Summary
Background
Remote patient monitoring (RPM) of symptoms using electronic patient reported outcomes (ePROs) has been shown to reduce symptom burden and hospitalizations, increase dose intensity and improve quality of life of patients during systemic therapy being recommended by international guidelines in routine oncology practice. However, implementation in routine care has been slow and faces several challenges. In this study we report on the real-world multi-center implementation of a RPM pathway encompassing weekly patient symptom ePRO reporting with electronic alert notifications triggered to providers for severe or worsening symptoms.
Methods
An RPM pathway was implemented in 33 European cancer centers in France and Belgium between November 2021 and August 2023. The implementation process followed a standardized phasic process of Exploration, Preparation, Implementation and Sustainment. Patient-level and system-level implementation metrics were collected and evaluated according to the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework.
Findings
Across the 33 cancer centers, the RPM pathway was implemented for 3015 patients cared for by 168 providers. The RPM pathway enabled effective and timely symptom management with 94.6% of all alerts (10,132/10,711) evolving to an improvement two weeks later, among which 88.4% (9468/10,711) showed ≥2 grades of improvement on the 5-point scale of the Patient-Reported Outcomes Common Terminology (PRO-CTCAE). The median time to alert management by the care team was 13 h 41 min (25th percentile: 1 h 42 min, 75th percentile: 1 day + 19 h 54 min), with 80% (36,269/45,334) of alerts managed by a nurse navigator telephone call. Patient adherence with weekly ePRO reporting was 82% (2472/3015). In an experience survey, 87% (32/38) of providers were satisfied with integrating the solution into their organization and 90% (276/307) of the patients felt that ePRO reporting positively impacted their care. As of March 2024, the pathway has been maintained in all participating centers, with activation of an additional 18 centers following data lock, and reimbursement for this RPM pathway approved in France in October 2023.
Interpretation
These findings demonstrate the feasibility of implementing and maintaining an RPM pathway during routine care across a diverse group of cancer centers in the European setting, with high levels of patient and provider engagement, and positive clinical impact.
Funding
Part of this work was funded Breast Cancer Research Foundation (Career Development Award to Maria Alice Franzoi) and Resilience (nurse navigation and technology support).
Keywords: Digital health, Remote symptom monitoring, Implementation science, RE-AIM framework, Oncology care delivery, Nurse navigation, ePROs, Routine care
Research in context.
Evidence before this study
Randomized clinical trials have demonstrated that remote patient monitoring (RPM) of symptoms using electronic patient-reported outcomes (ePROs) reduces symptom burden and emergency visits, increases dose intensity and improves quality of life of patients during systemic cancer therapy. This approach is recommended by international guidelines. However, the large-scale implementation of this evidence-based strategy requires modifications of workflow, personnel roles, and patient and provider engagement, and therefore needs further investigation to demonstrate feasibility of implementation in different healthcare settings. We searched PubMed and Google Scholar with the following terms (and associated MeSH terms): “remote patient monitoring”, “ePROs”, “Electronic Symptom Monitoring” and ‘‘oncology”, “cancer” and “routine care” for publication between January 1 2004 and June 1st 2024, in English. No studies reporting detailed implementation metrics of RPM pathways in routine care in a multicentric context in Europe was found.
Added value of this study
This study reports the implementation process (including actions and interactions between technology providers, implementation managers and hospitals) of a RPM pathway across 33 different cancer centers in two European countries reaching 3015 patients. It also provides a detailed evaluation of implementation metrics following the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework.1 Our findings report the feasibility of implementing RPM in routine oncology care with patient adherence with weekly ePRO reporting of 82% and significant improvement of patients’ symptom burden during participation in the pathway (94.6% of patients with symptom improvement two weeks after an alert was triggered to a provider, among which 88.4% showed ≥2 grades of improvement on a 5-point scale).
Implications of all the available evidence
This study is reassuring regarding the ability to use implementation science to adapt RPM pathways to different cancer sites and oncology centers to achieve smooth integration within routine care organization workflows. It also demonstrates that such pathway held a positive clinical impact. Future studies should focus on characterizing potential provider-related burden related to RPM processes, and the impact of patient and provider digital health-literacy and social determinants of health in the access, engagement and derived benefit of RPM in routine cancer care.
Introduction
Optimizing communication between patients and their clinical teams improves the quality and level of satisfaction and alleviates costs associated with medical care following cancer diagnosis.2,3 Patient-reported outcomes (PROs) are an established facilitator of such communication, revealing symptom patterns that significantly differ from those reported by clinicians.4, 5, 6, 7 In particular, PROs enable the healthcare team to more accurately understand the patient experience in terms of symptom incidence and trajectories.6,8
Remote patient monitoring (RPM) is an application of digital technology that allows patients to periodically report PROs through electronic devices (ePROs) thus enabling care teams to monitor patients' symptoms and intervene in real-time. A large group of randomized controlled trials and population research showed that the implementation of PROs and subsequently ePROs and RPM in oncology care leads to improved communication, symptom burden, quality of life, treatment adherence, health resources utilization, overall survival, and it is likely cost-effective.9, 10, 11, 12, 13, 14, 15 This value has been recognized by the European Society of Medical Oncology (ESMO), which published a clinical guideline in 2022 supporting the implementation of RPM based on ePROs in routine clinical care during systemic cancer treatment, specifically including digital systems connecting patients and health care teams with proactive alerts for severe and worsening symptoms.16
Despite this solid evidence base supporting the benefits of ePROs in oncology patient care, implementation in routine clinical practice lags behind and faces challenges ranging from providers, data accuracy, and privacy concerns, reimbursement and organizational issues, equity and technology illiteracy, as well as considerations related to the interoperability with medical records and local digital infrastructures.17 The COVID-19 pandemic accelerated the introduction of RPM systems in oncology due to the heightened need for decentralized care.18 Large multicentric initiatives mainly located in North America, revealed RPM's feasibility in routine oncology care but highlighted the need to optimize clinical pathways and digital systems in order to achieve optimal participation, adherence, and clinical impact.19, 20, 21, 22 A cluster, pragmatic randomized trial that included 52 U.S. community oncology practices and 1191 patients with metastatic cancer in the United States, recently revealed encouraging engagement metrics but also challenges regarding increased healthcare provider (HCP) burden.13,23
Here, we report on the real-world implementation of an RPM pathway across 33 centers in Europe using the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) framework.
Methods
Study design
This study prospectively reports the implementation of an RPM pathway for symptom monitoring via ePROs in support to the routine care of patients with cancer in France and Belgium. The primary aim was to describe the adoption of the solution and the secondary objectives were to describe reach, effectiveness, implementation, and maintenance.
Description of the RPM system
We used an evidence-based RPM system (Resilience PRO, a CE-marked, class IIa medical device) that includes a patient interface (mobile or web) for ePRO reporting and an HCP interface with different levels of integration with local electronic medical records (Supplementary Figure S1). The RPM solution was developed with direct input from a large variety of stakeholders including patients, their families and personal caregivers, healthcare professionals (oncologists, nurses, and supportive care specialists), and engagement experts. After the prescription of the RPM by the medical oncologist and subsequent patient registration on the digital device and education on its use by a nurse navigator, the system prompts patients weekly with a notification by text message, email or pushup notification in the mobilpe app as chosen by the patient to complete a survey including a core set of symptoms from the National Cancer Institute Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (NCI's PRO-CTCAE) questionnaire. The core set of symptom surveys included single PRO-CTCAE items for nausea, vomiting, diarrhea, constipation, dyspnea, fatigue, pain, depression, and anxiety, in addition to a single item from the Patient Health Questionnaire-9 (PHQ-9) focused on depressive symptoms, the patient-reported Eastern Cooperative Oncology Group [ECOG] performance status,24 temperature, weight and information on oral intake (food/drink). The PRO-CTCAE measurement system assesses the frequency, severity, interference, and presence/absence of symptomatic toxicities. A free text field allows for other symptoms or further symptom details to be provided. For applicable symptoms, severe or worsening symptoms i.e., questions with an absolute grade of 3 and 4 (2 or 3 for depression; severe) or moving 2 points (e.g., from 0 to 2; worsening) generate a real-time alert notification sent to the patient's cancer care team, based on prior research.25
Nurse navigators were responsible for onboarding patients in the RPM system (including training on how to report their symptoms and how to use the mobile app) and managing alerts generated by the RPM system. More precisely this included: first reaching up the patient to better understand and categorize the clinical status (through a phone call) and then using locally available symptom management decision trees to manage alerts generated (advising on symptom management by phone, contacting the medical team for prescriptions and advice, organizing a new consultation, hospitalization or emergency visit). Symptom management decision trees from Gustave Roussy were available to other clinical centers as a general guide but they were not required to be used. Of note, from the participating centers only Gustave Roussy already had nurse navigators specifically working in the setting of remote patient monitoring using digital health solutions following the activities of a prior randomized clinical trial performed in the institution.12 For all the other participating centers, nurse navigation using digital tools for ePROs reporting and RPM was a new activity/care role implemented. Existing nurse navigators in local centers were activated for this activity in 23 centers. For the 10 remaining centers, decentralized nurse navigators (Resilience-based) were responsible by onboarding and managing alerts. In general, one nurse navigator could manage up to 200 patients in the system. Nurse navigators advised patients that reported symptoms were reviewed by the care team only during business hours from Monday to Friday, and that the RPM should not be considered as the sole means of communicating problems or for directly contacting the care team or seeking emergency services. Additionally, an algorithm embedded in the RPM system provides guideline-concordant educational content on symptom-relevant self-management advice on the digital patient interface (mobile app). An HCP dashboard summarizes PRO grades and alert status information, and records the actions taken by providers in response to alert notifications.
Implementation process
The implementation of the RPM pathway followed the Exploration, Preparation, Implementation, Sustainment (EPIS) implementation science framework and other validated implementation techniques.17,26,27 A tailored implementation strategy was adopted to adapt to each center's local needs and existing clinical workflows, personnel roles, symptom management approaches, information systems, and patient population characteristics. The actions, interaction strategies and resources proposed for each clinical center during the exploration/preparation, implementation and sustainment phase are detailed in Table 1. A Scientific Committee and a Patients Committee provided oversight throughout the implementation and analysis processes.
Table 1.
Overview of the RPM pathway implementation process according to the exploration, preparation, implementation and sustainability (EPIS) framework.
| Pre-implementation phase (Corresponding EPIS framework: exploration and preparation) | |
| Actions | Frequency of interaction and resources proposed |
|
|
| Pilot implementation phase (Corresponding EPIS framework: implementation)—refers to the enrolment of the first 50 patients in each setting | |
| Actions | Frequency of interaction and strategies applied |
|
|
| Post-implementation phase (Corresponding EPIS framework phase: sustainability) | |
| Actions | Frequency of interactions and strategies applied |
|
|
Patient population and cohort definition
Adult patients with a cancer diagnosis who were beginning systemic therapy and who had received a prescription by their oncologist for the Resilience PRO RPM system as routine care were included in the study. System-level metrics were collected covering all participating hospitals. Considering that this was a real world, pragmatic study in routine care, inclusion criteria for the RPM pathway (such as the volume of patients for which the pathway was proposed and the profile including type of cancer, stage and treatment received) differed in each clinical center. This was defined locally according to organisational wishes and priorities regarding target population and resources available for remote symptom monitoring (existing nurse navigators). Two patient cohorts were established conditional on ethical approvals: cohort 1 including all registered patients (system-level, aggregated data; default cohort except if said otherwise) and cohort 2 including all consented patients for the participation in research-related initiatives (pseudo-anonymized data). Patients registering from November 2021 to August 2023 were included in the cohorts for analyses. A provider-level cohort, was included to conduct HCP experience surveys.
Study outcomes
The implementation evaluation was guided by the RE-AIM framework.1 The primary endpoint was adoption of the pathway as measured by patient adherence to weekly ePRO reporting. Adherence was defined as the number of surveys replied by patients divided by the overall number of surveys requested from patients (typically requested on a weekly basis). Secondarly, we assessed the reach (absolute number and proportion of patients and centers enrolled over a time period), and efficacy (post-alert grade and symptom burden, and median time for alert management by the care team). The alert rate is calculated at the questionnaire level: if a questionnaire has one or more ePRO items triggering an alert, the questionnaire is considered to be on alert and is calculated by dividing the number of surveys on alert by the number of surveys answered by patients.
Post-alert symptoms grade evaluation
PRO grade was compared before and after an alert notification to the care team to determine its variation directionality and the magnitude of variation. Specifically, the grade of the PRO triggering an alert episode was compared to the grade assessed 2 weeks prior to the date of the alert and 2 weeks later. Given the 4 week interval between administered PRO questions for anxiety and depression, these symptoms were excluded from the analysis. A clinically meaningful change was defined with the threshold of 1 point, following a recent study.28 A description of alerts included in the analysis is shown in Supplementary Table S1.
In addition, we evaluated patient and physician experience during implementation (satisfaction survey and Net Promoter Score [NPS]) as well as the potential of the RPM pathway to be permanently integrated in routine care (based on the proportion of centers that sustained RPM practice for more than 12 months).
Statistical analysis
All quantitative analyses were performed on the entire population and by prespecfified cohort. Quantitative variables were described using the mean and standard deviations if the normality assumption was met, otherwise other descriptive statistics (median, range, quartiles) were used. Categorical variables were described using frequency, percentage and 95% confidence interval (binomial distribution).
Ethics approval
This study was submitted to Resilience's Patients Committee and Scientific Committee and was registered in the French Health Data Hub (No. F20221025155833).
Role of the funding source
Part of this work was funded by a Conquer Cancer—Breast Cancer Research Foundation Career Development Award for Diversity and Inclusion, supported by Breast Cancer Research Foundation to Maria Alice Franzoi (personal salary). Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer, or Breast Cancer Research Foundation. Resilience provided funding for decentralized nurse navigation in 10 centers. The design of the study, analysis plan and interpretation of data, writing and decision to submit the paper for publication was performed by the primary investigators (MAF and IVL). Resilience participated in the technological development, data collection and data analysis.
Results
Reach of the RPM pathway
From November 2021 to August 2023, 33 hospitals, including two Organization of European Cancer Institutes (OECI)-designated comprehensive cancer centers, across two countries with substantial regional diversity (Table 2), deployed the RPM system in France and Belgium (Fig. 1). In France, the deployment covered 10 (83%) of the continental administrative regions. The per capita number of physicians as well as the population-level regular internet use across the regions where the pathway was implemented is described in the Supplementary Material (Supplementary Table S2). Eight (24.2%) centers achieved interoperability with the local electronic medical record.
Table 2.
Study cohort characteristics.
| Characteristics | Cohort 1—overall cohort (n = 3015; 100%) | Cohort 2 (n = 1332; 44.2%) |
|---|---|---|
| Age | ||
| Median (IQR) | 63 (52–72) | 60 (50–70) |
| Mean (SD) | 61.6 (13.26) | 59.5 (13.3) |
| Min—Max | 19–95 | 19–95 |
| ≥65 years, n (%) | 1361 (45.1) | 519 (39.0) |
| Sex, n (%) | ||
| Female | 1977 (65.6) | 907 (68.1) |
| Male | 1038 (34.4) | 425 (31.9) |
| Tumor type, n (%) | ||
| Breast | 1155 (38.3) | 564 (42.3) |
| Gastrointestinal | 696 (23.1) | 305 (22.9) |
| Genitourinary | 254 (8.4) | 105 (7.9) |
| Thoracic oncology | 116 (3.8) | 74 (5.6) |
| Gynecologic | 175 (5.8) | 70 (5.3) |
| Others | 433 (14.4) | 126 (9.4) |
| Stage, n (%) | ||
| Non-metastatic | 1087 (35.1) | 591 (44.4) |
| Metastatic | 567 (18.8) | 294 (22.1) |
| Missing | 1661 (55.1) | 447 (33.5) |
| Cancer center type, n (%) | ||
| Hospital center | 748 (24.8%) | 101 (7.6%) |
| Academic hospital | 163 (5.4) | 73 (5.5%) |
| Comprehensive cancer center | 1066 (35.4%) | 648 (48.6%) |
| Private hospital | 987 (32.7%) | 476 (35.7%) |
| Private nonprofit hospital | 51 (1.7%) | 34 (2.6%) |
Fig. 1.
Centers participating in the RPM pathway.
Overall, 3015 patients were registered across all sites. 1977 (65.6%) patients were female, and the median overall age was 63 (min–max: 19–95) years (Table 2) with a total of 1361 patients (45%) aged over 65 years old. Breast was the most represented cancer type (38.3%), followed by colon (8.6%) and pancreas (5.1%); 1087 (35%) of the patients presented early-stage disease and 18.8% metastatic disease.
There were 168 HCPs who engaged with the RPM system to deliver patient care including nurse navigators, oncologists and supportive care specialists.
Effectiveness of the RPM pathway on capturing and managing alerts
The most commonly reported symptoms of any grade by patients were pain (90.7%), diarrhea (84.4%), and dyspnea (83.1%) (cohort 2). In terms of alerts triggered to based on the prespecified thresholds for severity or worsening, the most common symptoms were pain (66.8%), nausea (48.8%), and diarrhea (41.4%) (cohort 2). In contrast, vomiting (12.9%), anxiety (15.7%), and depression (17.4%) were the least commonly identified severe/worsening symptoms (Supplementary Figure S2; cohort 2). During the overall study period, the total alert rate (for severe or worsening symptoms) was 49.2% (11,041/22,441), among which 9.5% yellow alerts (grade 2 PRO CTCAE-aggravating), 27.5% orange alerts (grade 3 PRO CTCAE) and 12.2% red alerts (grade 4 PRO CTCAE). Due to a learning curve associated with the use of the RPM solution by patients and refinements of the symptom survey and alerting processes, the overall alert rate decreased over time. Specifically, in the last 6 months of the study, the overall alert rate was 45.7%. Refinements in the alert system included substitution of the item “loss of apetite” to “reduction in food and water intake”, removal of alerts related uniquely to fatigue, a 24 h recall for the symptom item diahrrea and the addition of a free text field after nausea, vomiting, diarrhea, constipation and pain in case they were present, requiring details on medications taken for the symptom management to speed up symptom management. In addition, in the beginning of the RPM implementation, one large volume cancer center asked for the addition of questions of 0–10 numerical rating scale (NRS) for nausea, vomiting, diarrhea, pain and dyspnea (on top of the PRO-CTCAE questions), this was associated with a higher rate of alerts and was then removed during the implementation.
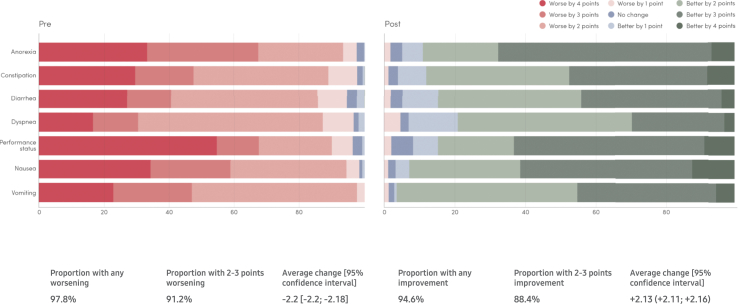
In the period of time subsequent to an alert being triggered to the care team, symptom grades improved in a substantial majority of cases. Specifically, 94.6% of alerts (10,132/10,711) demonstrated a clinically meaningful improvement two weeks later, among which 88.4% (9468/10,711) showed ≥2 grades of improvement on the 5-point scale of the PRO-CTCAE (cohort 2). On average, after an alert was triggered, an improvement of 2.13 (95% CI 2.11–2.16) grades was seen two weeks later (Fig. 2; cohort 2).
Fig. 2.
Pre- and post-alert PRO CTCAE grades.
The median time to alert management by the care team was 13 h 41 min (P25: 1 h 42 min, P75: 1 day +19 h 54 min), with 36,269/45,334 (80%) of alerts managed by a nurse navigator call, 1799/45,334 (4%) by a referral for internal/external medical appointments, 67/45,334 (0.15%) with an emergency room referral and 46/45,334 (0.1%) with an hospitalization referral (non-specified actions were found in approximately 455/45,334 (1.0%) of alerts.
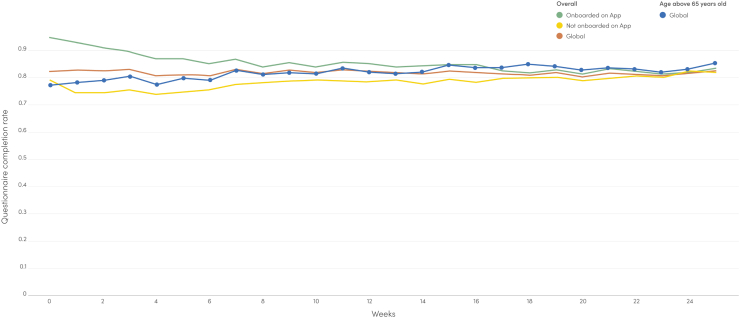
Adoption of the RPM pathway (primary study endpoint)
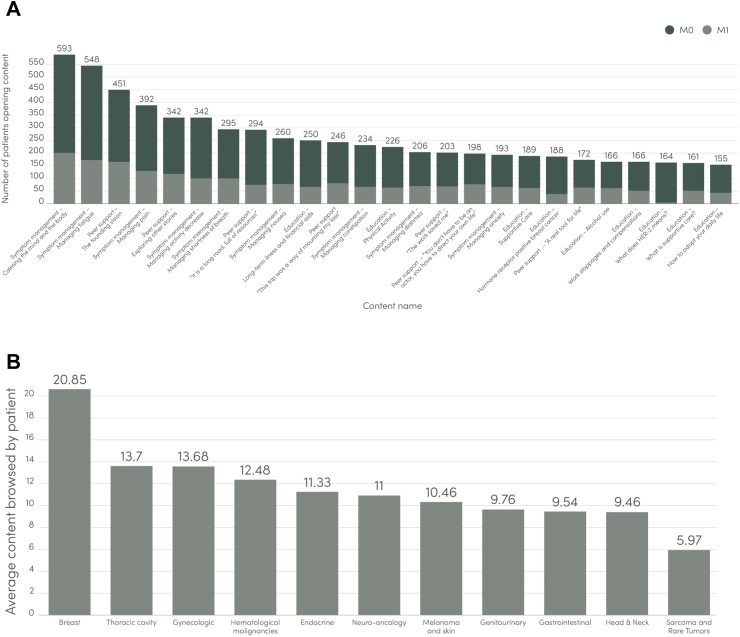
Average patient adherence with weekly ePRO reporting during 6 months of follow-up was 82% overall, varying from 85% for mobile application users to 78% for web users (Fig. 3). Among elderly patients (≥65 years old), adherence with weekly ePRO reporting was an average of 82%. A total of 2020/3015 patients (67.0%) chose to report ePROs through the mobile application and 33% through the web platform. RPM patient retention for ≥3 months, regardless of disease status, was 79%. For mobile app users, the overall median number of individual educational contents accessed was 14.1 (27,131 individual content opened/1921 content users). Overall, most accessed educational contents in the mobile app mirrored main reported symptoms in the cohort, with higher content consumption observed in patients with breast cancer (Fig. 4).
Fig. 3.
Adherence to ePRO surveys.
Fig. 4.
Patterns of content consumption according to metastatic disease status (A) and primary tumor site (B).
Provider and patient surveys on implementation experience
Among a total of 217 providers surveyed, 38 (17.5%) including 18 nurses, 15 physicians and 5 others healthcare providers from 12 centers participated in the HCP Experience Survey. Of these providers, 33 (87%) reported being satisfied with the Resilience RPM Pathway in their organization and 30 (78%) felt patients were better managed through the use of Resilience RPM. In addition, 24 (62%) reported that the Resilience RPM pathway enabled early detection of treatment-related side-effects or deterioration of patient health status.
From 843 Experience Surveys sent, a total of 307 (36.4%) patients from 18 centers replied. On the survey question, “How much of a benefit do you find the Resilience RPM system to be on a scale of 0–10?”, the average score was 8.2, with 270 patients (78%) rating this highly (score ≥7; with 199 [65%] rating this very highly [score 9–10]). In addition, 90% (276) of surveyed patients agreed that the RPM system had a positive impact on the care provided by their healthcare team.
Maintenance of the RPM pathway
All the participating centers (33/33, 100%) are continuing to enroll patients in the RPM Pathway after a period of 12 months with internal expansion of tumor types. The RPM pathway continues to be implemented in new cancer centers in France (18 new centers were activated after database lock). In France, local reimbursement was granted for Resilience RPM in October, 2023, which is intended to support the long-term implementation of the RPM pathway across French institutions. Notably, this reimbursement was not approved yet during the period of the implementation analysis.
Discussion
This study describes the real-world implementation of an RPM pathway in routine oncology care, which enrolled 3015 patients across 33 centers in France and Belgium during the period of November 2021 to August 2023. The RPM pathway implemented in this study demonstrated high levels of patient engagement (i.e., 82% average compliance with weekly ePROs), and effective alert management across a diverse group of participating centers (i.e., median time for nurse navigators to respond to patient's alerts: 13 h 41 min), translating into clinically meaningful benefits28 with decreases in the grade of patient-reported symptoms (i.e., 88.4% decreased by 2 or more grade points out of 5 within two weeks).
Although the benefits of RPM are widely proven, its implementation and adoption in routine care has remained challenging.26 A large cluster randomized trial reported encouraging rates of 91.5% adherence to patients' weekly ePRO reporting during RPM in the metastatic setting.13 However, Cherny et al. and Patt et al. have published their experiences implementing RPM in routine oncology care in community practices in the United States outside a clinical trial.20,29 In these studies, patients’ adherence to weekly ePRO reporting was approximately 64%, with lower engagement among elderly patients. In addition, smaller, single-institution studies of RPM in routine care in France reported an overall 66%–72% adherence rate with weekly ePRO reporting.30,31 In our study, the overall adherence to weekly ePRO reporting was 82% including elderly patients who represented 45% of the cohort. Several factors may have been associated with the high level of patient engagement observed in our study.
First, we employed a structured and well-defined implementation process that was center-adaptive and able to respond to a range of implementation barriers (Table 1). This process followed frameworks and considered software functionality, measured outcomes, personnel deployment, leadership and culture, practical workflow, and patient engagement and the internal and external contexts informing effective implementation.1,26 The present work also benefited from available resources such as the prior French experience of designing RPM systems in the setting of the CAPRI trial,32 the guidelines from the Patient-Reported Outcomes Tools, Engaging Users and Stakeholders Consortium (PROTEUS),27 the tenets for implementing electronic Patient-reported Outcomes for RPM during cancer treatment17 and recommendations for adaptation of RPM systems for real-world settings.21
Secondly, the RPM pathway used in this study was co-designed with a large variety of stakeholders including HCPs, researchers, patients, and technology and engagement experts. Patient representatives were included in the co-design process across all phases of the development (conceptualization, design, beta testing and implementation) either as part of the advisory patient committee or through the participation in formal qualitative usability studies.33 Also, the RPM technology solution (Resilience PRO) used in this study hosts more patient engagement and empowerment features than earlier RPM pathways described in literature. These features include a large library of educational, empowerment, and community-building content that is personalized and delivered in the mobile app when a patient reports relevant symptoms or symptom severities. In prior qualitative studies, patients revealed high satisfaction with the content, design, and usability of our mobile app including the features previously mentioned.33, 34, 35 Such aspects could have impacted engagement, as observed in our results (e.g., higher engagement rates among mobile app users vs. web users). Importantly, a high proportion of our patients (67%) chose to report ePROs through our mobile app interface, suggesting that our study population have had higher digital literacy rates and therefore may have been prone to higher engagement. Indeed, previous studies have demonstrated that when given an option, elderly patients, patients from lower socioeconomic backgrounds, and from black ethnicities tend to choose web-based or automated telephone interfaces for ePRO reporting compared to a mobile app interface.23,36,37 Dedicated studies are ongoing to evaluate in detail the usability and adoption metrics of the educational and empowerment content provided by the Resilience PRO and its impact on quality of life and symptom burden.
Third, the patients participating in our RPM pathway received feedback and evaluation from the care team within a median timeframe of 13 h 41 min. This can be considered effective care coordination and symptom management and may have affected patient engagement and adherence to weekly ePRO reporting. It is important to highlight that the implementation of RPM in routine care requires, besides the technological infrastructure, an organizational change within the cancer care centers. Centers must devote a team of HCPs and staff members to proper RPM planning, deployment, patient onboarding, monitoring and alert management. In July 2023, the French Ministry of Health announced the reimbursement of RPM for chronic health conditions, including cancer. The government now offers a reimbursement fee to the technology provider (if they meet the specified requirements [Art. R. 162–74-I]) and to the RPM deploying healthcare organization, in order to finance the provider in charge of symptom management care coordination. We believe that the launch of this reimbursement system will accelerate the implementation of RPM pathways in routine care in France because the financial support will help combat structural obstacles to the adoption of RPM such as a limited workforce, budgetary constraints, and lack of necessary resources. In the United States, the Centers for Medicare & Medicaid Services (CMS) has reimbursed the practice of remote therapeutic monitoring (RTM), corresponding to RPM in France, since late 2022, also following specific criteria. Since August 2023, a chat functionality was included in the Resilience PRO platform in pilot centers to allow direct information exchange between HCPs and patients. The impact of this feature on alert management as well as HCPs burden will be evaluated in an upcoming study.
A strength of our study was the involvement of a diverse group of cancer centers encompassing comprehensive cancer centers, academic centers, and community practices across a wide geographic distribution within France and Belgium including territories of varying levels of broadband internet access, availability of healthcare professionals and economic income.38,39 The diversity of centers participating in our study demonstrates the ability of the RPM pathway to onboard cross-regional centers and HCPs facing diverse patient needs. It also highlights the increased interest in PROs and RPM pathways from HCPs at wide-ranging, non-academic centers, which participated even before reimbursement was approved in France.
Considering the relative low rates of experience survey responses from HCPs, enrichment of the present analysis with qualitative interviews could bring further insights in contextual factors that may influence implementation and are planned.
Some limitations need to be acknowledged in our study. We lacked data regarding the penetration of the pathway in each institution not allowing us to understand the proportion of eligible patients that were offered to participate in RPM. In addition, individual patient level analyses was possible only with 44% of the cohort (consented patients). For this report, we lacked detailed sociodemographic data on participants due to our desire to minimize the reported burden of data entry for patients and HCPs.40 We recognize, however, that social determinants of health are important factors influencing care delivery, symptom management, and supportive care needs and also play an important role in the adoption and access to digital health solutions.41 We have recently enabled the collection of a set of parameters related to social determinants of health to address this issue42 and better understand engagement metrics, optimize alert management and enhance equitable supportive care delivery, this will be properly reported in an upcoming publication. In addition, although the vast majority of patients experienced improved symptom burden within 2 weeks after an alert was generated, we could not precisely evaluate the proportion of symptoms that would have been transitory in relationship to treatment and normally resolve within 2 weeks. However, several randomized clinical trials with similar interventions have proven that RPM significantly improves symptom burden in comparison with routine care.7,13,14 Furthermore, although cost-effectiveness was not the focus of the present manuscript, a paired analysis with the French nation-wide claims database (the French Social Security Database SNDS) is ongoing to obtain comparative data regarding crude costs of the RPM pathway compared to usual care, as well as on healthcare resources utilization (including hospitalizations, appointments, and emergency visits) that will further support cost-effectiveness analysis.
In conclusion, the implementation of this RPM pathway was feasible across a diverse group of 33 cancer centers in France and Belgium, with high levels of patient and provider participation and engagement and sustained practice, and meaningful improvements in symptom control.
Contributors
MAF: Conceptualisation, Methodology, Investigation, Data Processing and interpretation, Writing—Original Draft, Review and Editing, Visualisation; AF: Conceptualisation, Methodology, Investigation, Software, Formal analysis, Data Processing, Writing—Original Draft, Review and Editing, Visualisation; AL: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing, JR: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; JG: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; JMR: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; LP: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; TG: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; XA: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; KLD: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; MP: Investigation, Data collection, Data Processing and interpretation, Writing—Review and Editing; EN: Investigation, Data Processing and interpretation, Writing—Review and Editing; MA: Investigation, Data Processing and interpretation, Writing—Review and Editing; FA: Conceptualisation, Investigation, Data Processing and interpretation, Writing—Review and Editing; EB: Conceptualisation, Investigation, Data Processing and interpretation, Writing—Review and Editing; OM: Conceptualisation, Investigation, Data Processing and interpretation, Writing—Review and Editing; CF: Investigation, Data Processing and interpretation, Writing—Review and Editing; MDP: Conceptualisation, Investigation, Data Processing and interpretation, Writing—Review and Editing; FS: Conceptualisation, Investigation, Data Processing and interpretation, Writing—Review and Editing; IVL: Conceptualisation, Investigation, Data Processing and interpretation, Writing—Original Draft, Review and Editing, Visualisation.
Data sharing statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps.
Disclaimer/Prior presentation
Presented in part as a poster at the 2023 ASCO Conference (Poster Discussion, Care Delivery Session).
Declaration of interests
Maria Alice Franzoi: Research Funding: Resilience Care (Institution). Speaker honoraria: Novartis (Institution).
Arlindo Ferreira: Resilience (Employment).
Antoine Lemaire: Consultancy or speaker honoraria: Ethypharm, Kyowa Kirin, Baxter, Alfasigma, Mundi Pharma, Accord Healthcare.
Joseph Rodriguez: None.
Jessica Grosjean: None.
Joana M Ribeiro: Travel, Accommodations, Expenses: Gilead, Astra Zeneca, eESO, Exact Sciences, Novartis, Pfizer, Roche and Sanofi, MSD.
Laura Polastro: Speaker fees: Resilience; Research Funding: Resilience (institution).
Thomas Grellety: Speaker fees: Resilience.
Xavier Artignan: None.
Katell Le Du: None.
Martina Pagliuca: Travel expenses: Gilead.
Élodie Nouhaud: Resilience (Employment).
Maximilien Autheman: Resilience (Employment).
Fabrice André: Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), Roche (Inst), Daiichi (Inst). Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca. Ad boards or symposium compensated to the hospital: AstraZeneca, Lilly, Novartis, Pfizer, Daiichi-Sankyo, Relay Tx and Roche. Ad board compensated to the author: Lilly.
Ethan Basch: Stock and Other Ownership Interests: Vector Science. Consulting or Advisory Role: SIVAN Innovation, Navigating Cancer, AstraZeneca, Resilience Care. Other Relationship: Centers for Medicare and Medicaid Services, National Cancer Institute, American Society of Clinical Oncology, JAMA-Journal of the American Medical Association, Patient-Centered OUtcomes Research Institute (PCORI).
Otto Metzger: Dr Metzger reported receiving grant funding from Pfizer Inc and personal fees from Merck & Co and Oncoclinicas outside the submitted work.
Charles Ferté: Resilience (Employment).
Mario Di Palma: Honoraria: AstraZeneca, Novartis. Consulting or Advisory Role: Sandoz. Speakers' Bureau: Amgen, Kyowa Kirin International, MSD Oncology, Mundipharma, Sandoz, Roche. Research Funding: Bayer, Sandoz (Inst), Pierre Fabre (Inst), Fresenius Kabi, Astellas Pharma (Inst), Janssen Oncology (Inst), Roche (Inst), Sanofi (Inst). Travel, Accommodations, Expenses: Pfizer, Novartis.
Florian Scotté: Honoraria: Leo Phar, Viatris, Pharmanovia, Amgen, Gilead Sciences, B. MS GmbH & Co. KG, GlaxoSmithKline.
Ines Vaz-Luis: Speaker honoraria from Amgen, AstraZeneca, Pfizer/Edimark, Novartis, Sandoz (Institutional); Writing engagement from Pfizer/Edimark (Institutional); Research funding from Resilience Care (Institutional), Travelling Novartis.
Acknowledgements
Part of this work was funded by a Conquer Cancer—Breast Cancer Research Foundation Career Development Award for Diversity and Inclusion, supported by Breast Cancer Research Foundation to Maria Alice Franzoi. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of the American Society of Clinical Oncology or Conquer Cancer, or Breast Cancer Research Foundation. Resilience provided funding for decentralized nurse navigation in 10 centers.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.101005.
Appendix A. Supplementary data
References
- 1.Glasgow R.E., Harden S.M., Gaglio B., et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. doi: 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slavova-Azmanova N., Newton J.C., Hohnen H., Johnson C.E., Saunders C. How communication between cancer patients and their specialists affect the quality and cost of cancer care. Support Care Cancer. 2019;27:4575–4585. doi: 10.1007/s00520-019-04761-w. [DOI] [PubMed] [Google Scholar]
- 3.Rodin G., Zimmermann C., Mayer C., et al. Clinician–patient communication: evidence-based recommendations to guide practice in cancer. Curr Oncol. 2009;16:42–49. doi: 10.3747/co.v16i6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L.Y., Manhas D.S., Howard A.F., Olson R.A. Patient-reported outcome use in oncology: a systematic review of the impact on patient-clinician communication. Support Care Cancer. 2018;26:41–60. doi: 10.1007/s00520-017-3865-7. [DOI] [PubMed] [Google Scholar]
- 5.Fromme E.K., Eilers K.M., Mori M., Hsieh Y.-C., Beer T.M. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol. 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Basch E., Iasonos A., McDonough T., et al. Patient versus clinician symptom reporting using the national cancer institute common terminology criteria for adverse events: results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 7.Di Maio M., Gallo C., Leighl N.B., et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33:910–915. doi: 10.1200/JCO.2014.57.9334. [DOI] [PubMed] [Google Scholar]
- 8.Reilly C.M., Bruner D.W., Mitchell S.A., et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velikova G., Booth L., Smith A.B., et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 10.Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbera L., Sutradhar R., Seow H., et al. The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: results of a population-based retrospective matched cohort analysis. Cancer Med. 2020;9:7107–7115. doi: 10.1002/cam4.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mir O., Ferrua M., Fourcade A., et al. Digital remote monitoring plus usual care versus usual care in patients treated with oral anticancer agents: the randomized phase 3 CAPRI trial. Nat Med. 2022;28:1224–1231. doi: 10.1038/s41591-022-01788-1. [DOI] [PubMed] [Google Scholar]
- 13.Basch E., Schrag D., Henson S., et al. Effect of electronic symptom monitoring on patient-reported outcomes among patients with metastatic cancer: a randomized clinical trial. JAMA. 2022;327(24):2413–2422. doi: 10.1001/jama.2022.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire R., McCann L., Kotronoulas G., et al. Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART) BMJ. 2021;374:n1647. doi: 10.1136/bmj.n1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lizée T., Basch E., Trémolières P., et al. Cost-effectiveness of web-based patient-reported outcome surveillance in patients with lung cancer. J Thorac Oncol. 2019;14:1012–1020. doi: 10.1016/j.jtho.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Di Maio M., Basch E., Denis F., et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO clinical practice guideline. Ann Oncol. 2022;33(9):878–892. doi: 10.1016/j.annonc.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Basch E., Rocque G., Mody G., Mullangi S., Patt D. Tenets for implementing electronic patient-reported outcomes for remote symptom monitoring during cancer treatment. JCO Clin Cancer Inform. 2023;7 doi: 10.1200/CCI.22.00187. [DOI] [PubMed] [Google Scholar]
- 18.Pritchett J.C., Borah B.J., Desai A.P., et al. Association of a remote patient monitoring (RPM) program with reduced hospitalizations in cancer patients with COVID-19. JCO Oncol Pract. 2021;17 doi: 10.1200/OP.21.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doolin J.W., Berry J.L., Forbath N.S., et al. Implementing electronic patient-reported outcomes for patients with new oral chemotherapy prescriptions at an academic site and a community site. JCO Clin Cancer Inform. 2021:631–640. doi: 10.1200/CCI.20.00191. [DOI] [PubMed] [Google Scholar]
- 20.Cherny N.I., Parrinello C.M., Kwiatkowsky L., et al. Feasibility of large-scale implementation of an electronic patient-reported outcome remote monitoring system for patients on active treatment at a community cancer center. JCO Oncol Pract. 2022;18:e1918–e1926. doi: 10.1200/OP.22.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocque G.B., Dent D.N., Ingram S.A., et al. Adaptation of remote symptom monitoring using electronic patient-reported outcomes for implementation in real-world settings. JCO Oncol Pract. 2022;18:e1943–e1952. doi: 10.1200/OP.22.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offodile A.C., 2nd, Delgado D., Lin Y.L., et al. Integration of remote symptom and biometric monitoring into the care of adult patients with cancer receiving chemotherapy-A decentralized feasibility pilot study. JCO Oncol Pract. 2023;19:e811–e821. doi: 10.1200/OP.22.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basch E., Stover A.M., Schrag D., et al. Clinical utility and user perceptions of a digital system for electronic patient-reported symptom monitoring during routine cancer care: findings from the PRO-TECT trial. JCO Clin Cancer Inform. 2020;4:947–957. doi: 10.1200/CCI.20.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Rashdan A., Sutradhar R., Nazeri-Rad N., Yao C., Barbera L. Comparing the ability of physician-reported versus patient-reported performance status to predict survival in a population-based cohort of newly diagnosed cancer patients. Clin Oncol. 2021;33:476–482. doi: 10.1016/j.clon.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moullin J.C., Dickson K.S., Stadnick N.A., Rabin B., Aarons G.A. Systematic review of the exploration, preparation, implementation, sustainment (EPIS) framework. Implement Sci. 2019;14:1. doi: 10.1186/s13012-018-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brundage M., Snyder C., Advisory Group The PROTEUS guide to implementing patient-reported outcomes in clinical practice: a synthesis of resources. 2023. www.TheProteusConsortium.org
- 28.Lee M., Basch E., Thanarajasingam G., et al. Identification of meaningful individual-level change thresholds for worsening on the patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) Qual Life Res. 2023;32:S23–S220. [Google Scholar]
- 29.Patt D., Wilfong L., Hudson K.E., et al. Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: dancing the Texas two-step through a pandemic. JCO Clin Cancer Inform. 2021;5:615–621. doi: 10.1200/CCI.21.00063. [DOI] [PubMed] [Google Scholar]
- 30.Rivière C., Brureau L., Parnot C., et al. Effectiveness of a digital telemonitoring platform for cancer care of older patients: the ConnectElderlyPatientToDoctor study. Int J Cancer. 2023;152:504–510. doi: 10.1002/ijc.34196. [DOI] [PubMed] [Google Scholar]
- 31.Helissey C., Parnot C., Rivière C., et al. Effectiveness of electronic patient reporting outcomes, by a digital telemonitoring platform, for prostate cancer care: the Protecty study. Front Digit Health. 2023;5:1104700. doi: 10.3389/fdgth.2023.1104700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrua M., Minvielle E., Fourcade A., et al. How to design a remote patient monitoring system? A French case study. BMC Health Serv Res. 2020;20:434. doi: 10.1186/s12913-020-05293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin E., Di Meglio A., Lapidari P., et al. Abstract P4-11-27: a multimodal and personalized digital companion to help survivors of breast cancer (BC) manage side effects of adjuvant endocrine therapy (ET): a qualitative exploration. Cancer Res. 2022;82 P4-11-27-P4-11–27. [Google Scholar]
- 34.Franzoi M.A., Degousée L., Martin E., et al. Implementing a PROACTive care pathway to empower and support survivors of breast cancer. JCO Oncol Pract. 2023;19:353–361. doi: 10.1200/OP.23.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin E., Ferreira A.R., Zunz M., et al. Interventions psychocorporelles à l’aide d’outils numériques pour améliorer la qualité de vie des patientes atteintes d’un cancer du sein. Congrès AFSOS (Association Francophone Des Soins Oncologiques De Support) https://www.congres-afsos.com/eposter/interventions-psychocorporelles-a-laide-doutils-numeriques-pour-ameliorer-la-qualite-de-vie-des-patientes-atteintes-dun-cancer-du-sein/
- 36.Samuel C.A., Smith A.B., Elkins W., et al. Racial differences in user experiences and perceived value of electronic symptom monitoring in a cohort of black and white bladder and prostate cancer patients. Qual Life Res. 2021;30:3213–3227. doi: 10.1007/s11136-020-02442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cronin C., Tramontano A., Schrag D., et al. Evaluating the use of web versus mobile devices for ePRO reporting and severe symptom responses at 6 cancer centers. J Clin Orthod. 2022;40:241. [Google Scholar]
- 38.Maurin L. Observatoire des inégalités; Tours: 2018. Comprendre les inégalités. [Google Scholar]
- 39.Un niveau de vie et des disparités de revenus plus élevés en Île-de-France et dans les communes denses - Insee Focus - 196. https://www.insee.fr/fr/statistiques/4508514
- 40.Pritchett J.C., Patt D., Thanarajasingam G., Schuster A., Snyder C. Patient-reported outcomes, digital health, and the quest to improve health equity. Am Soc Clin Oncol Educ Book. 2023;43 doi: 10.1200/EDBK_390678. [DOI] [PubMed] [Google Scholar]
- 41.Richardson S., Lawrence K., Schoenthaler A.M., Mann D. A framework for digital health equity. NPJ Digit Med. 2022;5:1–6. doi: 10.1038/s41746-022-00663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franzoi M.A.B., Di Palma M., Ribeiro J.M., et al. The impact of self-reported social determinants of health (SDOH) on patient engagement and symptom burden across a remote patient monitoring (RPM) pathway in 42 European hospitals. J Clin Orthod. 2024;42:1506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.