Abstract
Background
Micro- and nanoplastics (MNPs) are emerging pollutants of concern with ubiquitous presence in global ecosystems. MNPs pose potential implications for human health; however, the health impacts of MNP exposures are not yet understood. Recent evidence suggests that MNPs can cross the placental barrier, underlying the urgent need to understand their impact on reproductive health and development.
Objective
The Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics (AURORA) project will investigate MNP exposures and their biological and health effects during pregnancy and early life, which are critical periods due to heightened vulnerability to environmental stressors. The AURORA project will enhance exposure assessment capabilities for measuring MNPs, MNP-associated chemicals, and plastic additives in human tissues, including placenta and blood.
Methods
In this interdisciplinary project, we will advance methods for in-depth characterization and scalable chemical analytical strategies, enabling high-resolution and large-scale toxicological, exposure assessment, and epidemiological studies. The AURORA project performs observational studies to investigate determinants and health impacts of MNPs by including 800 mother-child pairs from 2 existing birth cohorts and 110 women of reproductive age from a newly established cohort. This will be complemented by toxicological studies using a tiered-testing approach and epidemiological investigations to evaluate associations between maternal and prenatal MNP exposures and health perturbations, such as placental function, immune-inflammatory responses, oxidative stress, accelerated aging, endocrine disruption, and child growth and development. The ultimate goal of the AURORA project is to create an MNP risk assessment framework and identify the remaining knowledge gaps and priorities needed to comprehensively assess the impact of MNPs on early-life health.
Results
In the first 3 years of this 5-year project (2021-2026), progress was made toward all objectives. This includes completion of recruitment and data collection for new and existing cohorts, development of analytical methodological protocols, and initiation of the toxicological tiered assessments. As of September 2024, data analysis is ongoing and results are expected to be published starting in 2025.
Conclusions
As plastic pollution increases globally, it is imperative to understand the impact of MNPs on human health, particularly during vulnerable developmental stages such as early life. The contributions of the AURORA project will inform future risk assessment.
International Registered Report Identifier (IRRID)
DERR1-10.2196/63176
Keywords: epidemiology, pregnancy, toxicology, microplastics, placenta, risk assessment
Introduction
Plastic is pervasive in both built and natural environments. Global plastic production was estimated to be 400.3 million tons in 2022, with most plastic generated for single-use purposes [1]. Despite growing regulatory efforts to reduce plastic production and increase recycling, global plastic production is projected to increase to 1100 million tons by 2050 [2-4]. The lifetime cost of the plastics produced in 2019 alone exceeded US $3.7 trillion, including expenses such as greenhouse gas emissions, waste management, and environmental cleanup, and without accounting for potential costs related to impacts on human health [5]. Small plastic particles are generated by weathering and degradation or intentionally produced [4,6]. These particles, including fibers, are categorized based on their dimensions, with those ranging from 5 mm to 1 μm referred to as microplastics (MPs), and those smaller than 1 μm termed nanoplastics (NPs) [6]. Although there is growing evidence of micro- and nanoplastics (MNPs) in air, drinking water, and food [7], the scale of MNP exposure and behavior in the human body remains uncertain [8]. Furthermore, the potential risk MNPs pose to human health is largely unknown [9,10].
MNPs represent a complex class of pollutants, with a range of physical-chemical properties, such as morphology, composition, density, and surface chemistry. Human health risk assessment of MNPs presents unique challenges, primarily due to the complexity and diversity of these particles [9]. Established risk assessment frameworks, for example, for engineered nanomaterials and chemical pollution, are likely inadequate to account for the complex characteristics of MNPs [11,12]. Recent inventories document more than 4000 substances used in plastic packaging and more than 10,000 plastic-associated compounds, including organic polymers, additives such as plasticizers, and nonintentionally added substances such as reaction by-products [13,14]. Furthermore, MNPs in ecosystems may form eco-coronas, which may subsequently absorb other environmental pollutants and potentially facilitate human exposure to additional pollutants [15]. Current measurement approaches inadequately capture the full extent of MNP-associated chemical exposures in humans, as they focus on a limited set of known chemicals.
Various analytical techniques have provided the first insights into human exposure to MNPs. There is growing evidence that MNPs can be detected in human tissues, including blood and placenta tissue [16-18]. Mass-based assessment of MNPs using high-resolution mass spectrometry coupled with pyrolysis (Py-GC/HRMS), chemical profile assessment, and complementary spectro-microscopic characterization have emerged as promising approaches [19-21]. However, most MNP exposure assessments to date have been small scale (ie, less than 100 subjects), proof-of-concept studies, and have primarily focused on MPs rather than NPs [17,18,22]. Critical analytical advancements are necessary to reduce the uncertainty and error of exposure estimates and to enable comprehensive risk assessment [11,23].
During gestation and early life, the developing fetus is highly vulnerable to environmental stressors, including chemical exposures, due to rapid organogenesis and developmental plasticity [24]. Disruption by environmental toxicants during this window of heightened susceptibility can have a long-lasting impact on the molecular and physiological phenotype and health later in life [25]. The placenta is a unique organ at the maternal-fetal interface that facilitates gas exchange and the transport of nutrients, hormones, and other solutes essential for fetal growth and development, while also supporting maternal health [26]. It has been observed that not only endogenous but also exogenous compounds, including engineered metallic and carbonaceous nanoparticles, can cross the placenta barrier [27,28]. Growing evidence supports the placental translocation of MNPs in in vitro, in vivo, and ex vivo models [29]. Furthermore, MNPs have recently been detected in human placenta tissue, amniotic fluid, meconium, and breastmilk [18,22,30-32]. Given that pregnancy and early life are periods of heightened susceptibility to environmental stressors, evidence supporting the potential for transplacental transport of MNPs [33,34] and early-life exposure via ingestion and inhalation [35] provide impetus for investigating the implications of MNP exposure during these periods.
To overcome these gaps in MNP research, the Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics (AURORA) research project aims to create a comprehensive framework for evaluating the health risks associated with MNPs during early-life stages [36]. Emphasis is placed on advancing analytical techniques for characterizing MNPs and evaluating their potential hazards during pregnancy and early life (Figure 1). This article provides an overview of the objectives, approaches, methodologies, strengths, challenges, and anticipated impacts of the AURORA project.
Figure 1.
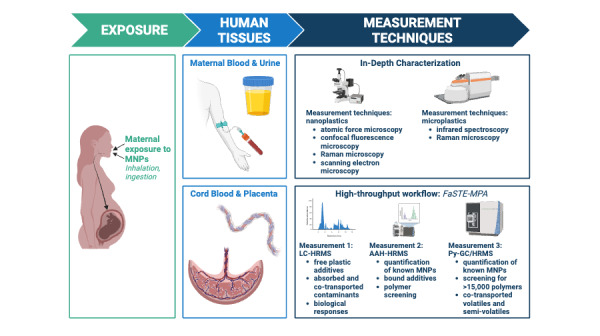
Overview of exposure assessment, sample types, and measurement techniques in AURORA. AAH-HRMS: alkaline-assisted hydrolysis with high resolution mass spectroscopy; AURORA: Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics; FaSTE-MPA: Fast, Single, Tissue Extraction for Multiplexed Plastic Analysis; LC-HRMS: liquid chromatography with high resolution mass spectroscopy; MNP: Micro- and nanoplastic; Py-GC/HRMS: pyrolysis gas chromatography high-resolution mass spectroscopy. Image created with BioRender.com [37].
Methods
The AURORA project will address fundamental knowledge gaps about the effects of MNPs on early-life health and inform future human health risk assessment [36]. This 5-year project (2021-2026) is funded by the European Union through the Horizon 2020 program and is one of the five projects that forms the CUSP European research cluster to study the health impacts of MNPs [38].
Objectives
The AURORA project has five core research objectives:
Exposure characterization: to develop analytical methods for in-depth characterization of MNPs in maternal and fetal human sample matrices, including placenta.
Scalable exposure assessment: to develop high-throughput analytical strategies for quantitative assessment of maternal and prenatal exposure to MNPs and biomonitoring in human populations.
Experimental toxicology: to assess toxicity, toxicokinetics, and toxicodynamics of MNPs in experimental models, focusing on placental models.
Epidemiology: to evaluate associations between MNP exposure and female reproductive and early-life health outcomes.
Advance risk assessment: to develop an actionable European framework for human health risk assessment of MNPs.
To address these objectives, the AURORA project was designed with an interdisciplinary approach, bringing together experts in nanoparticle characterization, inorganic chemistry, analytical chemistry, exposomics, exposure science, biology, toxicology, epidemiology, risk assessment, and science communication.
Study Populations and Samples
An extensive set of profiled human tissue samples, household samples, and biological markers and health outcomes will provide novel information about MNP exposure and health impacts. The AURORA project involves 2 richly phenotyped birth cohorts with unique biobanks, including placenta samples, and will establish a new cohort to assess determinants of MNP exposure.
The ENVIRonmental influence ON early AGEing (ENVIRONAGE) birth cohort has been recruiting pregnant women in the East Limburg Hospital, Genk, Belgium since 2010, and currently includes more than 2300 mother-child pairs [39]. This cohort was established to investigate the impact of air pollution and other environmental stressors on early biological aging. The Barcelona Life Study Cohort (BiSC) has recruited 1080 mother-child pairs residing in the Barcelona metropolitan area, Spain, during the years 2018-2021 with the overall aim of investigating the impact of the early-life exposome on maternal and child health and development [40]. Both cohorts collected fresh placenta tissue immediately after delivery. In addition to the standard biopsies sampled at fixed locations on the fetal and maternal side of the placenta [39], for a subset of women recruited since 2020, a de novo sample collection of the entire placenta was initiated with a sample collection procedure designed to minimize MNP contamination. In addition to placenta samples, cord blood, maternal urine, and blood samples from these cohorts will be analyzed to comprehensively characterize MNP exposures. To assess the impact of MNP exposures on maternal and early-life health, we will leverage the longitudinal assessments and novel health outcome data from the 2 birth cohorts, including markers of placental function, immune-inflammatory responses, oxidative stress, accelerated biological aging (telomere length), birth outcomes, and childhood growth and development.
Furthermore, a new cohort will be established in the Netherlands to assess determinants of MNP exposure, specifically among women of reproductive age (18-45 years). We will include 110 women living within 50 km of Utrecht, the Netherlands. Blood, urine, and household dust samples will be collected at baseline and after 3 months. In addition, a self-administered online questionnaire, taking approximately 15 minutes to complete, will be used to identify factors contributing to MNP exposure. This questionnaire will gather sociodemographic information, home environment characteristics, food consumption and preparation habits, as well as lifestyle and behavior patterns.
Quality Assurance and Quality Control
One of the considerations taken across the project is minimizing both primary MNP contamination during sample collection and secondary MNP contamination during processing and analysis with quality assurance and quality control (QA/QC) measures [41]. Plastic is commonly used for collecting biological samples and has many applications in laboratory settings. We use glass sample collection materials to collect de novo blood, cord blood, and urine samples and store placenta samples in aluminum foil. Cohort samples collected with plastic materials prior to the AURORA project will be compared to samples from the de novo collection to understand background contamination, and empty collection tubes will be analyzed to determine background MNP levels. In laboratory spaces, plastic is avoided and removed where possible, and work is done under a laminar flow hood. We implement QA/QC measures including field and procedural blanks at multiple stages of collection and analysis to account for any remaining background contamination. Quality control samples are prepared by spiking samples with known MNP concentrations to monitor the accuracy and performance of the method, ensuring the quality and reliability of the data across batches.
In-Depth Characterization of MNPs
The development of an analytical framework for the in-depth characterization of MNPs in maternal and fetal tissues is fundamental to providing measures of the particle characteristics potentially driving toxicological and health effects [9]. Key characteristics include particle quantity (mass and particle count), morphology (size, shape), chemical composition, surface chemistry, and state of degradation [19]. Low-throughput nondestructive particle-based approaches will be used for in-depth characterization.
Microscopic techniques are established for detecting and characterizing MNPs greater than ~1 μm in diameter [20]. However, additional advancements are required for the detection of NPs [20,21]. Thus, we will first focus on developing and applying innovative spectro-microscopic techniques, such as atomic force microscopy, confocal fluorescence microscopy, and scanning electron microscopy to characterize NPs [42,43]. To characterize MPs, we will leverage additional microscopy methods including Raman microscopy and infrared spectroscopy [44]. These methods will be optimized and validated using simple matrices (eg, salt and fresh water) to ensure the highest sensitivity before analyzing tissue samples.
Adding to the complexity of MNPs is that environmental MNPs undergo degradation, which makes understanding characteristics of MNPs at various stages of degradation essential for the identification of MNPs in complex matrices [45]. A vibrational spectroscopic library of MNPs from controlled degradation experiments will be established to facilitate identification of MNP type, regardless of MNP condition and state of degradation. Due to the complex nature of maternal and fetal tissues, sufficient sample digestion, filtration, and preconcentration is required to remove biological interferences prior to microspectroscopic analysis and imaging [46]. Tissue sample processing methods will be optimized to maximize sensitivity and minimize alteration of MNPs.
The spectroscopic methods for in-depth characterization of MNPs will be developed and validated with commercial polystyrene (PS) spheres. However, the advancement of toxicological models requires the availability of well-defined, comparable, and representative MNPs that span a diverse range of polymers, sizes, and shapes [47]. Synthesis of suitable MNPs is necessary due to the limited commercial availability of MNPs, which currently predominately limits research to PS spheres [48]. The synthesis of MNPs via nanoprecipitation for the most common polymer types, both with and without fluorescent tagging for detection with fluorescence microscopy, will be performed to support the development and testing of the toxicological models. These fluorescent tags are designed not to leach, ensuring they remain nontoxic.
Scalable Exposure Assessment
The AURORA project will develop and apply a high-throughput analytical workflow to quantify MNPs in human tissues. High throughput, robust, and quantitative methods are essential for human biomonitoring and to conduct informative epidemiological studies [49,50]. We will use this approach to assess the mass concentration of MNPs and associated chemicals in maternal blood, urine, placenta, and cord blood. These metrics are chosen because they are useful for biomonitoring studies (maternal urine, blood) and suitable for early-life exposure assessment (birth cohorts: placenta and cord blood).
Our approach (termed Fast, Single, Tissue Extraction for Multiplexed Plastic Analysis: FaSTE-MPA) combines three HRMS platforms to enable systematic characterization of MNPs in complex matrices: (1) Py-GC/HRMS to characterize MNP levels and cotransported volatiles and semivolatiles, (2) alkaline-assisted hydrolysis with liquid chromatography HRMS (AAH-HRMS) to measure particle monomers and additives, and (3) untargeted liquid chromatography with HRMS (LC-HRMS) to characterize the metabolome for the presence of MNP constituents, additives, nonintentionally added substances, and biological response profiles [51,52]. Analysis by Py-GC/HRMS will provide mass concentration estimates of common plastic polymers including PS, polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyamide (PA), polyethylene (PE), polypropylene (PP), polymethyl methacrylate (PMMA), and polycarbonate, as well as screen for other MNPs. Additionally, AAH-HRMS and LC-HRMS provide complementary information about both free and bounded plastic polymers and additives, such as phthalates. We will develop a comprehensive library containing the Py-GC/HRMS fingerprints of common MNP polymers and associated plastic additives. In this manner, maternal and prenatal MNP exposure and biological responses will be measured in 800 paired placenta and cord blood samples from the birth cohorts. Further, an in-depth investigation into the maternal-fetal transfer of MNPs from maternal blood to placenta to cord blood will be conducted in a subgroup of mother-child pairs, aiming to provide insight into kinetics and transfer efficiencies. Spatial distribution and accumulation of MNPs within the placenta will be assessed in a subset of placentas.
Despite the potential applications of Py-GC/HRMS for assessing MNPs, targeted Py-GC/HRMS is currently not routinely available in analytical laboratories designated for human samples. Furthermore, required sample volumes (currently >1 mL) may preclude its use in the analyses of precious biobanked samples. To identify the top chemical signals predicting MNP exposure, we will leverage untargeted HRMS data to build classification models for biomarkers exhibiting the highest sensitivity and selectivity for MNP exposure. These biomarkers will be used to establish a quantitative, targeted method that can be implemented in human biomonitoring studies (eg, Human Biomonitoring for Europe) and large-scale epidemiological studies. Results from these population-based studies are critical inputs for future etiological research and regulatory assessment of MNP exposure and health effects [10].
Hazard Assessment: Experimental Toxicology
Despite evidence suggesting that MNPs can cross the placental barrier, placental toxicokinetics and toxicity of MNPs remain largely unexplored [33,53-55]. Knowledge gaps in human placental transfer and toxicity of MNPs hinder comprehensive hazard characterization for early-life exposure [34]. We will apply a series of toxicological models for toxicokinetic and effect studies, focusing on the placental barrier and assessing the effects of MNPs on the placenta and the developing organism. Placenta is a morphologically and functionally complex organ; therefore, placental models with increasing level of complexity, that is, monolayers, cocultures, and placental perfusion, will be used to address the complex interplay between different placental cell types [34].
Considering the multitude of MNP characteristics, toxicity testing will be done in a tiered approach, using MNPs generated commercially and within AURORA (Figure 2). Many MNPs will be tested in the simple models, and based on uptake, transport and toxicity will be prioritized for further testing in more complex models. In Tier 1, toxicity end points (eg, cell viability, damage to the cell membrane, and oxidative stress) as well as uptake and transport of MNPs will be investigated in nonsyncytialized and syncytialized human choriocarcinoma cells (BeWo b30), representative of cytotrophoblasts and syncytiotrophoblasts, respectively [56]. In Tier 2, MNPs will be investigated with additional monolayer cell cultures, including the human adenocarcinoma cell line (H295R) and human trophoblast stem cells, as well as more complex coculture cellular models such as BeWo/Human umbilical vein endothelial cells (HUVEC) and BeWo/HUVEC/H295R triculture [57-59]. Tiers 1 and 2 will provide insight into a wide array of effects on placental integrity and function, immune response, endocrine functions, and other pathway perturbations. We will also perform mRNA sequencing and metabolomic analyses in selected samples to investigate system homeostasis.
Figure 2.
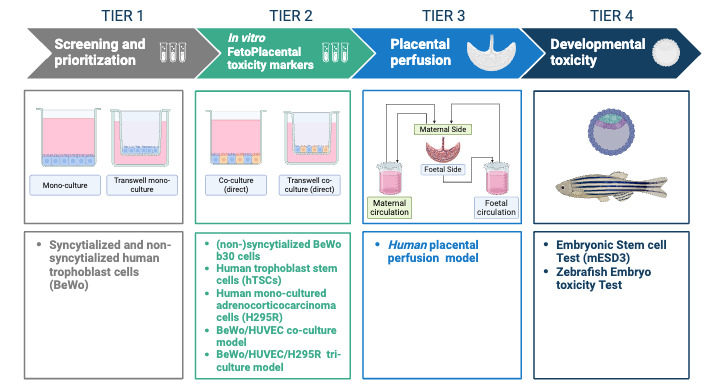
Tiered approach to investigate transport, toxicokinetics and toxicity of MNPs (micro- and nanoplastics) in toxicological models with varying complexity, including in vitro placental models, human placental models, and developmental toxicity tests. HUVEC: human umbilical vein endothelial cells. Image created with BioRender.com [37].
Further investigations will focus on selected MNPs in a human placental perfusion model (Tier 3), the embryonic stem cell test with mouse embryonic stem cells (mES-D3; Tier 4), and the zebrafish embryo toxicity model (Tier 4). Transplacental transfer and toxicity markers will be assessed upon exposure to selected MNPs in the human placental perfusion model [60].MNP concentrations in perfusion media and tissue will be analyzed by Py-GC/HRMS and LC-HRMS analysis. Untargeted LC-HRMS and biological pathway analyses will be performed on the embryonic stem cell test and zebrafish embryos exposed to MNPs [61]. Placental and developmental toxicity markers upon MNP exposure will be compared with the health outcome assessments in the epidemiological studies.
Epidemiological Studies
In epidemiological studies, we will evaluate the impact of MNPs and other plastic-related chemicals on multiple end points: (1) placental function; (2) system homeostasis; and (3) early-life growth and development (Figure 3). MNP exposure will be evaluated in 800 placenta samples from the ENVIRONAGE and BiSC cohorts. To examine the impact of (prenatal) MNP exposures on health, we will exploit the cohorts’ longitudinal health assessments to generate novel data on exposure-health associations. The children currently span from new-born to 14 years of age.
Figure 3.
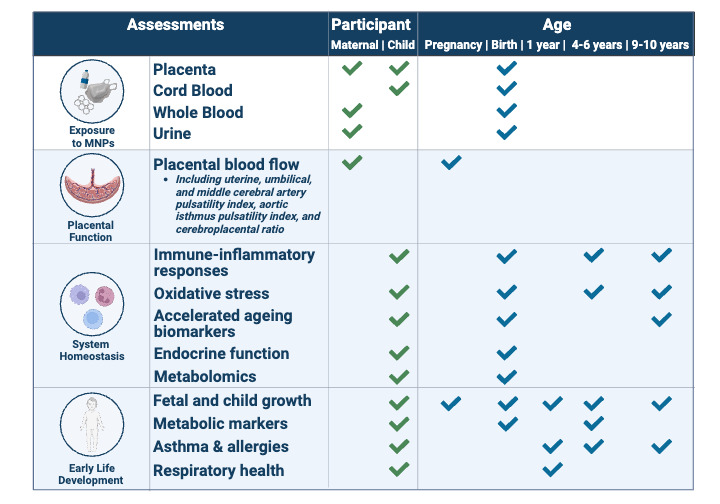
Overview of MNP (micro- and nanoplastic) exposure assessment and health outcomes to be evaluated in epidemiological studies in AURORA (Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics). Image created with BioRender.com [37].
When applicable, we will pool data from the 2 birth cohorts. We will use single-exposure-outcome regression modeling adjusted for covariates, as well as complementary exposure-wide modeling for MNPs and MNP-associated chemicals, including multivariable variable selection and machine learning approaches [62-64]. In addition, we will screen for associations between MNPs and metabolomic analytes to identify the overall biological alterations associated with MNP exposure and generate novel hypotheses on potential effects of MNPs [65,66]. Moreover, mediation analyses will be performed to evaluate system homeostasis markers, using models that accommodate multiple and high dimensional mediators [67,68].
Given that MNPs are a continuous exposure variable, the minimal sample size required to detect an effect for dichotomized outcomes depends on the prevalence of the outcome. The primary dichotomized outcomes to be examined are asthma and allergy, both prevalent in 5%-15% European pediatric populations [69-71]. Due to the absence of prior knowledge on the effect size of MNPs, we referred to a study which reported an increased risk of asthma (odds ratio [OR] 1.6-3.9) in Canadian children exposed to high phthalate concentrations in house dust [72]. Assuming a baseline outcome prevalence of 5%, an OR of 2, and a type I error of 0.05, the minimal statistical power obtained with 800 participants would be 0.77, which is deemed satisfactory. The statistical power for continuous outcomes is higher given the same sample size.
Identifying the contributing factors to MNP exposure levels provides insight into how MNP exposure may influence early-life health. Therefore, we will investigate determinants of MNP exposure, including maternal food consumption, sociodemographic and other lifestyle factors in the birth cohorts. The Dutch MNP exposure cohort, consisting of 110 women of reproductive age in the Netherlands, will allow for an in-depth assessment of the determinants of MNP exposure by combining levels of MNPs in blood, urine, and household dust samples, with a questionnaire investigating MNP exposure sources. We will assess the relative contribution of food packaging, food preparation methods, and indoor sources such as furnishing and frequency of cleaning to MNP and plastic-associated chemical body burdens.
Risk Assessment
Risk assessment of MNPs is challenging, because MNPs constitute a very broad class of substances with diverse physiochemical properties, making it difficult to apply standard regulatory risk assessment approaches [12,73,74]. Evaluation of the exposure and hazard accounting for both the polymers and MNP-associated chemicals (ie, mixture effects) is essential [11]. Risk assessment of MNPs is also constrained by limited availability of reference materials, analytical challenges, and insufficient information about key characteristics of MNPs [11]. Further, hazard characterization is currently limited by lack of information about the accumulation, persistence, and kinetics of inhaled and ingested MNPs [10]. To understand early-life effects, risk assessment must consider the exposure rates for both the mother and the fetus.
We will build a framework for performing human risk assessment for MNPs with a focus on direct risk to the fetus and maternal-mediated risk, including via the placenta (Figure 4). A systematic evidence mapping approach will be applied to evaluate relevant literature from organizations such as the World Health Organization, European Food Safety Authority, Organization for Economic Co-operation and Development, National Institute for Occupational Safety and Health, and the published scientific literature, and critically evaluate the available regulatory tools for their relevance and adequacy for assessing the risks of MNPs. The framework we develop will integrate the project’s updated methodologies and tools for assessing MNP exposures and risks from toxicological and epidemiological studies. Ultimately, we will determine the requirements to carry out a comprehensive risk assessment of MNPs and develop recommendations for advancing risk assessment for MNPs, focusing on early-life health.
Figure 4.
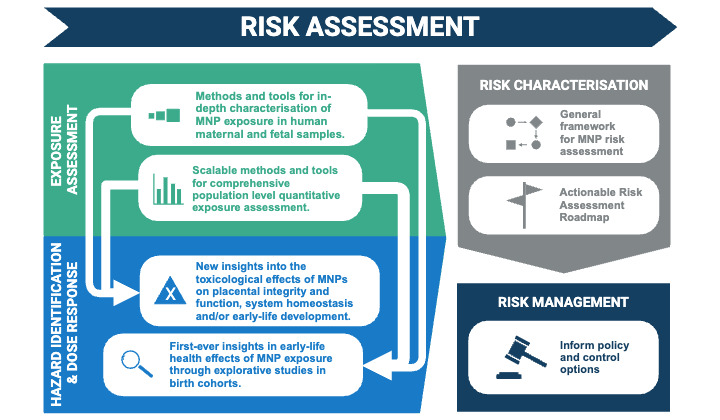
The anticipated output of the AURORA (Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics) project, which will contribute to the development of a human health risk assessment framework for evaluating the risks associated with MNP (micro- and nanoplastic) exposure in early life. Image created with BioRender.com [37].
Ethical Considerations
The research activities of AURORA comply with international conventions and ethical codes, including the Declaration of Helsinki (2013) and the Declaration of Taipei (2016). Local ethics committees will support participating research groups to address all ethics requirements. Measures for personal data protection and confidentiality comply with the European Union General Data Protection Regulation (2016/679) and the FAIR (findable, accessible, interoperable, reusable) Data Principles [75].
The toxicological studies adhere to the 3R principles emphasizing nonanimal, in vitro cell, or alternative animal models [76]. Zebrafish fall outside the definition of animal models, eliminating the need for a specific ethical permit [77]. Human ex vivo placental perfusions with MNPs received ethics committee approval from the Research Ethics Committee of Hospital District of Northern Savo (952/2022). The ENVIRONAGE and BiSC cohorts are approved by the Ethics Committee of Hasselt University and East-Limburg Hospital (EudraCT B37120107805) and Comite de Etica de la Investigation Parc de salut Mar (No. 2018/8050/I), respectively. The Dutch MNP exposure cohort received ethics approval from the Medical Research Ethics Committee NedMec (NL81071.041.22). Adult participants in all cohorts provided informed consent for themselves and where applicable, for their children. An incidental finding policy is in place for all cohorts. All participants can withdraw from the study at any time, for any reason and without any consequences. Participants in the birth cohorts receive no compensation, whereas participants in the Dutch MNP exposure cohort receive a €25 (as of July 12, 2023 1 EUR=US $1.1135) gift card upon completion of the study.
Results
In the first 3 years of this 5-year project (2021-2026), the following progress has been made. As of September 2024, data analysis has begun and results are expected to be published starting in 2025.
In-Depth Characterization
We established a database consisting of infrared spectroscopy data measured on various polymers treated with different conditions; created a sample preprocessing workflow for measuring MNPs in complex human sample matrices, notably placenta; assessed the gaps in the current field of NP synthesis and fluorescent detection, synthesized NPs for 7 polymers including PVC, PS, PET, PA, PP, PMMA, and low-density PE using 2 fluorescent dyes; and summarized microspectroscopic techniques for measuring NPs [20].
Scalable Exposure Assessment
We advanced critical operational procedures for scalable biomonitoring of MNPs in placenta tissue and blood, including developing high-throughput and robust sample preparation based upon microwave-assisted extraction. We developed a comprehensive MNPs library containing the Py-GC/HRMS signal fingerprints of 39 common polymers, alongside relevant information on their associated plastic additives. Sample analysis for the characterization of maternal and fetal MNP exposure and biological response in the BiSC and ENVIRONAGE cohorts and spatial analysis of whole placentas is ongoing.
Toxicological Hazard Assessment Studies
We reviewed the models for assessing placental uptake, transport, and toxicity of MNPs [34] and optimized the BeWo/HUVEC coculture model, and the BeWo/HUVEC/H295R triculture. The Tier 3 placenta perfusion model and the Tier 4 zebrafish and mESD3 models have also been optimized. Screening and prioritization of MNPs in Tier 1 and Tier 2 are ongoing, and Tier 4 experiments with zebrafish are in progress.
Epidemiological Studies
We recruited 110 participants for the Dutch MNP exposure cohort and collected blood, urine, and household dust samples. Sample collection from the birth cohorts for the epidemiological studies is complete; biobanked samples were selected from ENVIRONAGE, and de novo samples were collected from BiSC. Sample collection is complete for the spatial distribution study of MNPs in the placenta and ongoing for the study on the maternal-fetal transfer of MNPs. Follow-up of the birth cohorts is ongoing, and epidemiological analyses will proceed when MNP exposure estimates are available.
Risk Assessment Framework
We completed the systematic evidence mapping and initial roadmap addressing risk assessment for MNPs in early life, highlighting the current challenges and limitations [78]. This will be updated when results from AURORA-associated studies are completed, and with emerging external research and gaps in knowledge and future research needs will be identified.
Discussion
Principal Findings
The AURORA project brings together experts with diverse knowledge, from producing plastics and measuring plastics and associated chemicals, to quantifying the biological impacts of nanoparticles and chemicals on reproductive health and development in vitro, in vivo, and in humans, as well as experts in risk assessment and risk communication to assess exposures to and health effects of MNPs during the critical period of early-life development. Advancements in detection, quantification, and characterization of MNPs are crucial for understanding human exposure to MNPs. AURORA will develop complementary low-throughput nondestructive particle-based approaches for characterization of MNP properties and high-throughput Py-GC/HRMS mass-based measurements for scalable exposure assessment. Toxicological testing will provide foundational insight into the toxicity of a diverse range of MNPs in test systems with varying complexity. The epidemiological investigations will provide the first extensive evaluation of maternal and prenatal MNP exposures. While developing and applying the tools and methodological workflows, a risk assessment framework specific to MNPs will be established.
MNPs have recently been detected in human placenta tissue, meconium, amniotic fluid, and breastmilk, and cumulative evidence from aquatic species indicates reproductive effects of MNPs [18,22,30,32,79]. In mice, maternal exposure to microplastics has been demonstrated to induce placental dysfunction and result in metabolic disorders in both the placenta and fetus. [80,81]. The diverse in vitro and in vivo human placental models in AURORA show potential for more comprehensively assessing the potential hazards of MNPs in utero. Currently, research investigating the potential risk MNPs may pose to early-life health in mammals remains limited [18,22,30,32,79]. In recent small-scale human studies, an inverse association between MNPs in amniotic fluid and gestational age was found, and placental MNPs were linked to intrauterine growth restriction [82,83]. Our results will enrich the understanding of MNP exposure in human tissues and provide insight for the first time about health effects of MNPs during early life. Understanding the exposure and hazard of MNPs in early life is a crucial first step toward determining whether public health actions are needed and informing the urgency of regulatory responses to MNPs. As the burden of plastic in the environment increases, more evidence about how MNPs affect human health is essential [4,84].
Limitations
Given the complex nature of MNPs, there are challenging methodological advancements that need to be made during the project to accurately measure and evaluate early-life exposure to MNPs. Preliminary MNP measurements in human tissues suggest that exposure assessment is feasible; however, advancements in certain steps are necessary to develop workflows tailored to complex human matrices and ensure the reliability of exposure estimates. Additionally, the development of suitable test materials and workflows for toxicity testing, including dispersion protocols, requires significant methodological advancements. Another challenge we encounter is adequately addressing MNP contamination, which we do through the implementation of extensive QA/QC measures.
Dissemination
The results of the study will be published in peer-reviewed journals and further disseminated, for example, through webinars, and scientific conferences. Analytical protocols will be made available to the research community whenever possible. We will also engage with stakeholders, including health care professionals, industry, civil society organizations, and policy makers, using a multichannel approach including the project website, newsletters, press releases, workshops, and social media. We will liaise with the European Commission and its Joint Research Centre to ensure that the findings are translated into policy. We will publish open access, including analytical scripts. Data and metadata will be stored on the eNanoMapper [85] data repository.
Future Directions
AURORA supports the European Strategy for Plastics in a Circular Economy [86] by contributing to advancements of analytical methods for assessing thousands of chemicals, including potential unknown contaminants in future biodegradable and compostable plastics. The tiered approach for testing the toxicological effects of MNPs builds a framework for assessing the potential health impacts of measures taken under European policy. Aligned with the European Bioeconomy Strategy [87], AURORA addresses food and nutrition security, sustainable resource management, reduced reliance on nonrenewables, climate change mitigation, and European competitiveness. By providing novel information on health and safety risks of MNPs, AURORA will inform the development of safer plastics and bioalternatives for a circular economy.
Along with the gaps in health knowledge, policy and regulatory gaps must be addressed for systemic changes to be made. Harmonization of measurement techniques, exposure metrics, and terminology is essential to facilitate understanding between scientific and regulatory communities. Best practice is reporting both mass and particle counts, as will be done in AURORA [88]. Initiatives like an MNP-reporting guidelines checklist is a good example of moving MNP research forward in a valid, reproducible, and comparable way [89]. The harmonization of these key components will allow the newly generated research to collectively contribute to informing policy and control measures.
As the research on MNP and health is in its infancy, we acknowledge that not all open questions will be answered within AURORA or the CUSP cluster [38]. Therefore, in addition to the novel methods and tools and general framework for risk assessment of MNPs, we will develop an actionable risk assessment framework for MNP exposure in early life where the remaining knowledge gaps and priorities needed for comprehensively evaluating the impact of MNPs on early-life health are identified.
Conclusions
As plastic pollution increases globally, it is imperative to understand the impact of MNPs on human health, particularly during vulnerable developmental stages such as early life. The contributions of the AURORA project are important for understanding how MNPs may influence health in early life. We will advance the research field by advancing characterization and quantification methods, providing novel toxicological and human health outcome data, and ultimately design a risk assessment framework to inform future MNP research.
Acknowledgments
We would like to acknowledge the following additional members of the AURORA consortium: Ioannis Basinas, Zoe Coates Fuentes, Yvette Christopher-de Vries, Anitha Devadoss, Isabel Goßmann, Bethany Knox, Petr Kukucka, Brooklynn McNeil, Ali Mohammed, Shahzad Rashid, Danielle Roordink, Martin Scheringer, Joel Scheuchzer, and Kirsi Vähäkangas.
The authors acknowledge the AURORA Scientific Advisory Board for their feedback on the research tasks in AURORA.
The authors attest that there was no use of generative artificial intelligence technology in the generation of text, figures, or other informational content of this manuscript. This research was supported by funding from the European Union’s Horizon 2020 research and innovation programme (grant 964827; AURORA project, coordinated by University Medical Center Utrecht). RECETOX was supported by the European Union’s Horizon 2020 research and innovation programme (grant 857560; CETOCOEN Excellence).
Abbreviations
- AAH-HRMS
alkaline-assisted hydrolysis with high resolution mass spectroscopy
- AURORA
Actionable eUropean ROadmap for early-life health Risk Assessment of micro- and nanoplastics
- BeWo b30
human choriocarcinoma cell line
- BiSC
Barcelona Life Study Cohort
- ENVIRONAGE
ENVIRonmental influence ON early AGEing
- FAIR data
findable, accessible, interoperable, reusable data
- FaSTE-MPA
Fast, Single, Tissue Extraction for Multiplexed Plastic Analysis
- H295R
human adenocarcinoma cell line
- HUVEC
human umbilical vein endothelial cells
- LC-HRMS
liquid chromatography with high resolution mass spectroscopy
- mES-D3
mouse embryonic stem cell line
- MNP
micro- and nanoplastic
- MP
microplastic
- NP
nanoplastic
- OR
odds ratio
- PA
polyamide
- PE
polyethylene
- PET
polyethylene terephthalate
- PMMA
polymethyl methacrylate
- PP
polypropylene
- PS
polystyrene
- PVC
polyvinyl chloride
- Py-GC/HRMS
pyrolysis gas chromatography high-resolution mass spectroscopy
- QA/QC
quality assurance and quality control
Peer review.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during this study. Data from this project will be shared in future publications and data repositories including eNanoMapper.
Footnotes
Authors' Contributions: JMB, HMD, MvD, MMF, JL, VL, FM, JM, TSN, PP, BMS-B., RV, MV, and DIW contributed to the conceptualization of the AURORA Horizon 2020 project application. MSPB, JvB, MB, EAC, AMD, PD, KSG, LDBM, ARR, NDS, RZ, and LZ played an important role in further developing the methodology once the project started. AMD and VL drafted the manuscript. All authors have read, revised, and agreed to the published version of the manuscript.
Conflicts of Interest: None declared.
References
- 1.Plastics Europe. Plastics - The Facts 2023. 2023. [2024-08-14]. https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/
- 2.Lau WWY, Shiran Y, Bailey RM, Cook E, Stuchtey MR, Koskella J, Velis CA, Godfrey L, Boucher J, Murphy MB, Thompson RC, Jankowska E, Castillo Castillo A, Pilditch TD, Dixon B, Koerselman L, Kosior E, Favoino E, Gutberlet J, Baulch S, Atreya ME, Fischer D, He KK, Petit MM, Sumaila UR, Neil E, Bernhofen MV, Lawrence K, Palardy JE. Evaluating scenarios toward zero plastic pollution. Science. 2020;369(6510):1455–1461. doi: 10.1126/science.aba9475. https://eprints.whiterose.ac.uk/166149/ science.aba9475 [DOI] [PubMed] [Google Scholar]
- 3.MacLeod M, Arp HPH, Tekman MB, Jahnke A. The global threat from plastic pollution. Science. 2021;373(6550):61–65. doi: 10.1126/science.abg5433.373/6550/61 [DOI] [PubMed] [Google Scholar]
- 4.Geyer R. Production, use, and fate of synthetic polymers. In: Letcher TM, editor. Plastic Waste and Recycling. California: Academic Press; 2020. pp. 13–32. [Google Scholar]
- 5.DeWit W, Burns ET, Guinchard JC, Ahmed N. Plastics: The Costs to Society, the Environment and the Economy. Gland, Switzerland: World Wide Fund for Nature; 2021. [2024-08-14]. https://media.wwf.no/assets/attachments/Plastics-the-cost-to-so ciety-the-environment-and-the-economy-WWF-report.pdf . [Google Scholar]
- 6.Arp HPH, Kühnel D, Rummel C, MacLeod M, Potthoff A, Reichelt S, Rojo-Nieto E, Schmitt-Jansen M, Sonnenberg J, Toorman E, Jahnke A. Weathering plastics as a planetary boundary threat: exposure, fate, and hazards. Environ Sci Technol. 2021;55(11):7246–7255. doi: 10.1021/acs.est.1c01512. doi: 10.1021/acs.est.1c01512. [DOI] [PubMed] [Google Scholar]
- 7.Sewwandi M, Wijesekara H, Rajapaksha AU, Soysa S, Vithanage M. Microplastics and plastics-associated contaminants in food and beverages; global trends, concentrations, and human exposure. Environmental Pollution. 2023;317:120747. doi: 10.1016/j.envpol.2022.120747.S0269-7491(22)01961-3 [DOI] [PubMed] [Google Scholar]
- 8.Zarus GM, Muianga C, Hunter CM, Pappas RS. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Science of the Total Environment. 2021;756:144010. doi: 10.1016/j.scitotenv.2020.144010. https://europepmc.org/abstract/MED/33310215 .S0048-9697(20)37541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371(6530):672–674. doi: 10.1126/science.abe5041.371/6530/672 [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization. Geneva: World Health Organization; 2022. Sep, [2024-08-14]. Dietary and inhalation exposure to nano- and microplastic particles and potential implications for human health. https://www.who.int/publications/i/item/9789240054608 . [Google Scholar]
- 11.Brachner A, Fragouli D, Duarte IF, Farias PMA, Dembski S, Ghosh M, Barisic I, Zdzieblo D, Vanoirbeek J, Schwabl P, Neuhaus W. Assessment of human health risks posed by nano-and microplastics is currently not feasible. Int J Environ Res Public Health. 2020;17(23):8832. doi: 10.3390/ijerph17238832. https://www.mdpi.com/resolver?pii=ijerph17238832 .ijerph17238832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noventa S, Boyles MSP, Seifert A, Belluco S, Jiménez AS, Johnston HJ, Tran L, Fernandes TF, Mughini-Gras L, Orsini M, Corami F, Castro K, Mutinelli F, Boldrin M, Puntes V, Sotoudeh M, Mascarello G, Tiozzo B, McLean P, Ronchi F, Booth AM, Koelmans AA, Losasso C. Paradigms to assess the human health risks of nano- and microplastics. Micropl & Nanopl. 2021;1(1):9. doi: 10.1186/s43591-021-00011-1. [DOI] [Google Scholar]
- 13.Groh KJ, Backhaus T, Carney-Almroth B, Geueke B, Inostroza PA, Lennquist A, Leslie HA, Maffini M, Slunge D, Trasande L, Warhurst AM, Muncke J. Overview of known plastic packaging-associated chemicals and their hazards. Sci Total Environ. 2019;651(Pt 2):3253–3268. doi: 10.1016/j.scitotenv.2018.10.015. https://linkinghub.elsevier.com/retrieve/pii/S0048-9697(18)33882-8 .S0048-9697(18)33882-8 [DOI] [PubMed] [Google Scholar]
- 14.Wiesinger H, Wang Z, Hellweg S. Deep dive into plastic monomers, additives, and processing aids. Environ Sci Technol. 2021;55(13):9339–9351. doi: 10.1021/acs.est.1c00976. doi: 10.1021/acs.est.1c00976. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Chen Z, Wei W, Chen J, Ni BJ. Toxicity of micro/nanoplastics in the environment: roles of plastisphere and eco-corona. Soil & Environmental Health. 2023;1(1):100002. doi: 10.1016/j.seh.2023.100002. [DOI] [Google Scholar]
- 16.De Boever S, Devisscher L, Vinken M. Unraveling the micro- and nanoplastic predicament: a human-centric insight. Sci Total Environ. 2024;916:170262. doi: 10.1016/j.scitotenv.2024.170262.S0048-9697(24)00397-8 [DOI] [PubMed] [Google Scholar]
- 17.Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199. https://linkinghub.elsevier.com/retrieve/pii/S0160-4120(22)00125-8 .S0160-4120(22)00125-8 [DOI] [PubMed] [Google Scholar]
- 18.Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi S, D'Amore E, Rinaldo D, Matta M, Giorgini E. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. https://linkinghub.elsevier.com/retrieve/pii/S0160-4120(20)32229-7 .S0160-4120(20)32229-7 [DOI] [PubMed] [Google Scholar]
- 19.Huber MJ, Ivleva NP, Booth AM, Beer I, Bianchi I, Drexel R, Geiss O, Mehn D, Meier F, Molska A, Parot J, Sørensen L, Vella G, Prina-Mello A, Vogel R, Caputo F. Physicochemical characterization and quantification of nanoplastics: applicability, limitations and complementarity of batch and fractionation methods. Anal Bioanal Chem. 2023;415(15):3007–3031. doi: 10.1007/s00216-023-04689-5. https://europepmc.org/abstract/MED/37106123 .10.1007/s00216-023-04689-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandemaker LDB, Meirer F. Spectro-microscopic techniques for studying nanoplastics in the environment and in organisms. Angew Chem Int Ed Engl. 2023;62(2):e202210494. doi: 10.1002/anie.202210494. https://europepmc.org/abstract/MED/36278811 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivleva NP. Chemical analysis of microplastics and nanoplastics: challenges, advanced methods, and perspectives. Chem Rev. 2021;121(19):11886–11936. doi: 10.1021/acs.chemrev.1c00178. doi: 10.1021/acs.chemrev.1c00178. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y, Chen B, Dong R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci Total Environ. 2023;854:158699. doi: 10.1016/j.scitotenv.2022.158699.S0048-9697(22)05798-9 [DOI] [PubMed] [Google Scholar]
- 23.Provencher JF, Covernton GA, Moore RC, Horn DA, Conkle JL, Lusher AL. Proceed with caution: the need to raise the publication bar for microplastics research. Sci Total Environ. 2020;748:141426. doi: 10.1016/j.scitotenv.2020.141426.S0048-9697(20)34955-X [DOI] [PubMed] [Google Scholar]
- 24.Lite C, Raja GL, Juliet M, Sridhar VV, Subhashree KD, Kumar P, Chakraborty P, Arockiaraj J. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ Toxicol Pharmacol. 2022;89:103779. doi: 10.1016/j.etap.2021.103779.S1382-6689(21)00197-6 [DOI] [PubMed] [Google Scholar]
- 25.Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2796.2007.01809.x .JIM1809 [DOI] [PubMed] [Google Scholar]
- 26.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5-6):397–407. doi: 10.1016/j.thromres.2004.06.038.S0049-3848(04)00342-1 [DOI] [PubMed] [Google Scholar]
- 27.Bové H, Bongaerts E, Slenders E, Bijnens EM, Saenen ND, Gyselaers W, Van Eyken P, Plusquin M, Roeffaers MBJ, Ameloot M, Nawrot TS. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10(1):3866. doi: 10.1038/s41467-019-11654-3. doi: 10.1038/s41467-019-11654-3.10.1038/s41467-019-11654-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aengenheister L, Favaro RR, Morales-Prieto DM, Furer LA, Gruber M, Wadsack C, Markert UR, Buerki-Thurnherr T. Research on nanoparticles in human perfused placenta: state of the art and perspectives. Placenta. 2021;104:199–207. doi: 10.1016/j.placenta.2020.12.014. https://linkinghub.elsevier.com/retrieve/pii/S0143-4004(20)30467-7 .S0143-4004(20)30467-7 [DOI] [PubMed] [Google Scholar]
- 29.Medley EA, Spratlen MJ, Yan B, Herbstman JB, Deyssenroth MA. A systematic review of the placental translocation of micro- and nanoplastics. Curr Environ Health Rep. 2023;10(2):99–111. doi: 10.1007/s40572-023-00391-x. https://europepmc.org/abstract/MED/36848019 .10.1007/s40572-023-00391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun T, Ehrlich L, Henrich W, Koeppel S, Lomako I, Schwabl P, Liebmann B. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics. 2021;13(7):921. doi: 10.3390/pharmaceutics13070921. https://www.mdpi.com/resolver?pii=pharmaceutics13070921 .pharmaceutics13070921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C, Zucchelli E, De Luca C, D'Avino S, Gulotta A, Carnevali O, Giorgini E. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers (Basel) 2022;14(13):2700. doi: 10.3390/polym14132700. https://www.mdpi.com/resolver?pii=polym14132700 .polym14132700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halfar J, Čabanová K, Vávra K, Delongová P, Motyka O, Špaček R, Kukutschová J, Šimetka O, Heviánková S. Microplastics and additives in patients with preterm birth: the first evidence of their presence in both human amniotic fluid and placenta. Chemosphere. 2023;343:140301. doi: 10.1016/j.chemosphere.2023.140301. https://linkinghub.elsevier.com/retrieve/pii/S0045-6535(23)02571-7 .S0045-6535(23)02571-7 [DOI] [PubMed] [Google Scholar]
- 33.Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, Diener P, Zisch A, Krug HF, von Mandach U. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect. 2010;118(3):432–436. doi: 10.1289/ehp.0901200. https://ehp.niehs.nih.gov/doi/10.1289/ehp.0901200?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dusza HM, van Boxel J, van Duursen MB, Forsberg MM, Legler J, Vähäkangas KH. Experimental human placental models for studying uptake, transport and toxicity of micro- and nanoplastics. Sci Total Environ. 2023;860:160403. doi: 10.1016/j.scitotenv.2022.160403. https://linkinghub.elsevier.com/retrieve/pii/S0048-9697(22)07505-2 .S0048-9697(22)07505-2 [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wang L, Trasande L, Kannan K. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ Sci Technol Lett. 2021;8(11):989–994. doi: 10.1021/acs.estlett.1c00559. [DOI] [Google Scholar]
- 36.AURORA Researching Early Life Health Impacts of Micro- and Nanoplastics. AURORA Research. 2024. [2024-08-14]. https://auroraresearch.eu/
- 37.BioRender. [2024-10-01]. https://www.biorender.com/
- 38.CUSP research. CUSP. 2023. [2024-01-30]. https://cusp-research.eu/
- 39.Janssen BG, Madhloum N, Gyselaers W, Bijnens E, Clemente DB, Cox B, Hogervorst J, Luyten L, Martens DS, Peusens M, Plusquin M, Provost EB, Roels HA, Saenen ND, Tsamou M, Vriens A, Winckelmans E, Vrijens K, Nawrot TS. Cohort Profile: the ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol. 2017;46(5):1386–1387. doi: 10.1093/ije/dyw269.dyw269 [DOI] [PubMed] [Google Scholar]
- 40.Dadvand P, Gascon M, Bustamante M, Rivas I, Foraster M, Basagaña X, Cosín M, Eixarch E, Ferrer M, Gratacós E, Gómez Herrera L, Jimenez-Arenas P, Júlvez J, Morillas À, Nieuwenhuijsen MJ, Persavento C, Pujol J, Querol X, Sánchez García O, Vrijheid M, Llurba E, Gómez-Roig MD, Sunyer J, BiSC Group Cohort profile: Barcelona life study cohort (BiSC) Int J Epidemiol. 2024;53(3):dyae063. doi: 10.1093/ije/dyae063.7667946 [DOI] [PubMed] [Google Scholar]
- 41.Brander SM, Renick VC, Foley MM, Steele C, Woo M, Lusher A, Carr S, Helm P, Box C, Cherniak S, Andrews RC, Rochman CM. Sampling and quality assurance and quality control: a guide for scientists investigating the occurrence of microplastics across matrices. Appl Spectrosc. 2020;74(9):1099–1125. doi: 10.1177/0003702820945713. [DOI] [PubMed] [Google Scholar]
- 42.Bové H, Steuwe C, Fron E, Slenders E, D'Haen Jan, Fujita Y, Uji-I H, vandeVen M, Roeffaers M, Ameloot M. Biocompatible label-free detection of carbon black particles by femtosecond pulsed laser microscopy. Nano Lett. 2016;16(5):3173–3178. doi: 10.1021/acs.nanolett.6b00502. doi: 10.1021/acs.nanolett.6b00502. [DOI] [PubMed] [Google Scholar]
- 43.Sifat AA, Jahng J, Potma EO. Photo-induced force microscopy (PiFM) - principles and implementations. Chem Soc Rev. 2022;51(11):4208–4222. doi: 10.1039/d2cs00052k. [DOI] [PubMed] [Google Scholar]
- 44.Prata JC, da Costa JP, Duarte AC, Rocha-Santos T. Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends in Analytical Chemistry. 2019;110:150–159. doi: 10.1016/j.trac.2018.10.029. [DOI] [Google Scholar]
- 45.Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JK, Wu C, Lam PK. Understanding plastic degradation and microplastic formation in the environment: a review. Environ Pollut. 2021;274:116554. doi: 10.1016/j.envpol.2021.116554.S0269-7491(21)00132-9 [DOI] [PubMed] [Google Scholar]
- 46.Renner G, Schmidt TC, Schram J. Analytical methodologies for monitoring micro(nano)plastics: which are fit for purpose? Current Opinion in Environmental Science & Health. 2018;1:55–61. doi: 10.1016/j.coesh.2017.11.001. [DOI] [Google Scholar]
- 47.Parker LA, Höppener EM, van Amelrooij EF, Henke S, Kooter IM, Grigoriadi K, Nooijens MGA, Brunner AM, Boersma A. Protocol for the production of micro- and nanoplastic test materials. Micropl & Nanopl. 2023;3(1):10. doi: 10.1186/s43591-023-00058-2. [DOI] [Google Scholar]
- 48.Rozman U, Kalčíková G. Seeking for a perfect (non-spherical) microplastic particle - the most comprehensive review on microplastic laboratory research. J Hazard Mater. 2022;424(Pt C):127529. doi: 10.1016/j.jhazmat.2021.127529. https://linkinghub.elsevier.com/retrieve/pii/S0304-3894(21)02497-3 .S0304-3894(21)02497-3 [DOI] [PubMed] [Google Scholar]
- 49.Andra SS, Austin C, Patel D, Dolios G, Awawda M, Arora M. Trends in the application of high-resolution mass spectrometry for human biomonitoring: an analytical primer to studying the environmental chemical space of the human exposome. Environ Int. 2017;100:32–61. doi: 10.1016/j.envint.2016.11.026. https://europepmc.org/abstract/MED/28062070 .S0160-4120(16)30902-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker DI, Valvi D, Rothman N, Lan Q, Miller GW, Jones DP. The metabolome: a key measure for exposome research in epidemiology. Curr Epidemiol Rep. 2019;6:93–103. https://europepmc.org/abstract/MED/31828002 . [PMC free article] [PubMed] [Google Scholar]
- 51.Hermabessiere L, Himber C, Boricaud B, Kazour M, Amara R, Cassone AL, Laurentie M, Paul-Pont I, Soudant P, Dehaut A, Duflos G. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal Bioanal Chem. 2018;410(25):6663–6676. doi: 10.1007/s00216-018-1279-0.10.1007/s00216-018-1279-0 [DOI] [PubMed] [Google Scholar]
- 52.Fischer M, Scholz-Böttcher BM. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry. Environ Sci Technol. 2017;51(9):5052–5060. doi: 10.1021/acs.est.6b06362. [DOI] [PubMed] [Google Scholar]
- 53.Fournier SB, D'Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, Yurkow EJ, Stapleton PA. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol. 2020;17(1):55. doi: 10.1186/s12989-020-00385-9. https://particleandfibretoxicology.biomedcentral.com/articles/10.1186/s12989-020-00385-9 .10.1186/s12989-020-00385-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bongaerts E, Nawrot TS, Van Pee T, Ameloot M, Bové H. Translocation of (ultra)fine particles and nanoparticles across the placenta; a systematic review on the evidence of in vitro, ex vivo, and in vivo studies. Part Fibre Toxicol. 2020;17(1):56. doi: 10.1186/s12989-020-00386-8. https://particleandfibretoxicology.biomedcentral.com/articles/10.1186/s12989-020-00386-8 .10.1186/s12989-020-00386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, Jochum W, Krug HF, Buerki-Thurnherr T, von Mandach U, Wick P. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ Health Perspect. 2015;123(12):1280–1286. doi: 10.1289/ehp.1409271. https://ehp.niehs.nih.gov/doi/10.1289/ehp.1409271?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dusza HM, Katrukha EA, Nijmeijer SM, Akhmanova A, Vethaak AD, Walker DI, Legler J. Uptake, transport, and toxicity of pristine and weathered micro- and nanoplastics in human placenta cells. Environ Health Perspect. 2022;130(9):97006. doi: 10.1289/EHP10873. https://ehp.niehs.nih.gov/doi/10.1289/EHP10873?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong MK, Li EW, Adam M, Selvaganapathy PR, Raha S. Establishment of an in vitro placental barrier model cultured under physiologically relevant oxygen levels. Mol Hum Reprod. 2020;26(5):353–365. doi: 10.1093/molehr/gaaa018. https://europepmc.org/abstract/MED/32159799 .5802692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazakidou P, Koopmans C, Grimberg S, Evangelista S, Koekkoek J, Lamoree M, Leonards P, van Duursen M. Expanding the H295R steroidogenic assay using LC-MS/MS and an ER-alpha reporter gene assay as read-outs using azole fungicides as test compounds. Toxicology Letters. 2021;350:S65–S66. doi: 10.1016/s0378-4274(21)00401-x. [DOI] [Google Scholar]
- 59.Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22(1):50–63.e6. doi: 10.1016/j.stem.2017.11.004. https://linkinghub.elsevier.com/retrieve/pii/S1934-5909(17)30456-3 .S1934-5909(17)30456-3 [DOI] [PubMed] [Google Scholar]
- 60.Karttunen V, Sahlman H, Repo JK, Woo C, Myöhänen K, Myllynen P, Vähäkangas KH. Criteria and challenges of the human placental perfusion – data from a large series of perfusions. Toxicol In Vitro. 2015;29(7):1482–1491. doi: 10.1016/j.tiv.2015.06.001.S0887-2333(15)00128-9 [DOI] [PubMed] [Google Scholar]
- 61.Bhagat J, Zang L, Nishimura N, Shimada Y. Zebrafish: an emerging model to study microplastic and nanoplastic toxicity. Sci Total Environ. 2020;728:138707. doi: 10.1016/j.scitotenv.2020.138707.S0048-9697(20)32224-5 [DOI] [PubMed] [Google Scholar]
- 62.Chaibub Neto E, Bare JC, Margolin AA. Simulation studies as designed experiments: the comparison of penalized regression models in the "large p, small n" setting. PLoS One. 2014;9(10):e107957. doi: 10.1371/journal.pone.0107957. https://dx.plos.org/10.1371/journal.pone.0107957 .PONE-D-14-17293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agier L, Portengen L, Chadeau-Hyam M, Basagaña X, Giorgis-Allemand L, Siroux V, Robinson O, Vlaanderen J, González JR, Nieuwenhuijsen MJ, Vineis P, Vrijheid M, Slama R, Vermeulen R. A systematic comparison of linear regression-based statistical methods to assess exposome-health associations. Environ Health Perspect. 2016;124(12):1848–1856. doi: 10.1289/EHP172. https://ehp.niehs.nih.gov/doi/10.1289/EHP172?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .EHP172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lenters V, Vermeulen R, Portengen L. Performance of variable selection methods for assessing the health effects of correlated exposures in case-control studies. Occup Environ Med. 2018;75(7):522–529. doi: 10.1136/oemed-2016-104231.oemed-2016-104231 [DOI] [PubMed] [Google Scholar]
- 65.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142. doi: 10.1186/1471-2164-7-142. https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-7-142 .1471-2164-7-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chadeau-Hyam M, Ebbels TMD, Brown IJ, Chan Q, Stamler J, Huang CC, Daviglus ML, Ueshima H, Zhao L, Holmes E, Nicholson JK, Elliott P, De Iorio M. Metabolic profiling and the metabolome-wide association study: significance level for biomarker identification. J Proteome Res. 2010;9(9):4620–4627. doi: 10.1021/pr1003449. https://europepmc.org/abstract/MED/20701291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai A, Remy S, Lenters V, Cox B, Schoeters G, Covaci A, Vermeulen R, Portengen L. Exposure to a mixture of endocrine-disrupting chemicals and metabolic outcomes in Belgian adolescents. Environ Sci Technol. 2023;57(48):19871–19880. doi: 10.1021/acs.est.3c07607. doi: 10.1021/acs.est.3c07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellavia A, Valeri L. Decomposition of the total effect in the presence of multiple mediators and interactions. Am J Epidemiol. 2018;187(6):1311–1318. doi: 10.1093/aje/kwx355. https://europepmc.org/abstract/MED/29140421 .4622592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spolidoro GCI, Amera YT, Ali MM, Nyassi S, Lisik D, Ioannidou A, Rovner G, Khaleva E, Venter C, van Ree R, Worm M, Vlieg-Boerstra B, Sheikh A, Muraro A, Roberts G, Nwaru BI. Frequency of food allergy in Europe: an updated systematic review and meta-analysis. Allergy. 2023;78(2):351–368. doi: 10.1111/all.15560. https://europepmc.org/abstract/MED/36271775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.González-de Paz L, Valdesoiro-Navarrete L, Roma J, Blat-Guimerà E, Benavent-Areu J, Bartra J, Sisó-Almirall A. Prevalence and impact of asthma and allergy on daily life, health outcomes and use of healthcare services in children: a population-based study. Arch Bronconeumol. 2023;59(8):481–487. doi: 10.1016/j.arbres.2023.05.005.S0300-2896(23)00163-1 [DOI] [PubMed] [Google Scholar]
- 71.Selroos O, Kupczyk M, Kuna P, Łacwik P, Bousquet J, Brennan D, Palkonen S, Contreras J, FitzGerald M, Hedlin G, Johnston SL, Louis R, Metcalf L, Walker S, Moreno-Galdó A, Papadopoulos NG, Rosado-Pinto J, Powell P, Haahtela T. National and regional asthma programmes in Europe. Eur Respir Rev. 2015;24(137):474–483. doi: 10.1183/16000617.00008114. http://err.ersjournals.com/lookup/pmidlookup?view=long&pmid=26324809 .24/137/474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Navaranjan G, Diamond ML, Harris SA, Jantunen LM, Bernstein S, Scott JA, Takaro TK, Dai R, Lefebvre DL, Azad MB, Becker AB, Mandhane PJ, Moraes TJ, Simons E, Turvey SE, Sears MR, Subbarao P, Brook JR. Early life exposure to phthalates and the development of childhood asthma among Canadian children. Environ Res. 2021;197:110981. doi: 10.1016/j.envres.2021.110981.S0013-9351(21)00275-9 [DOI] [PubMed] [Google Scholar]
- 73.Koelmans AA, Redondo-Hasselerharm PE, Nor NHM, de Ruijter VN, Mintenig SM, Kooi M. Risk assessment of microplastic particles. Nat Rev Mater. 2022;7(2):138–152. doi: 10.1038/s41578-021-00411-y. [DOI] [Google Scholar]
- 74.World Health Organization . WHO Human Health Risk Assessment Toolkit: Chemical Hazards. USA: World Health Organization; 2021. [2024-08-14]. https://www.who.int/publications/i/item/9789240035720 . [Google Scholar]
- 75.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten J, da Silva Santos LB, Bourne PE, Bouwman J, Brookes AJ, Clark T, Crosas M, Dillo I, Dumon O, Edmunds S, Evelo CT, Finkers R, Gonzalez-Beltran A, Gray AJ, Groth P, Goble C, Grethe JS, Heringa J, 't Hoen PAC, Hooft R, Kuhn T, Kok R, Kok J, Lusher SJ, Martone ME, Mons A, Packer AL, Persson B, Rocca-Serra P, Roos M, van Schaik R, Sansone S, Schultes E, Sengstag T, Slater T, Strawn G, Swertz MA, Thompson M, van der Lei J, van Mulligen E, Velterop J, Waagmeester A, Wittenburg P, Wolstencroft K, Zhao J, Mons B. The FAIR guiding principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. doi: 10.1038/sdata.2016.18.sdata201618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hubrecht RC, Carter E. The 3Rs and humane experimental technique: implementing change. Animals (Basel) 2019;9(10):754. doi: 10.3390/ani9100754. https://www.mdpi.com/resolver?pii=ani9100754 .ani9100754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes. 2010. [2010-09-22]. https://www.fao.org/faolex/results/details/en/c/LEX-FAOC098296/
- 78.Christopher EA, Christopher-de Vries Y, Devadoss A, Mandemaker LD, van Boxel J, Copsey HM, Dusza HM, Legler J, Meirer F, Muncke J, Nawrot TS, Saenen ND, Scholz-Böttcher BM, Tran L, Weckhuysen BM, Zou R, Zimmermann L, Galea KS, Vermeulen R, Boyles MSP. Impacts of micro- and nanoplastics on early-life health: a roadmap towards risk assessment. Micropl.&Nanopl. 2024 Jul 02;4(1):13. doi: 10.1186/s43591-024-00089-3. [DOI] [Google Scholar]
- 79.Yang J, Kamstra J, Legler J, Aardema H. The impact of microplastics on female reproduction and early life. Anim Reprod. 2023;20(2):e20230037. doi: 10.1590/1984-3143-AR2023-0037. https://europepmc.org/abstract/MED/37547566 .arAR20230037_EN [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen G, Xiong S, Jing Q, van Gestel CAM, van Straalen NM, Roelofs D, Sun L, Qiu H. Maternal exposure to polystyrene nanoparticles retarded fetal growth and triggered metabolic disorders of placenta and fetus in mice. Sci Total Environ. 2023;854:158666. doi: 10.1016/j.scitotenv.2022.158666.S0048-9697(22)05765-5 [DOI] [PubMed] [Google Scholar]
- 81.Dibbon KC, Mercer GV, Maekawa AS, Hanrahan J, Steeves KL, Ringer LCM, Simpson AJ, Simpson MJ, Baschat AA, Kingdom JC, Macgowan CK, Sled JG, Jobst KJ, Cahill LS. Polystyrene micro- and nanoplastics cause placental dysfunction in mice†. Biol Reprod. 2024;110(1):211–218. doi: 10.1093/biolre/ioad126.7277078 [DOI] [PubMed] [Google Scholar]
- 82.Xue J, Xu Z, Hu X, Lu Y, Zhao Y, Zhang H. Microplastics in maternal amniotic fluid and their associations with gestational age. Sci Total Environ. 2024;920:171044. doi: 10.1016/j.scitotenv.2024.171044.S0048-9697(24)01183-5 [DOI] [PubMed] [Google Scholar]
- 83.Amereh F, Amjadi N, Mohseni-Bandpei A, Isazadeh S, Mehrabi Y, Eslami A, Naeiji Z, Rafiee M. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ Pollut. 2022;314:120174. doi: 10.1016/j.envpol.2022.120174.S0269-7491(22)01388-4 [DOI] [PubMed] [Google Scholar]
- 84.Microplastics are everywhere - we need to understand how they affect human health. Nat Med. 2024;30(4):913. doi: 10.1038/s41591-024-02968-x.10.1038/s41591-024-02968-x [DOI] [PubMed] [Google Scholar]
- 85.eNanoMapper. [2024-08-14]. https://enanomapper.adma.ai/
- 86.European Commission A European strategy for plastics in a circular economy. 2018. [2018-01-16]. https://research-and-innovation.ec.europa.eu/research-area/environment/circular-economy/plastics-circular-economy_en .
- 87.European Commission . A Sustainable Bioeconomy for Europe Strengthening the Connection Between Economy, Society and the Environment : Updated Bioeconomy Strategy. Europe: Publications Office of the European Union; 2018. [2024-08-14]. https://www.qualenergia.it/wp-content/uploads/2018/10/ec_bioeconomy_strategy_2018.pdf . [Google Scholar]
- 88.de Ruijter VN, Redondo-Hasselerharm PE, Gouin T, Koelmans AA. Quality criteria for microplastic effect studies in the context of risk assessment: a critical review. Environ Sci Technol. 2020;54(19):11692–11705. doi: 10.1021/acs.est.0c03057. doi: 10.1021/acs.est.0c03057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cowger W, Booth AM, Hamilton BM, Thaysen C, Primpke S, Munno K, Lusher AL, Dehaut A, Vaz VP, Liboiron M, Devriese LI, Hermabessiere L, Rochman C, Athey SN, Lynch JM, De Frond H, Gray A, Jones OA, Brander S, Steele C, Moore S, Sanchez A, Nel H. Reporting guidelines to increase the reproducibility and comparability of research on microplastics. Appl Spectrosc. 2020;74(9):1066–1077. doi: 10.1177/0003702820930292. https://journals.sagepub.com/doi/abs/10.1177/0003702820930292?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer review.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during this study. Data from this project will be shared in future publications and data repositories including eNanoMapper.


