Abstract
We present here the first-ever fossil flies from the family Deuterophlebiidae. The recent adults have an exceptionally brief lifespan, with males surviving only two hours. Their distinctive features include a complete reduction of mouthparts, and wing venation characterized by a dense net of false veins replacing most of true veins. Due to this distinctive venation the phylogenetic position of Deuterophlebiidae was unclear, compounded by the absence of fossils that could shed light on the early development of these characters. Two new genera and species are described from Burmese amber, Protodeuterophlebia oosterbroeki Krzemiński, Krzemińska & Soszyńska, gen. et sp. nov. and Cretodeuterophlebia courtneyi, Krzemiński, Skibińska & Kopeć, gen. et sp. nov. They date back the age of the family to the mid-Cretaceous. Notably, the fossils reveal first false veins and reduced mouthparts, suggesting a short lifespan in these Cretaceous mountain midges. A comparative analysis of wing venation indicated the Hennigmatidae as a plausible ancestral group to the Deuterophlebiidae. A syninclusion of mayfly indicates the coexistence of these short-lived insects during the same flight period. This synchrony extends to their brief time in flight aligning with the flow of fluid resin. The occurrence of these simultaneous events is extremely low, emphasizing the significance of these findings.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75389-y.
Keywords: Cretaceous, Burmese amber, New species, New genus, Palaeoecology, Vetuformosa
Subject terms: Evolution, Zoology
Introduction
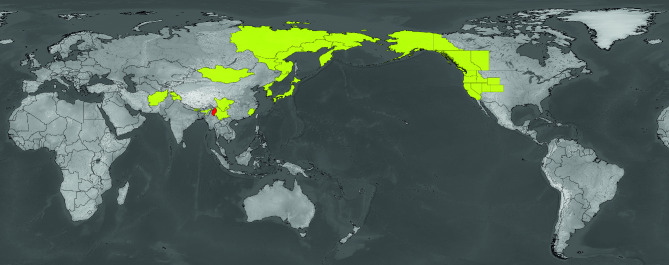
Deuterophlebiidae, the mountain midges, is a monotypic family with 19 recent species of the genus Deuterophlebia1. Most of these species are distributed around the circumpacific Holarctic regions, while several occur deep in the Asiatic continent (Fig. 1).
Fig. 1.
Distribution of Deuterophlebiidae: recent (yellow) and fossil (red).
Mountain midges are known for several characteristics of morphology, behaviour, and life cycle, setting them apart among the Diptera. Deuterophlebiidae are rheophiles, and the morphology of larvae, pupae and adults is adapted to life in swift running, cold streams. The larvae possess prolegs ending with hooks, and the ventral side of the pupae is equipped with adhesive discs. Both adaptations enable the juvenile stages to cling to the rocky bottom, preventing them from being carried away by the torrent. The lifespan of adult males is remarkably short, lasting only two hours after emergence2. Due to the extremely short life of the adults, complete reduction of the mouthparts3, and superficially similar wing venation, Deuterophlebiidae have been compared to Ephemeroptera2,4.
These characters have also contributed to their disputed phylogenetic position, a topic under discussion since 1960s5,6. Hennig7,8 placed this family within the Psychodomorpha, and Courtney9,10 supported the sister-group position of Blephariceromorpha (including Deuterophlebiidae) to Psychodomorpha based on morphological features in both immature and adult stages. This position was further confirmed11,12, incorporating analyses of adult morphology and including fossil species to their assessments.
Much of the discussions revolved around the presumed relationship with Nymphomyiidae and Blephariceridae9,10,13–15. Molecular analyses have indicated a basal position, possibly even a sister-group relationship of Deuterophlebiidae to the remaining Diptera groups16–18. The ambiguity surrounding this family persisted due to the absence of fossils for Deuterophlebiidae, which could provide insights into the early stages of their distinctive characteristics and unequivocally link the family to the already known Diptera groups. Until now, Deuterophlebiidae was one of two recent dipteran families without known fossil representatives, the other being the recently described Rangomaramidae19.
This study presents the first description of fossil deuterophlebiids, providing evidence of the family’s presence in the Cretaceous, approximately 100 Ma. Two new genera and species are described. The comparative analysis of wing venation, involving these newly described specimens and other fossil representatives of psychodomorphs, helps identify a probable ancestral group to Deuterophlebiidae. One notable finding is a male deuterophlebiid accompanied by an ephemeropteran wing. The co-occurrence of these two short-lived insects within the same piece of amber, known as a syninclusion, suggests a shared flight period, which for both species was remarkably brief.
Results
Systematic part
Order: Diptera Linnaeus, 1758.
Infraorder: Psychodomorpha Newman, 1834.
The Psychodomorpha, dating back to the Lower/Middle Triassic, represents one of the earliest arising lineages of Diptera11,20,21. Members of this infraorder are distinguished by all five radial veins being long and terminating in wing margin. This character is a basal plesiomorphy of the Diptera20,21 and a character state unique to the Psychodomorpha. In the remaining Diptera, the vein R2 is either positioned as a cross vein or absent/fused with other veins. The Psychodomorpha encompass four extant families: Psychodidae, Tanyderidae, Blephariceridae, and Deuterophlebiidae, along with seven extinct families: Grauvogeliidae, Nadipteridae, Hennigmatidae, Kuperwoodiidae, Tilliardipteridae, Rhaetaniidae and Ansorgidae. Last three families were included, even though the long vein R2 is absent or fused in these groups11,12,22.
Family
Deuterophlebiidae Edwards, 1922.
Type genus: Deuterophlebia Ewards, 1922.
Amended diagnosis. Body 2–4 mm long, with subtriangular wings longer than body and widest across the greatly expanded anal lobe. The membrane is delicate and clear, with thin wing veins exhibiting additional forkings and pseudoveins. Vein Mb is notably short, approximately 0.1 times the wing length or less. The d-cell is either open or partially closed, with its inner angle close to the wing base and very narrow. Mouthparts are greatly reduced, and palpi are absent. Until now, the family was monotypic and included 19 recent species of Deuterophlebia in the Holarctic and Oriental Regions. Two new fossil genera are described herein, Protodeuterophlebia gen. nov. and Cretodeuterophlebia gen. nov., from Burmese amber aged Upper Cretaceous (earliest Cenomanian; 98.79 ± 0.62 Ma)23.
Genus: Protodeuterophlebia Krzemiński, Krzemińska et Soszyńska, gen. nov.
LSID urn:lsid:zoobank.org:pub:BC559013-1255-42BB-A984-AF821539A7AF
Diagnosis. Same as its only species, by monotypy.
Type species. Protodeuterophlebia oosterbroeki n. sp., Burmese amber Upper Cretaceous.
Etymology. The genus name alludes to the basal position of the taxon in relation to recent Deuterophlebiidae.
Protodeuterophlebia oosterbroeki Krzemiński, Krzemińska et Soszyńska, sp. nov.
LSID urn:lsid:zoobank.org:pub:BC559013-1255-42BB-A984-AF821539A7AF
Diagnosis. Antennae of male are thin, nearly as long as body and c.1.3× longer than wings, consist of 14 flagellomeres. Head is flat, partially hidden under vaulted scutum, with distal margin of scutum bearing nine sharp, spur-like projections, central one being longest. Wing venation: R2 forked at end; pseudoveins present in radial section, d-cell partially open, only one anal vein present. Male terminalia: narrow gonocoxites slightly curved to inside; gonostyles 1/3 as wide as, and only slightly longer than gonocoxites, slightly dilated at end. Female unknown.
Etymology. The new species name is dedicated to Pjotr Oosterbroek, the world known specialist in the dipteran family Tipulidae and its phylogeny, and the creator of the webpage “Catalogue of the Craneflies of the world” (https://ccw.naturalis.nl/), an immense help to dipterists.
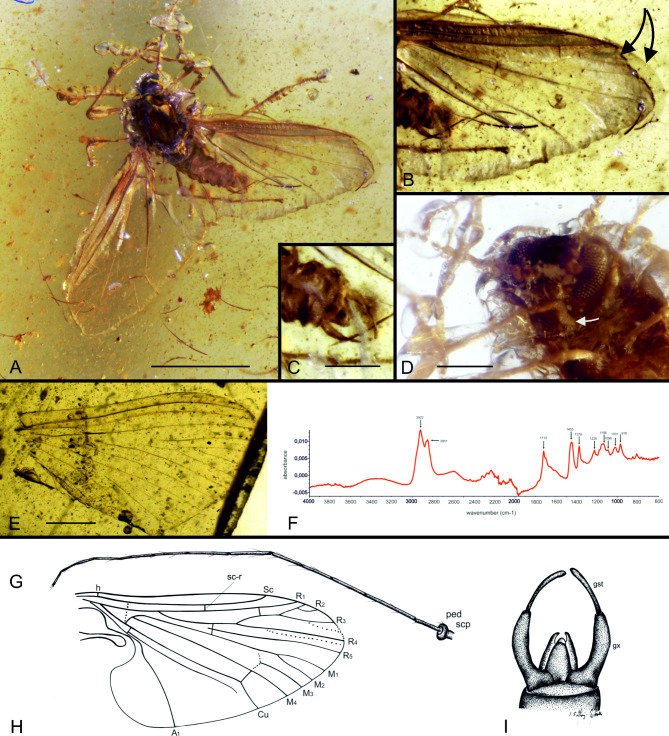
Material. Holotype NIGP177894-A male, Burmese amber, age Upper Cretaceous: earliest Cenomanian; 98.79 ± 0.62 Ma 23. The holotype is housed at NIGP. Within the same amber piece, a single wing of Vetuformosa buckleyi24 (Ephemeroptera; NIGP177894-B) is embedded (Fig. 2E). The amber was cut to facilitate the study of both specimens, and currently the deuterophlebiid and ephemeropteran specimens are held in separate pieces labeled with the suffix letters “A” and “B”, respectively. Originally, both specimens were embedded approximately 4 mm apart from each other.
Fig. 2.
Syninclusions of Protodeuterophlebia oosterbroeki gen. et sp. nov., holotype (A‒D), photographs; (G‒I), reconstructions) and a mayfly wing Vetuformosa buckleyi (E). (A) specimen; (B), wing fragment (arrows indicate upper and lower membranes partially separated); (C), genitalia dorsally; (D), head in frontal view (arrow: note proximity of eye and front coxa). ( F), spectrum of Burmese amber piece. (G) antenna; (H) wing; (I) genitalia in dorsal view. Abbreviations: gst, gonostylus; gx, gonocoxite; ped, pedicel; scp, scapus. Bars: (A) 1 mm; (C, D) 0.2 mm; (E) 0.5 mm.
Description. While the specimen is well-preserved, it is surrounded by numerous gas bubbles, which hinder the observation of some details under the microscope (Fig. 2A, D) and further complicate examination under the synchrotron imaging (Fig. 3A, C).
Fig. 3.
Protodeuterophlebia oosterbroeki, gen. et sp. nov., male, holotype. (A‒B), dorsal side: (A), habitus (shapes of flagellomeres are dilated due to adhering gas bubbles), (B), thorax (arrows mark symmetrical spines at posterior border of scutum; note central portion of scutum pressed to the inside in course of fossilization); (C‒E) ventral side: (C) habitus, (D) genitalia, (E) proximity of prothoracic coxa (c) and eye (e) (ped, pedicel). (B is an exact, cropped version of (A) and (D‒E), of (C).
Head is small and flattened, broader than long in frontal view, partially hidden under vaulted scutum, bent down, a feature evident when comparing the very short distance between the eyes and the front coxa (Figs. 2D and 3E). Mouthparts are invisible, and palpi are absent. Eyes are large, dioptic, positioned on lateral regions of the head, extending over full height of head, composed of large ommatidia arranged in rows. Antennae are thin, nearly as long as body, approximately 1.3 × longer than wings; scapus is short and cylindrical; pedicel is rounded; 14 thin, long flagellomeres, borders between them are poorly discernible (Fig. 2G).
Scutum appears to have collapsed post mortem (Fig. 3A, B), indicating that its authentic shape must have been highly vaulted; posterior margin of scutum exhibits nine symmetrically arranged spurs, with central one and two lateralmost being strongest (Fig. 3B). Legs are thin and rather short, without tibial spurs; tarsi with large empodia.
Wing (Fig. 2A, B, H) 2 mm long, maximal width 1.1 mm across anal field, wing longer than body. Anal field is expanded, deeply incised at base. Membrane is delicate (see remarks below), covered by numerous microtrichia. Veins are very thin, with only Sc, R1, R5, Cu and A1 are stronger; R2 and R3 are forked into additional pseudoveins; of four medial veins, only M4 is distinct; Mb is very short, strongly bent to Cu; d-cell is partially open due to partial reductions of basal section of M3 and of cross vein m-m; m-cu in fork of M3 + 4 into M3 and M4; Cu is unforked; fold parallel to Cu is visible; A1 spans the largest width of anal field, wavy basally, unforked.
Male terminalia are visible in dorsal and ventral views (Figs. 2C and I and 3D, respectively): gonocoxites are narrow, slightly curved to inside; gonostyles are half as wide as and only slightly longer than gonocoxites, with a slight inflation at apex; central triangular projection holding inner genitalia is flanked by two symmetrical structures that are curved to inside, possibly parameres.
Remarks on taphonomy and preservation
The upper and lower wing membranes in this specimen are partially separated and folded around the wing margin (Fig. 2B). In each layer, some details of venation are preserved. Under synchrotron, antennal flagellomeres appear globular due to adhering gas bubbles (Fig. 3A, C); actual shapes of the antennae and bubbles are visible using transmitted light (Fig. 2A, D).
Genus: Cretodeuterophlebia Krzemiński, Skibińska et Kopeć, gen. nov.
LSID urn:lsid:zoobank.org:pub:BC559013-1255-42BB-A984-AF821539A7AF
Diagnosis of the genus is the same as its only species, by monotypy.
Type species.Cretodeuterophlebia courtneyi gen. et sp. n. NIGP177893 male, found in Burmese amber Upper Cretaceous (earliest Cenomanian; 98.79 ± 0.62 Ma)24. The holotype is housed at NIGP.
Etymology. Genus name alludes to the Cretaceous age.
Cretodeuterophlebia courtneyi Krzemiński, Skibińska et Kopeć, sp. nov.
LSID urn:lsid:zoobank.org:act:4E0EB968-1288-4C39-BF4B-7438C021C15E
Diagnosis. Head free (not hidden under scutum); mouthparts reduced, palpi absent. Antennae ~ 2/3 of wing length; scape narrow, pedicel large, round; 14 flagellomeres, 2‒6 oval, remaining ones are cylindrical. Wings 1.3 × as long as body; R2 and R3 fused over entire length; R4, R5 with additional forkings; d-cell open; distal sections of medial, cubital and anal veins reduced to pseudo veins and distally forked. Male genitalia with slender gonostylus and large, forked genital plate.
Etymology. The species is named after Gregory W. Courtney, the renowned specialist on Deuterophlebiidae and author of most papers on this family.
Material. Holotype NIGP177893 male, in Burmese amber Upper Cretaceous (earliest Cenomanian; 98.79 ± 0.62 Ma)24. The specimen is entirely preserved, although gas bubbles obscure some details of the head and thorax. The holotype is housed at NIGP.
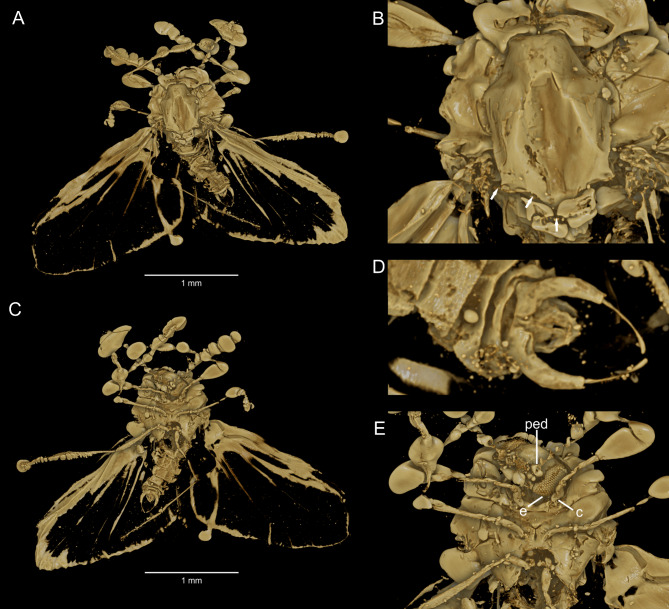
Description. Head is free (not hidden under the scutum) (Fig. 4 D, E), pronotum is not reduced. Mouthparts are reduced, a flat gas bubble covers some details (Fig. 4G), but the face region appears as flat as in recent species and in Protodeuterophlebia oosterbroeki, sp. nov. (compare Fig. 2D). Antennae are short, only ~ 2/3 of wing length; scapus is narrow, pedicel is large and round; 14 flagellomeres, initial 2‒6 flagellomeres are oval, remaining ones cylindrical (Figs. 4B, D). Eyes large and oval, composed of big ommatidia. Wing 1.7 mm long, 0.7 mm wide; wings c.~1.3 × as long as body, both membrane and veins are very delicate. Venation is challenging to decipher because some veins, especially peripheral ones, are diffused into pseudoveins, apparently composed of short sclerotizations (Fig. 4A, H). In our reconstruction (Fig. 4A), some numerous additional forkings of these pseudoveins are omitted. Four radial veins are present; R2 and R3 are entirely fused into R2 + 3; R4 and R5 exhibit additional forkings; Mb is very short and strongly curved to Cu; M1 + 2 is fairly strong, remaining medial veins are transformed into very delicate pseudoveins and terminally forked; d-cell is almost open, but its length can be estimated due to traces of cross vein m-m and basal M3; anal lobe is dilated and deeply incised at base; several anal veins present.
Fig. 4.
Cretodeuterophlebia courtneyi gen. et sp. nov., male holotype, Burmese amber. (A‒C) recontructions: (A) wing (false veins are dotted); (B) antenna; (C) genitalia in dorsal view. (D‒K) photographs: (D) habitus; (E) thorax (note dilated front femur); (G) head enlarged, ventral view (note flat gas bubble covering the mouth region); (F, H, I) wing: (F) fragment (marked in H) shows difference between true vein (Mb) and pseudo vein (pv) under transmittent light; (H) entire wing; (I) distal wing fragment in dark field shows thickenings of membrane aligned into transverse line (highlighted); (J) male genitalia in laterodorsal view; (K) empodium in front leg. Abbreviations: ae, aedeagus; dp, dorsal plate; f, femur; gst, gonostylus; gx, gonocoxite; ped, pedicel; pv, pseudo vein; scp, scapus. Bars: (D) − 1 mm, (E) J − 0.2 mm, (K) − 0.05 mm.
Legs: front femora are dilated (Fig. 4B); fifth tarsomeres have large empodia and two small claws (Fig. 4K).
Male terminalia (Figs. 4C, J): gonocoxites are narrow, gonostyles are approximately 1/4 as wide and not much longer, slightly dilated in distal half; dorsal side with large dorsal plate forked in half its length.
Phylogenetic position of the family
Although, until now, fossil Deuterophlebiidae were unknown, the family was considered to be at the basal position inside Diptera, possibly being the sister group to the remaining extant species. This classification dates back to the first phylogenies based on morphological features by Hennig7,8 and Rohdendorf6. Some molecular analyses have supported this perspective, and even a sister-group position to the remaining Diptera was proposed16–18. Hennig8 placed Deuterophlebiidae in the Psychodomorpha, and a sister-group position close to this infraorder was suggested by Courtney9. A phylogeny based on morphological characters of all stages showed Deuterophlebiidae + Blephariceridae + Nymphomyiidae (Blephariceromorpha) as a monophyletic group in a sister group position to Psychodomorpha10. The similar result was obtained by Blagoderov et al.12, which was an only study incorporating 45 fossil taxa out of a total of 75.
Taking these views into account it seemed plausible to us to incorporate representatives of the oldest Diptera beginning from the Triassic into phylogenetic considerations. From these times only the wings are preserved, and the phylogeny is based on the venation, as observed in other insect phylogenies derived from imprints in sediments12. The species included in the analysis are: two here described species, a recent deuterophlebiid Deuterophlebia mirabilis Edwards, 19224 and following fossil species: Grauvogelia arzvilleriana Krzemiński, Krzemińska & Papier, 199420 (Grauvogeliidae; Lower/Middle Triassic), Tanus triassicus Krzemiński & Krzemińska, 200321 (Nadipteridae; Lower/Middle Triassic), Metaptychopteridium confusum Handlirsch, 1939 (Hennigmatidae; Lower Jurassic25,26), Kuperwoodia benefica Lukashevich, 199511 (Kuperwoodiidae; Middle/Upper Triassic), Ansorgius predictus Krzemiński & Lukashevich, 199327 (Ansorgiidae; Upper Jurassic), Tillyardiptera prima Lukashevich & Shcherbakov, 199928 (Tillyardipteridae; Upper Triassic), Rhaetania dianae Krzemiński & Krzemińska, 200321 (Rhaetaniidae; Upper Triassic), Praemacrochile chinensis Krzemiński & Ren, 200129 (Tanyderidae; Middle Jurassic), Nannotanyderus krzeminskii Ansorge, 199430 (Tanyderidae; Lower Jurassic), Liassopsychodina pommerana Ansorge, 199430 (Psychodidae; Lower Jurassic), Blephadejura propria Lukashevich, Huang & Lin, 200631 (Blephariceridae; Middle Jurassic).
The wing venation of the analysed species is depicted in Figs. 5 and 6, and the character states are as follows.
Fig. 5.
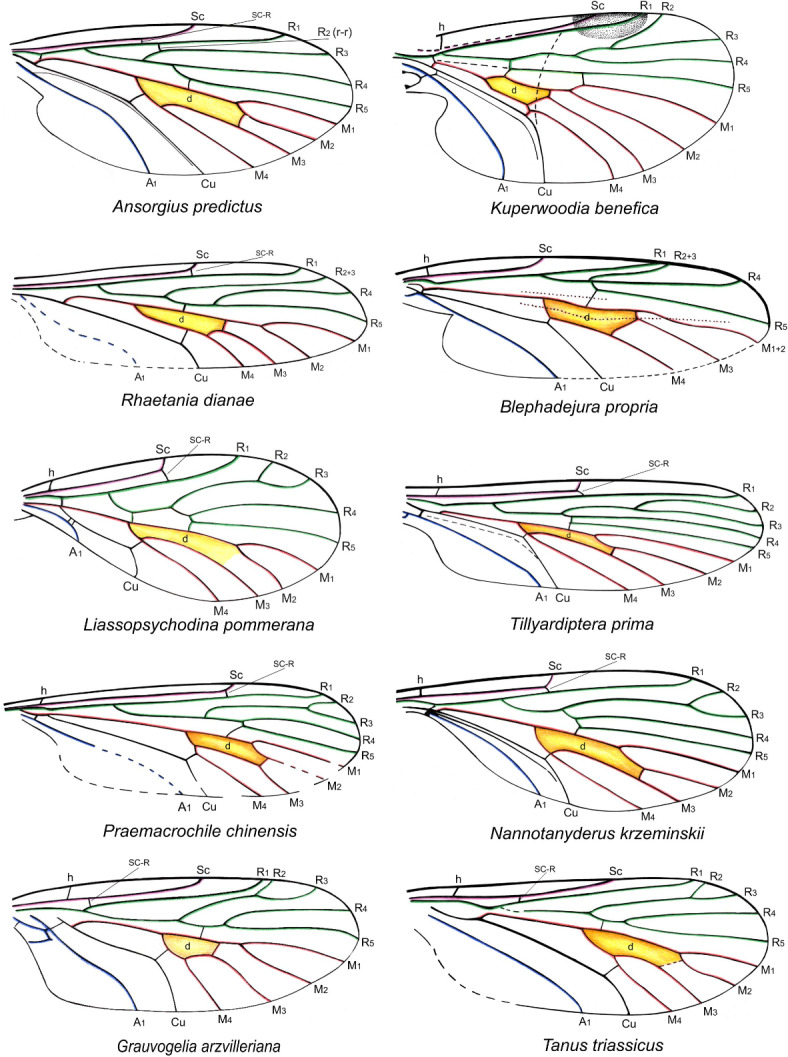
Wing venation of species included in the phylogenetic analysis. Color legend for veins: green - radial, red - medial, black - cubital, blue – anal; yellow area – discal cell.
Fig. 6.
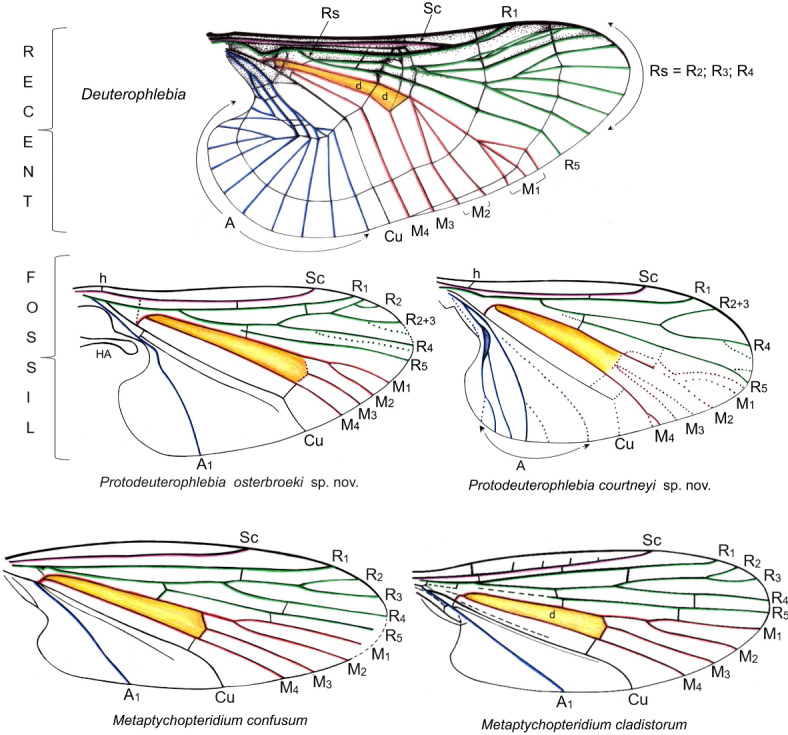
Wing venation of species included in the phylogenetic analysis: comparison of Hennigmatidae and Deuterophlebiidae. Color legend as in Fig. 5.
Characters:
Number of radial veins terminating in wing margin: 5 veins (0); less than 5 (1); more than 5 (2);
Sc ending: in C (0); in R1 (1);
Sc length: reaching level of mid wing (0); 2/3 wing length (1); distinctly before mid wing (2);
sc-r positioned: close to base of Sc (0); in mid of Sc (1); at end of or close to Sc (2);
R1 ending: opposite or before level of end of M3 (0); opposite end of M2 (1); opposite end of M1 (2);
Rs length: equal to or longer than R2 + 3 (0); shorter than R2 + 3 (1);
R2 terminating in wing margin: present (0); absent (1);
R2 single (0); forked (1);
R2 + 3 present (0); absent (1);
R2 + 3 not reaching wing margin (0); reaching it (1).
R2 + 3 longer than R3 (0); shorter or equal R3 (1);
R4 + 5 present (0); absent (1);
Mb length compared to wing length: Mb up to 7 times shorter than the wing length (0); Mb very short, more than 20 times shorter than the wing length (1);
M3 + 4: shorter to equal 1/6 wing length (0); c. ¼ of wing length (1); c. 1/2 wing length (2);
Shape of d cell: lower margin of d cell (= shape of M3 + 4 within d cell): curved (0); straight (1).
d-cell length compared to Mb length: less than 2x length of Mb (0); more than 10x Mb (1);
m-cu positioned: in mid of M3 + 4 (0); before (1); behind (2);
anal veins: one (0); more (1).
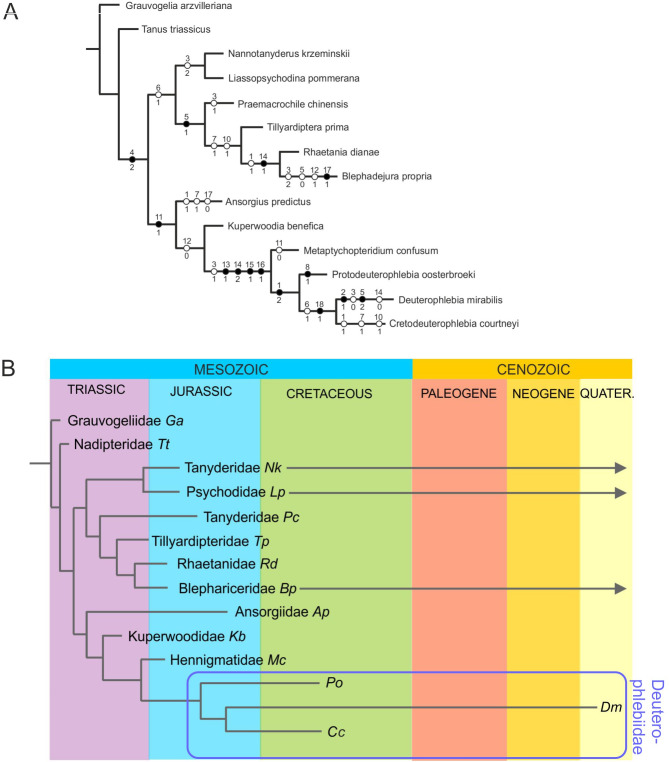
One optimal tree was obtained, illustrated in Fig. 7. All branches bear boostrap support = 100. Tree indices: L = 37; Ci = 62; Ri = 68. The character matrix is available in the Supplementary file 2.
Fig. 7.
Phylogenetic position of the Deuterophlebiidae. (A) cladogram; only unambiguous apomorphies are indicated; all main branches bear boostrap support = 100. Tree indices: L = 37; Ci = 62; Ri = 68. (B) Phylogenetic tree in chronological context (based on the cladogram A). Species are denoted by first letters of their generic and species names (Ap Ansorgius predictus; Bp Blephadejura propria; Cc Cretodeuterophlebia courtneyi n. sp.; Dm Deuterophlebia mirabilis; Ga Grauvogelia arzvilleriana; Kb Kuperwoodia benefica; Lp Liassopsychodina pommerana; Mc Metaptychopteridium confusum; Nk Nannotanyderus krzeminskii; Pc Praemacrochile chinensis; Po Protodeuterophlebia oosterbroeki n. sp.; Rd Rhaetania dianae; Tp Tillyardiptera prima; Tt Tanus triassicus).
Comments on the topology of the tree. In our tree, the Psychodomorpha are divided into two well-supported lineages, both with bootstrap index 100. Both branches terminate with crown taxa represented by recent species: Deuterophlebiidae in one and Blephariceridae in the other. The immediate sister group to Deuterophlebiidae are the Hennigmatidae, and three unique synapomorphies of both families concern the construction of the discal-cell and the veins around it (characters 13, 14, 15, 16; all unambiguous synapomorphies). The shared arrangement of a very long and narrow discal cell reaching close to the wing base is attributed to an extremely short Mb (approximately 1/10 of wing length) and an unusually long M3 + 4; these characters are unique to this clade among the Diptera and support the placement of Deuterophlebiidae within the Psychodomorpha, indicating an ancient origin of this group. In all other dipteran wings included here, the fork of Mb into M1 + 2 and M3 + 4 is positioned deeper in the wing, and the d cell is shorter.
The Hennigmatidae are one of the geologically oldest dipteran families, and in the Mesozoic they had a wide distribution documented by the following fossils in Europe, Asia, and South America: Trihennigma zavattieri Lara and Lukashevich, 201332, from the lower Upper Triassic of Argentina; Metaptychopteridium confusum25, from the early Jurassic of Germany (Toarcian) (originally described in the Trichoptera and revised)26; Daohennigma panops Lukashevich, Huang and Lin, 200631, from the Middle or Late Jurassic of China; and the youngest known species, Metaptichopteridium cladistorum Shcherbakov, 199511 described from the Early Cretaceous of Mongolia. This dispersion indicates an original Pangean distribution of this family.
The second main branch of the tree comprises the Blephariceridae, a family often discussed in terms of its close relationship with the Deuterophlebiidae due to similar adaptations to torrential habitats in the larvae and net-like pattern of false veins in adults’ wings9,10,12. In the tree presented here, the Blephariceridae hold the crown position in the second branch of the Psychodomorpha and appear a sister group to the Tillyardipteridae, an extinct Triassic family. Other members of this branch include Tanyderidae and Psychodidae, whose sister relationship was generally accepted11,21,30,33, also supported by molecular analyses16,17; their venation in oldest representatives is very similar.
Discussion
Evolution of the family Deuterophlebiidae
Position within the Psychodomorpha. The Psychodomorpha is a highly diversified infraorder, whose members first appeared in the early Middle Triassic20,21. Taxa with complete wing venation, whether fossil or recent, can be easily classified to the Psychodomorpha based on the radial field with five veins terminating in the wing margin. However, during independent evolution of particular families, numerous modifications in venation have occurred, which now obscure its earlier stages. The most significant changes are observed in the recent Deuterophlebiidae and Blephariceridae. Until recently, the fossil Deuterophlebiidae were unknown, and fossil Blephariceridae are scarce34. Consequently, tracing the gradual changes in venation in these families is challenging and hinders our understanding of relationships within the Psychodomorpha.
According to the results presented herein, the Deuterophlebiidae exhibit closest relation with the Hennigmatidae, an extinct family of the Triassic origin, widespread in the Mesozoic. Although the venation of recent deuteroplebiid species is hardly comparable to other Diptera, it still shows similarity to the Hennigmatidae, particularly in an extremely short Mb and a very narrow d-cell, which are two strong synapomorphies absent from any other dipteran family. This similarity is even more evident in the fossil taxa described here, as their venation retained plesiomorphic features (such as the absence of transverse rings of cross veins).
In the phylogeny presented herein (Fig. 7) the Psychodomorpha are divided into two main branches; the crown taxon of one of them are the Deuterophlebiidae. However, in some phylogenies, the family was positioned basally inside Diptera8, also in the studies based on molecular analyses16–18. This uncertainty regarding the families` position may be explained by the fact that most representatives of the Psychodomorpha are extinct. Consequently, the DNA of the Deuterophlebiidae could only be compared only to living descendants of Psychodomorpha, namely Tanyderidae, Psychodidae, and Blephariceridae.
Phylogenetical relations within the family Deuterophlebiidae, based on morphological features, were examined by Courtney14; both sexes and all developmental stages of 14 species were included. The state of preservation of our fossil species allows only for a comparison of antennal length; other characters of adult males, such as leg setation and mouth region features, are not preserved. However, it is interesting to note that two recent species, which exhibit the most plesiomorphic character states (Deuterophlebia brachyrhina Courtney and D. oporina Courtney) live in the Himalayan region, close to the locality of origin of Burmese amber. This locality is also only known fossil site for deuterophlebiids, hitherto the oldest members of the family.
Development of ecological adaptations in mountain midges. Today, the juvenile and adult Deuterophlebiidae exhibit strong adaptions to habitats with fast running waters. These adaptations in immature stages are well known since long35 and allow to thrive in torrents where current velocity reaches up to 3–4 m/s.
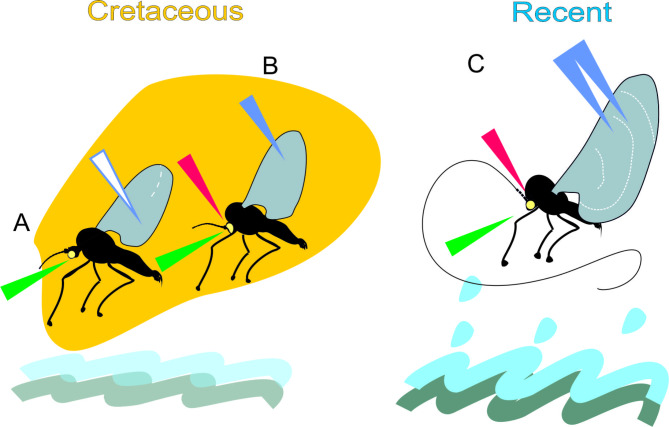
The two fossil deuterophlebiids preserved in Burmese amber, described here, stand as the sole extinct members of this family. Both specimens share similar age, each providing insight into an earlier stage of evolutionary adaptation of the head, tarsi and wings - distinctive structures in contemporary Deuterophlebia species. They are overviewed below and summarized in Fig. 8.
Fig. 8.
Ecological adapations and external morphology of fossil (A‒B) and recent Deuterophlebiidae (C). (A) Cretodeuterophlebia courtneyi sp.nov.; (B) Protodeuterophlebia oosterbroeki sp. nov., both in Burmese amber; (C) Deuterophlebia coloradensis, recent species. Note: complete reduction of mouthparts in all species; reduction of the neck region from (A) to (C); differences in antennae; proportions of wings and body lengths in fossil and recent species; early stages of development of false veins net. Legend: yellow irregular shape symbolises amber; red arrow - reduction of the neck region; green arrow - reduction of mouthparts; white and blue arrows - presence of false veins: white - false veins are scarce; single blue arrow - abundant; double blue arrow -prevailing. Wavy blue lines with splashes symbolize torrent water inhabited by recent deuterophlebiids; waves weaker in shape and colour indicate our supposition on similar habitat in Cretaceous deuterophlebiids. White dashed lines across wings in (C) represent transverse false veins; the possible early stage of this structure is marked in (A).
Head. In recent mountain midges the neck region (pronotum) is reduced so that the head forms a single entity with thorax3. Large, dioptic eyes and extremely long antennae (up to 10x exceeding body length) compensate for the immobility of head and enable for quick finding of the females. Both sexes do not feed; their mouthparts are completely reduced. The main function in their brief life is the mating, and in females, the oviposition. Of both fossil males here described, only Protodeuterophlebia oosterbroeki sp. nov. has a reduced neck region, while in Cretodeuterophlebia courtneyi gen. et sp. nov., the neck is exposed, indicating a fully movable head. The eyes are dioptic and large, but proportionally smaller than in recent Deuterophlebia. Antennae in both Cretaceous males are shorter than body and consist of 13–16 flagellomeres of oval to cylindrical shape which is common among nematoceran flies. They do not show any resemblance to recent 4‒6-segmented antennae with the ultimate segment transformed into a filament up to 10x longer than the body. Nevertheless, reduction of mouthparts was already advanced during this period, as both specimens bear no trace of any elongated structures on the facial region or palpi. Although the mouth openings are obscured by gas bubbles, the surface is undoubtedly flat (Figs. 2D, 3E and 4G).
Legs. In Deuterophlebia the tarsi of males end with hairy empodia, serving as extremely large attachment pads, at least 8x wider than the diameter of the fifth tarsus over the terminal swelling (measure based on36). Both long antennae and enlarged empodia, in addition to their plausible role in mating behaviour, help males to quickly rise from turbulent water in case of accidental falls, which occur frequently. Recovery from such falls is usually rapid, and males can resume flight within a few seconds37. A pad-like shaped empodium is visible in both fossil males, but is better preserved in the holotype of Cretodeuterophlebia courtneyi, gen. et sp. nov. (Fig. 4A, K); the empodium is approximately 3x wider than the last tarsal segment, but its shape is similar to that in recent taxa.
Wing size, shape and venation. Efficient flight is of utmost importance for deuterophlebiids, as they most likely mate in flight2. The wings in both sexes are almost twice as long as their bodies (Fig. 8C). Maneuvering such wings must therefore exert tremendous effort on these insects, likely leading to rapid exhaustion and a subsequent fall into water, thus concluding their life. The wings of both Cretaceous deuterophlebiids were proportionally smaller and only slightly exceeded the body length (Fig. 8A, B). Noteworthy, their shape widest across the anal field makes them similar to subtriangular wings in recent deuterophlebiids.
The venation of recent mountain midges (Fig. 6) is characterized by only vestiges of main veins, especially in distal portions, where they are replaced by abundant untracheated false veins38. In the anal region the false veins are arranged in fan-like pattern to spread the large anal lobe, partially separated from the wing base by a deep incision. Across the wing the false veins form three concentric lines. Due to this venation the phylogenetic position of Deuterophlebiidae was unclear, compounded by the absence of fossils that could shed light on the early development of these characters.
It is now fascinating to see the early stages of development of this distinctive venation in the two Cretaceous deuterophlebiids. Two different transformation stages of the venation are observed. In Protodeuterophlebia oosterbroeki gen. et sp. nov., a few false veins appear in the radial field, while in Cretodeuterophlebia courtneyi gen. et sp. nov., they emerge abundantly in the medial and anal fields, in place of distal sections of true veins. Under high magnification, their appearance is noticeably dissimilar from true veins; the thickenings of membrane are arranged linearly but remain unconnected. In the latter species, a curved, discontinuous line transverses the wing (Figs. 4I and 8A), providing suggestive origin for the concentric arrangement visible in recent wing (Fig. 8C).
Materials and methods
Material
Two inclusions of deuterophlebiid males in Burmese amber: #NIGP177893 and #NIGP177894-A. The latter is accompanied by a syninclusion, of a separate wing of the mayfly Vetuformosa buckleyi, #NIGP177894-B. These specimens originate from the Hukawng Valley in Kachin State, northern Myanmar, dating to the Upper Cretaceous (earliest Cenomanian; 98.79 ± 0.62 Ma)23. They are housed in the Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210,008, China (NIGP). Collected in 2015, the specimens underwent collection and storage procedures in full compliance with the institute`s regulations (NIGP) (see the statement in the Supplementary file No. 1).
For comparative purpose, a recent specimen, Deuterophlebia shasta39 male, USA: CA. Humboldt Co., Van Duzen River & Hwy. 36 was included in the study (G.W. Courtney).
Methods
Synchrotron X-ray microtomography was conducted using the IPS UFO station at KIT Light Source using a parallel polychromatic X-ray beam produced by a 1.5 T bending magnet that was spectrally filtered by 0.5 mm aluminum to remove low energy components from the beam. The resulting spectrum exhibited a peak at approximately 15 keV, with a full-width at half maximum bandwidth of about 10 keV. To achieve a magnification of 10x, providing an effective pixel size of 1.22 μm, we employed a fast indirect detector system. This system comprised a 13 μm LSO: Tb scintillator40, a diffraction-limited optical microscope (Optique Peter), and a 12 bit pco.dimax S4 high-speed camera with a resolution of 2016 × 2016 pixels41. To cover the entire specimen, four individual scans were performed. Each scan involved the acquisition of 200 dark field images, 200 flat field images, and 3000 equiangularly spaced radiographic projections over a range of 180°. The exposure time for each radiographic projections was 10 milliseconds, resulting in scan durations of 34 s per scan. The concert control system42 facilitated data acquisition and online reconstruction of tomographic slices for data quality assurance. The final 3D reconstruction was executed using tofu43 and involved phase retrieval44, ring removal, 8-bit conversion, and blending of phase and absorption 3D reconstructions to enhance contrast. The merging and registration of four tomograms into a single image volume was performed using Amira 5.6. The gray values were then inverted and the sample was digitally isolated from the background. Subsequently, the specimen was digitally isolated from the background, and volume renderings of the processed data were created with Drishti 2.5.145. The wings of Deuterophlebia were dyed through a seven-day soak in 70% alcohol solution of toluidine blue. The images were captured using a Nikon SMZ25 stereoscopic microscope equipped with a Nikon DSRi2 digital camera at ISEA PAS. Additional pictures in Fig. 4F, I were taken using the Delta Optical Genetic Pro microscope with an MP3 camera at the Pedagogical University, Kraków. The images were further processed using Scopelmage 9.0 Professional Imaging Software. The map was generated using using simplemappr.net46 and modified using Corel Draw and Corel Photopaint X7. Phylogenetic analysis was performed in a graphical software application TNT 1.547. The morphological data for the matrix were compiled into a Nexus file using Mesquite v.3.0148. All 18 characters of the imagines used in the analysis were treated as unordered; the implied weighting was applied with K = 12, following recommendation for morphological characters49. A single optimal tree was obtained and its features were studied using WinClada 1.00.08 and ASADO 1.61. The nomenclature for male genitalia follows Courtney37.
Identification of fossil resin
Recording and archiving the infrared (IR) spectra of fossil resins is a recommended practice for museum material to validate the authenticity of newly described taxa50. Consequently, amber pieces containing the described inclusions underwent testing for their IR spectrum. Fourier transform infrared spectroscopy (attenuated total reflectance) was conducted using a Nicolet iS5 FTIR spectrometer with a diamond crystal ATR attachment. The resulting spectrum exhibited characteristic bands for Burmese amber, with distinct absorption peaks at 1226, 1155, 1031, and 974 cm− 1, confirming the origin of this specimen (Fig. 2F).
This published work and the associated nomenclatural acts have been registered in ZooBank, the online registration system for the International Code of Zoological Nomenclature (ICZN). The LSID (Life Science Identifier) for this publication is: LSID urn:lsid:zoobank.org:pub:BC559013-1255-42BB-A984-AF821539A7AF.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Angelica Cecilia and Marcus Zuber (both KIT) for their support during beamtime and Tomáš Faragó (KIT) for tomographic reconstructions. We gratefully acknowledge the data storage service SDS@hd supported by the Ministry of Science, Research and the Arts Baden-Württemberg (MWK). Further, we acknowledge the KIT Light Source for provision of instruments at their beamlines and we would like to thank the Institute for Beam Physics and Technology (IBPT) for the operation of the storage ring, the Karlsruhe Research Accelerator (KARA). Marzena Albrycht is thanked for help in taking microscope camera pictures at the Pedagogical University, Kraków. Roman Godunko University of Lodz, Poland confirmed identification of a wing of Vetuformosa buckleyi in Burmese amber.
Author contributions
Conceptualization: W.K., E.K., A.S. Methodology: W.K., T.K., E.K., K.S., A.S. Investigation: E.K., W.K., I.K.K., K.S., K.K., T.K., Q.Z., A.S. Visualization: E.K., I.K.K., K.S., T.K. Supervision: E.K., W.K. Writing—original draft: W.K., I.K.K., E.K., Q.Z., A.S.; writing—review & editing: E.K., A.S.
Funding
The research was supported by the grant from the Polish National Science Center No. UMO-2016/23/B/NZ8/00936] (UMO-2020/37/B/NZ8/03042) and the German Research Foundation (DFG) through grant INST 35/1503-1 FUGG.
Data availability
Synchrotron X-ray microtomographic data are deposited at the RADAR4KIT repository of Karlsruhe Institute of Technology: (https://doi.org/10.35097/vPFKYFgwPUznpZiP). All other data generated or analysed during this study are loaded in this published article and its supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng, X., Chen, Z., Mu, P., Ma, Z. & Zhou, C. Descriptions and barcoding of five new Chinese Deuterophlebia species revealing this genus in both Holarctic and oriental realms (Diptera: Deuterophlebiidae). Insects. 13, 593. 10.3390/insects13070593 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Courtney, G. W. Life history patterns of Nearctic mountain midges (Diptera: Deuterophlebiidae). J. North. Am. Benthol Soc.10, 177–197 (1991b). [Google Scholar]
- 3.Schneeberg, K., Courtney, G. W. & Beutel, R. G. Adult head structures of Deuterophlebiidae (Insecta), a highly derived ancestral dipteran lineage. Arthropod Struct. Dev.40, 93–104 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Edwards, F. W. DeuterophMirabilisabilis, gen. et sp. n., a remarkable dipterous insect from Kashmir. Ann. Mag Nat. Hist.9, 379–387 (1922). [Google Scholar]
- 5.Rohdendorf, B. B. Historical development of dipterous insects. Trudy Paleont Inst.100, 1–311 (1964). [In Russian; English translation 1974]. [Google Scholar]
- 6.Rohdendorf, B. B. The Historical Development of Diptera (University of Alberta, 1974).
- 7.Hennig, W. Kritische Bemerkungen über den Bau Der Flügelwurzel bei den Dipteren Und die Frage Nach Der Monophylie Der Nematocera. Stutt Beitr. Naturkd. 193, 1–23 (1968). [Google Scholar]
- 8.Hennig, W. Diptera (Zweiflügler) in Handbuch Der Zoologie, IV. Band: Arthropoda e2. Hälfte: Insecta, 2. Teil. (eds Helmcke, J. G., Starck, D. & Wermuth, H.) 1–200 (Walter de Gruyter, 1973).
- 9.Courtney, G. W. Cuticular morphology of larval mountain midges (Diptera: Deuterophlebiidae): implications for the phylogenetic relationships of Nematocera. Can. J. Zool.68, 556–578 (1990). [Google Scholar]
- 10.Courtney, G. W. Phylogenetic analysis of the Blephariceromorpha, with special reference to mountain midges (Deuterophlebiidae). Syst. Ent. 16, 137–172 (1991). [Google Scholar]
- 11.Shcherbakov, D. E., Lukashevich, E. D. & Blagoderov, V. A. Triassic Diptera and initial radiation of the order. J. Dipt.Res.6 (2), 75–115 (1995). [Google Scholar]
- 12.Blagoderov, V., Grimaldi, D. A. & Fraser, N. C. How time flies for flies: diverse Diptera from the Triassic of Virginia and early radiation of the order. Am. Mus. Nov. 3572, 1–39 (2007). [Google Scholar]
- 13.Wood, D. M. & Borkent, A. 114. Phylogeny and classification of the Nematocera in Manual of Nearctic Diptera, vol. 3. (ed. McAlpine, J.F.) 209–212 (Research Branch, Agriculture Canada, 1989).
- 14.Courtney, G. W. Revision of Palaearctic mountain midges (Diptera: Deuterophlebiidae), with phylogenetic and biogeographic analyses of world species. Syst. Ent.19, 1–24 (1994). [Google Scholar]
- 15.Oosterbroek, P. & Courtney, G. Phylogeny of the nematocerous families of Diptera (Insecta). Zool. J. Linn. Soc.115, 267–311 (1995). [Google Scholar]
- 16.Bertone, M. A., Courtney, G. W. & Wiegmann, B. W. Phylogenetics and temporal diversification of the earliest true flies (Insecta: Diptera) based on multiple nuclear genes. Syst. Ent. 33, 668e687 (2008). [Google Scholar]
- 17.Wiegmann, B. M. et al. Episodic radiations in the fly tree of life. PNAS. 108(14), 5690–5695 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegmann, B. M. & Yeates, D. K. Phylogeny of Diptera in Manual of Afrotropical Diptera, Vol.1. Introductory chapters and keys to Diptera families. Suricata 4. (eds Kirk-Spriggs, A.H. & Sinclair, B.J.) 253–265 (South African National Biodiversity Institute, 2017).
- 19.Jaschhof, M. & Didham, R. K. Rangomaramidae fam. nov. from New Zealand and implications for the phylogeny of the Sciaroidea (Diptera: Bibionomorpha). Stud. Dipterol Suppl.11, 1–60 (2002). [Google Scholar]
- 20.Krzemiński, W., Krzemińska, E. & Papier, F. Grauvogelia arzvilleriana sp. n. - the oldest Diptera species (Lower/Middle Triassic of France). Acta Zool. crac. 37(2), 95–99 (1994). [Google Scholar]
- 21.Krzemiński, W. & Krzemińska, E. Triassic Diptera: review, revisions and descriptions. Acta Zool. crac (Suppl Fossil Insects). 46, 153–184 (2003). [Google Scholar]
- 22.Lukashevich, E. D. Phylogeny of Ptychopteroidea (Insecta: Diptera). Paleont. J.46(5), 476–484 (2012). [Google Scholar]
- 23.Shi, G. et al. Age constraint on Burmese amber based on U–Pb dating of zircons. Cret Res.37, 155–163 (2012). [Google Scholar]
- 24.Poinar, G. Jr. Vetuformosa buckleyi n. gen., n. sp. (Ephemeroptera: Baetidae; Vetuformosinae n. subfam.), a new subfamily of mayflies in early cretaceous Burmese amber. Hist. Biol.23(4), 369–374 (2011). [Google Scholar]
- 25.Handlirsch, A. Neue Untersuchungen über die fossilen Insekten, 2. Teil. Ann. Naturhist Mus. Wien. 49, 1–240 (1939). [Google Scholar]
- 26.Ansorge, J. Lower Jurassic Hennigmatidae (Diptera) from Germany. Stud. Dipt.8 (1), 97–102 (2001). [Google Scholar]
- 27.Krzemiński, W. & Lukashevich, E. D. Ansorgiidae, a new family from the Upper Cretaceous of Kazakhstan (Diptera, Ptychopteromorpha). Acta Zool. Crac. 35 (3), 593–596 (1993). [Google Scholar]
- 28.Lukashevich, E. D. & Shcherbakov, D. E. A new Triassic family of Diptera from Australia in Proceedings of the First Palaeontomological Conference Moscow 81–89 (AMBA Projects International, 1999). (1999).
- 29.Krzemiński, W. & Ren, D. Praemacrochile chinensis sp. n. from Middle Jurassic of China (Diptera: Tanyderidae). Pol. J. Ent. 70, 127–129 (2001). [Google Scholar]
- 30.Ansorge, J. Tanyderidae and Psychodidae (Insecta: Diptera) from the Lower Jurassie of northeastern Germany. Paläont Zeit. 68, 199–210 (1994). [Google Scholar]
- 31.Lukashevich, L., Huang, D-Y. & Lin, Q-B. Rare families of nematoceran Diptera (Hennigmatidae, Blephariceridae, Perissommatidae) from the jurassic of China. Stud. Dipter.13(1), 555–666 (2006). [Google Scholar]
- 32.Lara, M. B. & Lukashevich, E. D. The first triassic dipteran (Insecta) from South America, with review of Hennigmatidae. Zootaxa. 3710(1), 081–092 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi, D. & Engel, M. S. Evolution of the Insects (Cambridge University Press, 2005).
- 34.Lukashevich, E. D. & Vorontsov, D. D. A new genus of net-winged midges (Diptera: Blephariceridae) from mid-cretaceous amber of Myanmar. Cret Res.144, 105447 (2023). [Google Scholar]
- 35.Hora, S. L. & Ecology Bionomics and evolution of the torrential fauna, with special reference to the organs of attachment. Philos. Trans. R Soc. Lond.218, 171–282 (1930). [Google Scholar]
- 36.Friedemann, K., Schneeberg, K. & Beutel, R. G. Fly on the wall – attachment structures in lower Diptera. Syst. Ent. 39, 460–473 (2014). [Google Scholar]
- 37.Courtney, G. W. Revision of Nearctic mountain midges (Diptera: Deuterophlebiidae). J. Nat. Hist.24, 81–118 (1990).
- 38.Kennedy, H. D. 9. Deuterophlebiidae in Manual of Nearctic Diptera, vol. 1. (eds McAlpine et al.) 199–202 (Ottawa: Research Branch Agriculture, 1981).
- 39.Wirth, W. W. A new mountain midge from California (Diptera: Deuterophlebiidae). Pan-Pac Entomol.27, 49–57 (1951). [Google Scholar]
- 40.Cecilia, A. et al. LPE grown LSO:Tb scintillator films for high resolution X-ray imaging applications at synchrotron light sources. Nucl. Instrum. Methods Phys. Res. A. 648 (Suppl. 1), S321–S323 (2021). [Google Scholar]
- 41.Douissard, P-A. et al. A versatile indirect detector design for hard X-ray microimaging. J. Instr.7, P09016 (2012). [Google Scholar]
- 42.Vogelgesang, M. et al. Real-time image-content-based beamline control for smart 4D X-ray imaging. J. Synch. Rad. 23, 1254–1263 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Faragó, T. et al. Tofu: a fast, versatile and user-friendly image processing toolkit for computed tomography. J. Synch. Rad. 29, 916–927 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paganin, D. et al. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Micr. 206, 33–40 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Limaye, A. Drishti: a volume exploration and presentation tool. Proceedings SPIE. Developments in X-Ray Tomography VIII, 85060X (2012).
- 46.Shorthouse, D. P. SimpleMappr, an online tool to produce publication-quality point maps https://www.simplemappr.net (2010).
- 47.Goloboff, P. A., Farris, J. S. & Nixon, K. C. TNT, a free program for phylogenetic analysis. Cladistics. 24, 774–786 (2008). [Google Scholar]
- 48.Maddison, W. P. & Maddison, D. R. Mesquite: a modular system for evolutionary analysis. Version 3.81 http://www.mesquiteproject.org (2014).
- 49.Goloboff, P. A., Torres, A. & Arias, S. J. Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics. 34, 407–437 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Giłka, W. et al. Wanted, tracked down and identified: mesozoic non-biting midges of the subfamily Chironominae (Chironomidae, Diptera). Zool. J. Linn. Soc.20, 1–19 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Synchrotron X-ray microtomographic data are deposited at the RADAR4KIT repository of Karlsruhe Institute of Technology: (https://doi.org/10.35097/vPFKYFgwPUznpZiP). All other data generated or analysed during this study are loaded in this published article and its supplementary information files.