endocrinol. 2009;161(4):599–605. doi: 10.1530/EJE-09-0410. https://doi.org/10.1530/EJE-09-0410. [DOI] [PubMed] [Google Scholar]
- 25.Auchincloss AH, Diez Roux AV, Brown DG, Erdmann CA, Bertoni AG. Neighborhood resources for physical activity and healthy foods and their association with insulin resistance. Epidemiology. 2008;19(1):146–57. doi: 10.1097/EDE.0b013e31815c480. https://doi.org/10.1097/EDE.0b013e31815c480. [DOI] [PubMed] [Google Scholar]
- 26.Belfiore A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des. 2007;13(7):671–86. doi: 10.2174/138161207780249173. https://doi.org/10.2174/138161207780249173. [DOI] [PubMed] [Google Scholar]
- 27.Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu-Perrard M, Leenhardt L, et al. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol. 2013;168(5):649–55. doi: 10.1530/EJE-12-0936. https://doi.org/10.1530/EJE-12-0936. [DOI] [PubMed] [Google Scholar]
- 28.Yildirim Simsir I, Cetinkalp S, Kabalak T. Review of factors contributing to nodular goiter and thyroid carcinoma. Med Princ Pract. 2020;29(1):1–5. doi: 10.1159/000503575. https://doi.org/10.1159/000503575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Zeng F, Wang Y, Bai Y, Shan X, Kong L. Prevalence and associated metabolic factors for thyroid nodules: a cross-sectional study in Southwest of China with more than 120 thousand populations. BMC Endocr Disord. 2021;21(1):175. doi: 10.1186/s12902-021-00842-2. https://doi.org/10.1186/s12902-021-00842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39(8):699–706. doi: 10.1111/j.1365-2362.2009.02162.x. https://doi.org/10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 31.Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab. 2015;100(12):4434–40. doi: 10.1210/jc.2015-3100. https://doi.org/10.1210/jc.2015-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sari R, Balci MK, Altunbas H, Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol (Oxf) 2003;59(2):258–62. doi: 10.1046/j.1365-2265.2003.01836.x. https://doi.org/10.1046/j.1365-2265.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- 33.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–72. doi: 10.1158/1055-9965.EPI-10-1220. https://doi.org/10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MT, Wang CY. Concomitant Graves hyperthyroidism with thyrotrophin-secreting pituitary adenoma. South Med J. 2010;103(4):347–9. doi: 10.1097/SMJ.0b013e3181d3ce93. https://doi.org/10.1097/SMJ.0b013e3181d3ce93. [DOI] [PubMed] [Google Scholar]
- 35.Yang HX, Zhong Y, Lv WH, Zhang F, Yu H. Association of adiposity with thyroid nodules: a cross-sectional study of a healthy population in Beijing, China. BMC Endocr Disord. 2019;19(1):102. doi: 10.1186/s12902-019-0430-z. https://doi.org/10.1186/s12902-019-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macvanin MT, Gluvic ZM, Zaric BL, Essack M, Gao X, Isenovic ER, et al. New biomarkers: prospect for diagnosis and monitoring of thyroid disease. Front Endocrinol (Lausanne) 2023;14:1218320. doi: 10.3389/fendo.2023.1218320. https://doi.org/10.3389/fendo.2023.1218320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexander EK, Doherty GM, Barletta JA. Management of thyroid nodules. Lancet Diabetes Endocrinol. 2022;10(7):540–8. doi: 10.1016/S2213-8587(22)00139-5. https://doi.org/10.1016/S2213-8587(22)00139-5. [DOI] [PubMed] [Google Scholar]
- 38.Welch HG, Doherty GM. Saving Thyroids—overtreatment of small papillary cancers. N Engl J Med. 2018;379(4):310–2. doi: 10.1056/NEJMp1804426. https://doi.org/10.1056/NEJMp1804426. [DOI] [PubMed] [Google Scholar]
- 39.Singh Ospina N, Brito JP, Maraka S, Espinosa de Ycaza AE, Rodriguez-Gutierrez R, Gionfriddo MR, et al. Diagnostic accuracy of ultrasound-guided fine needle aspiration biopsy for thyroid malignancy: systematic review and meta-analysis. Endocrine. 2016;53(3):651–61. doi: 10.1007/s12020-016-0921-x. https://doi.org/10.1007/s12020-016-0921-x. [DOI] [PubMed] [Google Scholar]
- 40.Grani G, Lamartina L, Cantisani V, Maranghi M, Lucia P, Durante C. Interobserver agreement of various thyroid imaging reporting and data systems. Endocr Connect. 2018;7(1):1–7. doi: 10.1530/EC-17-0336. https://doi.org/10.1530/EC-17-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoang JK, Middleton WD, Farjat AE, Teefey SA, Abinanti N, Boschini FJ, et al. Interobserver variability of sonographic features used in the American College of Radiology Thyroid Imaging Reporting and Data System. AJR Am J Roentgenol. 2018;211(1):162–7. doi: 10.2214/AJR.17.19192. https://doi.org/10.2214/AJR.17.19192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.