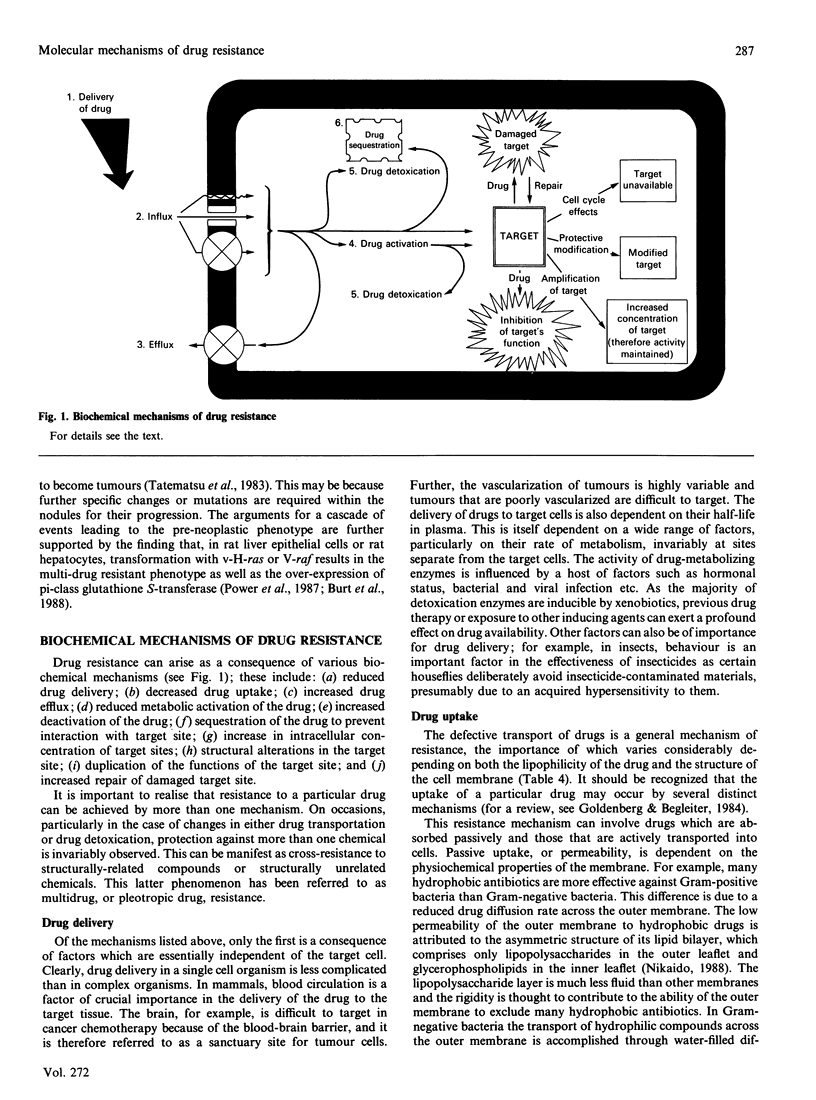
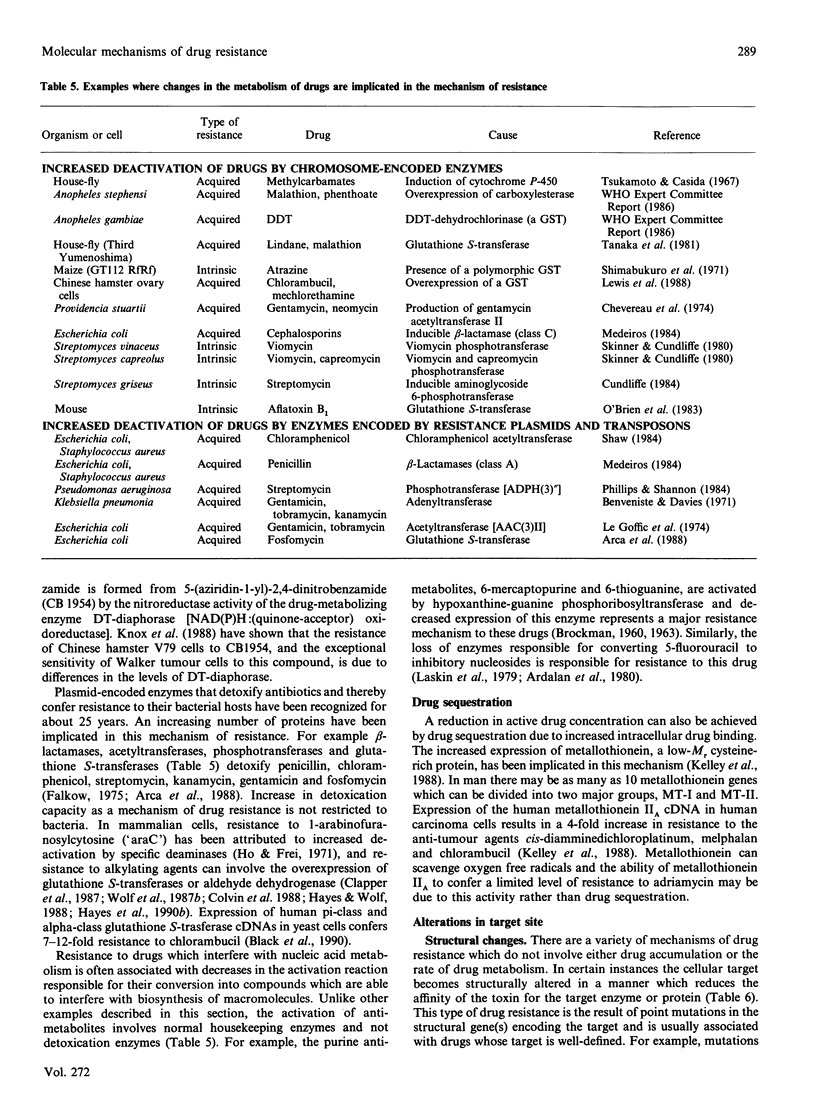
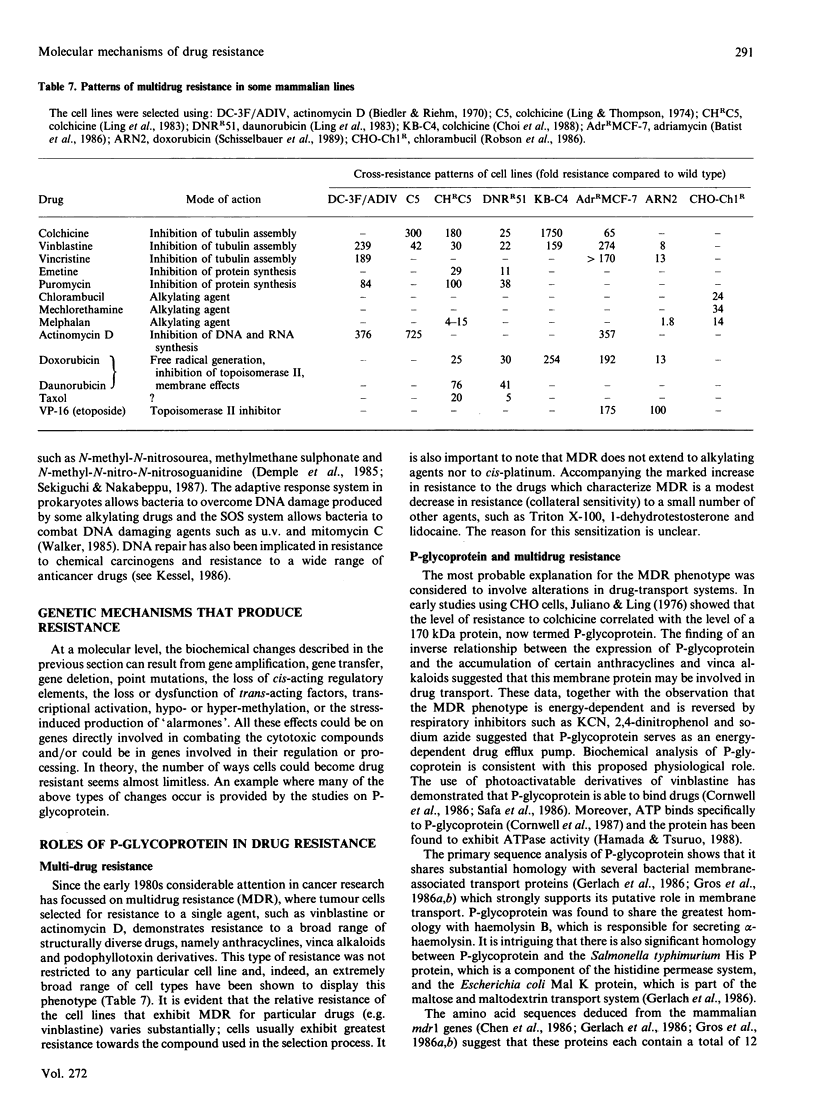
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams K. J., Carmichael J., Wolf C. R. Altered mouse bone marrow glutathione and glutathione transferase levels in response to cytotoxins. Cancer Res. 1985 Apr;45(4):1669–1673. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alvares K., Carrillo A., Yuan P. M., Kawano H., Morimoto R. I., Reddy J. K. Identification of cytosolic peroxisome proliferator binding protein as a member of the heat shock protein HSP70 family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5293–5297. doi: 10.1073/pnas.87.14.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Amyes S. G. The success of plasmid-encoded resistance genes in clinical bacteria. An examination of plasmid-mediated ampicillin and trimethoprim resistance genes and their resistance mechanisms. J Med Microbiol. 1989 Feb;28(2):73–83. doi: 10.1099/00222615-28-2-73. [DOI] [PubMed] [Google Scholar]
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Arca P., Rico M., Braña A. F., Villar C. J., Hardisson C., Suárez J. E. Formation of an adduct between fosfomycin and glutathione: a new mechanism of antibiotic resistance in bacteria. Antimicrob Agents Chemother. 1988 Oct;32(10):1552–1556. doi: 10.1128/aac.32.10.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardalan B., Cooney D. A., Jayaram H. N., Carrico C. K., Glazer R. I., Macdonald J., Schein P. S. Mechanisms of sensitivity and resistance of murine tumors to 5-fluorouracil. Cancer Res. 1980 May;40(5):1431–1437. [PubMed] [Google Scholar]
- BROCKMAN R. W. A mechanism of resistance to 6-mercaptopurine: metabolism of hypoxanthine and 6-mercaptopurine by sensitive and resistant neoplasms. Cancer Res. 1960 Jun;20:643–653. [PubMed] [Google Scholar]
- BROCKMAN R. W. MECHANISMS OF RESISTANCE TO ANTICANCER AGENTS. Adv Cancer Res. 1963;7:129–234. doi: 10.1016/s0065-230x(08)60983-5. [DOI] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Beer D. G., Pitot H. C. Biological markers characterizing the development of preneoplastic and neoplastic lesions in rodent liver. Arch Toxicol Suppl. 1987;10:68–80. doi: 10.1007/978-3-642-71617-1_6. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. R-factor mediated gentamicin resistance: A new enzyme which modifies aminoglycoside antibiotics. FEBS Lett. 1971 May 20;14(5):293–296. doi: 10.1016/0014-5793(71)80282-x. [DOI] [PubMed] [Google Scholar]
- Bertino J. R. Approaches to drug selectivity in cancer therapy on the basis of differences between membranes of normal vs. neoplastic cells. Adv Pathobiol. 1980;7:377–386. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Bitonti A. J., Sjoerdsma A., McCann P. P., Kyle D. E., Oduola A. M., Rossan R. N., Milhous W. K., Davidson D. E., Jr Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science. 1988 Dec 2;242(4883):1301–1303. doi: 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]
- Black S. M., Beggs J. D., Hayes J. D., Bartoszek A., Muramatsu M., Sakai M., Wolf C. R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J. 1990 Jun 1;268(2):309–315. doi: 10.1042/bj2680309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon R. H. Heat shock and the heat shock proteins. Biochem J. 1986 Dec 1;240(2):313–324. doi: 10.1042/bj2400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt R. K., Garfield S., Johnson K., Thorgeirsson S. S. Transformation of rat liver epithelial cells with v-H-ras or v-raf causes expression of MDR-1, glutathione-S-transferase-P and increased resistance to cytotoxic chemicals. Carcinogenesis. 1988 Dec;9(12):2329–2332. doi: 10.1093/carcin/9.12.2329. [DOI] [PubMed] [Google Scholar]
- Burt R. K., Thorgeirsson S. S. Coinduction of MDR-1 multidrug-resistance and cytochrome P-450 genes in rat liver by xenobiotics. J Natl Cancer Inst. 1988 Nov 2;80(17):1383–1386. doi: 10.1093/jnci/80.17.1383. [DOI] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Campbell S. D., Hilliker A. J., Phillips J. P. Cytogenetic analysis of the cSOD microregion in Drosophila melanogaster. Genetics. 1986 Feb;112(2):205–215. doi: 10.1093/genetics/112.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper S. W., Duffy J. J., Gerner E. W. Heat shock proteins in thermotolerance and other cellular processes. Cancer Res. 1987 Oct 15;47(20):5249–5255. [PubMed] [Google Scholar]
- Carr B. I., Huang T. H., Buzin C. H., Itakura K. Induction of heat shock gene expression without heat shock by hepatocarcinogens and during hepatic regeneration in rat liver. Cancer Res. 1986 Oct;46(10):5106–5111. [PubMed] [Google Scholar]
- Carr B. I. Pleiotropic drug resistance in hepatocytes induced by carcinogens administered to rats. Cancer Res. 1987 Nov 1;47(21):5577–5583. [PubMed] [Google Scholar]
- Center M. S. Evidence that adriamycin resistance in Chinese hamster lung cells is regulated by phosphorylation of a plasma membrane glycoprotein. Biochem Biophys Res Commun. 1983 Aug 30;115(1):159–166. doi: 10.1016/0006-291x(83)90983-x. [DOI] [PubMed] [Google Scholar]
- Center M. S. Mechanisms regulating cell resistance to adriamycin. Evidence that drug accumulation in resistant cells is modulated by phosphorylation of a plasma membrane glycoprotein. Biochem Pharmacol. 1985 May 1;34(9):1471–1476. doi: 10.1016/0006-2952(85)90686-0. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Chevereau M., Daniels P. J., Davies J., LeGoffic F. Aminoglycoside resistance in bacteria medicated by gentamicin acetyltransferase II, an enzyme modifying the 2'-amino group of aminoglycoside antibiotics. Biochemistry. 1974 Jan 29;13(3):598–603. doi: 10.1021/bi00700a030. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Tanaka S., Darlington G., Pastan I., Gottesman M. M. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990 Jan 5;265(1):221–226. [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Choi K. H., Chen C. J., Kriegler M., Roninson I. B. An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell. 1988 May 20;53(4):519–529. doi: 10.1016/0092-8674(88)90568-5. [DOI] [PubMed] [Google Scholar]
- Chopra I. Antibiotic resistance resulting from decreased drug accumulation. Br Med Bull. 1984 Jan;40(1):11–17. doi: 10.1093/oxfordjournals.bmb.a071940. [DOI] [PubMed] [Google Scholar]
- Chopra I. Mechanisms of resistance to fusidic acid in Staphylococcus aureus. J Gen Microbiol. 1976 Oct;96(2):229–238. doi: 10.1099/00221287-96-2-229. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Storz G., Ames B. N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin M., Russo J. E., Hilton J., Dulik D. M., Fenselau C. Enzymatic mechanisms of resistance to alkylating agents in tumor cells and normal tissues. Adv Enzyme Regul. 1988;27:211–221. doi: 10.1016/0065-2571(88)90018-0. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Gottesman M. M., Pastan I. H. Increased vinblastine binding to membrane vesicles from multidrug-resistant KB cells. J Biol Chem. 1986 Jun 15;261(17):7921–7928. [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Coulson A. Beta-lactamases: molecular studies. Biotechnol Genet Eng Rev. 1985;3:219–253. doi: 10.1080/02648725.1985.10647814. [DOI] [PubMed] [Google Scholar]
- Croop J. M., Guild B. C., Gros P., Housman D. E. Genetics of multidrug resistance: relationship of a cloned gene to the complete multidrug resistant phenotype. Cancer Res. 1987 Nov 15;47(22):5982–5988. [PubMed] [Google Scholar]
- Cullmann W., Dalhoff A., Dick W. Nonspecific induction of beta-lactamase in Enterobacter cloacae. J Gen Microbiol. 1984 Jul;130(7):1781–1786. doi: 10.1099/00221287-130-7-1781. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. Self defence in antibiotic-producing organisms. Br Med Bull. 1984 Jan;40(1):61–67. doi: 10.1093/oxfordjournals.bmb.a071949. [DOI] [PubMed] [Google Scholar]
- Demple B., Sedgwick B., Robins P., Totty N., Waterfield M. D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(9):2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988 Sep 15;155(2):801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Rushmore T., Lee G., Koo P., Goldsmith M. E., Myers C. E., Farber E., Cowan K. H. Carcinogen-induced mdr overexpression is associated with xenobiotic resistance in rat preneoplastic liver nodules and hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7701–7705. doi: 10.1073/pnas.84.21.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber E. Pre-cancerous steps in carcinogenesis. Their physiological adaptive nature. Biochim Biophys Acta. 1984;738(4):171–180. doi: 10.1016/0304-419x(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Fine R. L., Patel J., Chabner B. A. Phorbol esters induce multidrug resistance in human breast cancer cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):582–586. doi: 10.1073/pnas.85.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Fox M., Roberts J. J. Drug resistance and DNA repair. Cancer Metastasis Rev. 1987;6(3):261–281. doi: 10.1007/BF00144267. [DOI] [PubMed] [Google Scholar]
- Funatsu G., Wittmann H. G. Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J Mol Biol. 1972 Jul 28;68(3):547–550. doi: 10.1016/0022-2836(72)90108-8. [DOI] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Georges E., Bradley G., Gariepy J., Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Jan;87(1):152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Glisson B. S., Sullivan D. M., Gupta R., Ross W. E. Mediation of multi-drug resistance in a Chinese hamster ovary cell line by a mutant type II topoisomerase. NCI Monogr. 1987;(4):89–93. [PubMed] [Google Scholar]
- Goldenberg G. J., Vanstone C. L., Bihler I. Transport of nitrogen mustard on the transport-carrier for choline in L5178Y lymphoblasts. Science. 1971 Jun 11;172(3988):1148–1149. doi: 10.1126/science.172.3988.1148. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. J., Vanstone C. L., Israels L. G., Iise D., Bihler I. Evidence for a transport carrier of nitrogen mustard in nitrogen mustard-sensitive and -resistant L5178Y lymphoblasts. Cancer Res. 1970 Sep;30(9):2285–2291. [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C. Characterization of beta-lactamase induction in Enterobacter cloacae. Antimicrob Agents Chemother. 1983 Jan;23(1):91–97. doi: 10.1128/aac.23.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M. Multidrug resistance during chemical carcinogenesis: a mechanism revealed? J Natl Cancer Inst. 1988 Nov 2;80(17):1352–1353. doi: 10.1093/jnci/80.17.1352. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Gupta R. Mutants of chinese hamster ovary cells affected in two different microtubule-associated proteins. Genetic and biochemical studies. J Biol Chem. 1984 Feb 10;259(3):1882–1890. [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988 Sep 1;48(17):4926–4932. [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Harrison D. J., Cronshaw A. D., Ross A. G., Neal G. E. Preferential over-expression of the class alpha rat Ya2 glutathione S-transferase subunit in livers bearing aflatoxin-induced pre-neoplastic nodules. Comparison of the primary structures of Ya1 and Ya2 with cloned class alpha glutathione S-transferase cDNA sequences. Biochem J. 1990 Jun 1;268(2):295–302. doi: 10.1042/bj2680295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. T., Bailey B. D., White J. C., Goldman I. D. Characteristics of transport of 4-amino antifolates and folate compounds by two lines of L5178Y lymphoblasts, one with impaired transport of methotrexate. Cancer Res. 1979 Jul;39(7 Pt 1):2440–2446. [PubMed] [Google Scholar]
- Ho D. H., Frei E., 3rd Clinical pharmacology of 1-beta-d-arabinofuranosyl cytosine. Clin Pharmacol Ther. 1971 Nov-Dec;12(6):944–954. doi: 10.1002/cpt1971126944. [DOI] [PubMed] [Google Scholar]
- Houghton J. A., Houghton P. J., Hazelton B. J., Douglass E. C. In situ selection of a human rhabdomyosarcoma resistant to vincristine with altered beta-tubulins. Cancer Res. 1985 Jun;45(6):2706–2712. [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kane S. E., Gottesman M. M. Multidrug resistance in the laboratory and clinic. Cancer Cells. 1989 Sep;1(1):33–36. [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Keates R. A., Sarangi F., Ling V. Structural and functional alterations in microtubule protein from Chinese hamster ovary cell mutants. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5638–5642. doi: 10.1073/pnas.78.9.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley S. L., Basu A., Teicher B. A., Hacker M. P., Hamer D. H., Lazo J. S. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988 Sep 30;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Kempe T. D., Swyryd E. A., Bruist M., Stark G. R. Stable mutants of mammalian cells that overproduce the first three enzymes of pyrimidine nucleotide biosynthesis. Cell. 1976 Dec;9(4 Pt 1):541–550. doi: 10.1016/0092-8674(76)90036-2. [DOI] [PubMed] [Google Scholar]
- Kessel D. Circumvention of resistance to anthracyclines by calcium antagonists and other membrane-perturbing agents. Cancer Surv. 1986;5(1):109–127. [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Kjeldsen E., Bonven B. J., Andoh T., Ishii K., Okada K., Bolund L., Westergaard O. Characterization of a camptothecin-resistant human DNA topoisomerase I. J Biol Chem. 1988 Mar 15;263(8):3912–3916. [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Jarman M., Roberts J. J. A new cytotoxic, DNA interstrand crosslinking agent, 5-(aziridin-1-yl)-4-hydroxylamino-2-nitrobenzamide, is formed from 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB 1954) by a nitroreductase enzyme in Walker carcinoma cells. Biochem Pharmacol. 1988 Dec 15;37(24):4661–4669. doi: 10.1016/0006-2952(88)90335-8. [DOI] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin J. D., Evans R. M., Slocum H. K., Burke D., Hakala M. T. Basis for natural variation in sensitivity to 5-fluorouracil in mouse and human cells in culture. Cancer Res. 1979 Feb;39(2 Pt 1):383–390. [PubMed] [Google Scholar]
- Le Goffic F., Martel A., Witchitz J. 3-N enzymatic acetylation of gentamicin, tobramycin, and kanamycin by Escherichia coli carrying an R factor. Antimicrob Agents Chemother. 1974 Dec;6(6):680–684. doi: 10.1128/aac.6.6.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Oppermann H., Jackson J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim Biophys Acta. 1980;606(1):170–180. doi: 10.1016/0005-2787(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Hahn G. M. Ethanol-induced tolerance to heat and to adriamycin. Nature. 1978 Aug 17;274(5672):699–701. doi: 10.1038/274699a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Ling V., Kartner N., Sudo T., Siminovitch L., Riordan J. R. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983 Oct;67(10):869–874. [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A. Beta-lactamases. Br Med Bull. 1984 Jan;40(1):18–27. doi: 10.1093/oxfordjournals.bmb.a071942. [DOI] [PubMed] [Google Scholar]
- Mellado W., Horwitz S. B. Phosphorylation of the multidrug resistance associated glycoprotein. Biochemistry. 1987 Nov 3;26(22):6900–6904. doi: 10.1021/bi00396a005. [DOI] [PubMed] [Google Scholar]
- Millar J. L., Hudspith B. N., Blackett N. M. Reduced lethality in mice receiving a combined dose of cyclophosphamide and busulphan. Br J Cancer. 1975 Aug;32(2):193–198. doi: 10.1038/bjc.1975.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. H., Edberg S. C., Mandel L. J., Behar C. F., Steigbigel N. H. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1980 Nov;18(5):722–729. doi: 10.1128/aac.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien K., Moss E., Judah D., Neal G. Metabolic basis of the species difference to aflatoxin B1 induced hepatotoxicity. Biochem Biophys Res Commun. 1983 Jul 29;114(2):813–821. doi: 10.1016/0006-291x(83)90854-9. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M., Ueda K., Lovelace E., Rutherford A. V., Willingham M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4486–4490. doi: 10.1073/pnas.85.12.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984 Dec 20;3(13):3095–3100. doi: 10.1002/j.1460-2075.1984.tb02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Walliker D., Wellems T. E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I., Shannon K. Aminoglycoside resistance. Br Med Bull. 1984 Jan;40(1):28–35. doi: 10.1093/oxfordjournals.bmb.a071943. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. A., Wang C. C. A Trypanosoma brucei mutant resistant to alpha-difluoromethylornithine. Mol Biochem Parasitol. 1987 Jan 2;22(1):9–17. doi: 10.1016/0166-6851(87)90064-8. [DOI] [PubMed] [Google Scholar]
- Power C., Sinha S., Webber C., Manson M. M., Neal G. E. Transformation related expression of glutathione-S-transferase P in rat liver cells. Carcinogenesis. 1987 Jun;8(6):797–801. doi: 10.1093/carcin/8.6.797. [DOI] [PubMed] [Google Scholar]
- Rabussay D., Zillig W. A rifampicin resistent rna-polymerase from E. coli altered in the beta-subunit. FEBS Lett. 1969 Oct 21;5(2):104–106. doi: 10.1016/0014-5793(69)80305-4. [DOI] [PubMed] [Google Scholar]
- Redwood W. R., Colvin M. Transport of melphalan by sensitive and resistant L1210 cells. Cancer Res. 1980 Apr;40(4):1144–1149. [PubMed] [Google Scholar]
- Reynolds P. E. Resistance of the antibiotic target site. Br Med Bull. 1984 Jan;40(1):3–10. doi: 10.1093/oxfordjournals.bmb.a071944. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Alexander J., Harris A. L., Hickson I. D. Isolation and characterization of a Chinese hamster ovary cell line resistant to bifunctional nitrogen mustards. Cancer Res. 1986 Dec;46(12 Pt 1):6290–6294. [PubMed] [Google Scholar]
- Rolinson G. N. The Garrod lecture. The influence of 6-aminopenicillanic acid on antibiotic development. J Antimicrob Chemother. 1988 Jul;22(1):5–14. doi: 10.1093/jac/22.1.5. [DOI] [PubMed] [Google Scholar]
- Rolinson G. N. The Garrod lecture. The influence of 6-aminopenicillanic acid on antibiotic development. J Antimicrob Chemother. 1988 Jul;22(1):5–14. doi: 10.1093/jac/22.1.5. [DOI] [PubMed] [Google Scholar]
- Rossouw F. T., Rowbury R. J. Effects of the resistance plasmid R124 on the level of the OmpF outer membrane protein and on the response of Escherichia coli to environmental agents. J Appl Bacteriol. 1984 Feb;56(1):63–79. doi: 10.1111/j.1365-2672.1984.tb04697.x. [DOI] [PubMed] [Google Scholar]
- Rowe T. C., Chen G. L., Hsiang Y. H., Liu L. F. DNA damage by antitumor acridines mediated by mammalian DNA topoisomerase II. Cancer Res. 1986 Apr;46(4 Pt 2):2021–2026. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Microbial resistance to newer generation beta-lactam antibiotics: clinical and laboratory implications. J Infect Dis. 1985 Mar;151(3):399–406. doi: 10.1093/infdis/151.3.399. [DOI] [PubMed] [Google Scholar]
- Sanders P. G., Wilson R. H. Amplification and cloning of the Chinese hamster glutamine synthetase gene. EMBO J. 1984 Jan;3(1):65–71. doi: 10.1002/j.1460-2075.1984.tb01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisselbauer J. C., Crescimanno M., D'Alessandro N., Clapper M., Toulmond S., Tapiero H., Tew K. D. Glutathione, glutathione S-transferases, and related redox enzymes in Adriamycin-resistant cell lines with a multidrug resistant phenotype. Cancer Commun. 1989;1(2):133–139. doi: 10.3727/095535489820875291. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., van der Hoeven J. J., Pinedo H. M., Lankelma J. Potentiation of doxorubicin cytotoxicity by the calcium antagonist bepridil in anthracycline-resistant and -sensitive cell lines. A comparison with verapamil. Cancer Chemother Pharmacol. 1987;20(4):285–290. doi: 10.1007/BF00262578. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Robins P. Isolation of mutants of Escherichia coli with increased resistance to alkylating agents: mutants deficient in thiols and mutants constitutive for the adaptive response. Mol Gen Genet. 1980;180(1):85–90. doi: 10.1007/BF00267355. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Bacterial resistance to chloramphenicol. Br Med Bull. 1984 Jan;40(1):36–41. doi: 10.1093/oxfordjournals.bmb.a071945. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Shimabukuro R. H., Frear D. S., Swanson H. R., Walsh W. C. Glutathione conjugation. An enzymatic basis for atrazine resistance in corn. Plant Physiol. 1971 Jan;47(1):10–14. doi: 10.1104/pp.47.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M. Obligate genetic expression in tumor cells of a fetal membrane property mediating "folate" transport: biological significance and implications for improved therapy of human cancer. Cancer Res. 1985 Sep;45(9):3992–4000. [PubMed] [Google Scholar]
- Skinner R. H., Cundliffe E. Resistance to the antibiotics viomycin and capreomycin in the Streptomyces species which produce them. J Gen Microbiol. 1980 Sep;120(1):95–104. doi: 10.1099/00221287-120-1-95. [DOI] [PubMed] [Google Scholar]
- Smith J. T., Amyes S. G. Bacterial resistance to antifolate chemotherapeutic agents mediated by plasmids. Br Med Bull. 1984 Jan;40(1):42–46. doi: 10.1093/oxfordjournals.bmb.a071946. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Zaidenzaig Y., Ziegler-Schlomowitz R., Abramova N. Mechanism of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):351–362. doi: 10.1099/00221287-62-3-351. [DOI] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Escherichia coli resistance to beta-lactam antibiotics through a decrease in the affinity of a target for lethality. Nature. 1978 Aug 17;274(5672):713–715. doi: 10.1038/274713a0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., Finer-Moore J. Acetylcholine receptor structure, function, and evolution. Annu Rev Cell Biol. 1985;1:317–351. doi: 10.1146/annurev.cb.01.110185.001533. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., O'Brien W. E., Beaudet A. L. Cloning of cDNA for argininosuccinate synthetase mRNA and study of enzyme overproduction in a human cell line. J Biol Chem. 1981 Nov 25;256(22):11826–11831. [PubMed] [Google Scholar]
- Sugawara I., Kataoka I., Morishita Y., Hamada H., Tsuruo T., Itoyama S., Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988 Apr 1;48(7):1926–1929. [PubMed] [Google Scholar]
- Talalay P., De Long M. J., Prochaska H. J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Nagamine Y., Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983 Nov;43(11):5049–5058. [PubMed] [Google Scholar]
- Thompson J., Schmidt F., Cundliffe E. Site of action of a ribosomal RNA methylase conferring resistance to thiostrepton. J Biol Chem. 1982 Jul 25;257(14):7915–7917. [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Tsui F. W., Andrulis I. L., Murialdo H., Siminovitch L. Amplification of the gene for histidyl-tRNA synthetase in histidinol-resistant Chinese hamster ovary cells. Mol Cell Biol. 1985 Sep;5(9):2381–2388. doi: 10.1128/mcb.5.9.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T. Mechanisms of multidrug resistance and implications for therapy. Jpn J Cancer Res. 1988 Mar;79(3):285–296. doi: 10.1111/j.1349-7006.1988.tb01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Vistica D. T., Toal J. N., Rabinovitz M. Amino acid-conferred protection against melphalan--characterization of melphalan transport and correlation of uptake with cytotoxicity in cultured L1210 murine leukemia cells. Biochem Pharmacol. 1978;27(24):2865–2870. doi: 10.1016/0006-2952(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Wadler S., Wiernik P. H. Partial reversal of doxorubicin resistance by forskolin and 1,9-dideoxyforskolin in murine sarcoma S180 variants. Cancer Res. 1988 Feb 1;48(3):539–543. [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner K., Li G. C. Adriamycin resistance, heat resistance and radiation response in Chinese hamster fibroblasts. Int J Radiat Oncol Biol Phys. 1986 May;12(5):829–833. doi: 10.1016/0360-3016(86)90043-x. [DOI] [PubMed] [Google Scholar]
- White T. C., Fase-Fowler F., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988 Nov 15;263(32):16977–16983. [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., McIvor R. S., Plagemann P. G. Facilitated transport of uracil and 5-fluorouracil, and permeation of orotic acid into cultured mammalian cells. J Cell Physiol. 1980 Sep;104(3):309–319. doi: 10.1002/jcp.1041040305. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Lewis A. D., Carmichael J., Adams D. J., Allan S. G., Ansell D. J. The role of glutathione in determining the response of normal and tumor cells to anticancer drugs. Biochem Soc Trans. 1987 Aug;15(4):728–730. doi: 10.1042/bst0150728. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. K., Amyes S. G. A new mechanism of plasmid trimethoprim resistance. Characterization of an inducible dihydrofolate reductase. J Biol Chem. 1986 Feb 25;261(6):2503–2505. [PubMed] [Google Scholar]
- van der Bliek A. M., Borst P. Multidrug resistance. Adv Cancer Res. 1989;52:165–203. doi: 10.1016/s0065-230x(08)60213-4. [DOI] [PubMed] [Google Scholar]



