Summary
Multiple sclerosis is a chronic, inflammatory, and neurodegenerative disease of the central nervous system and a major cause of neurological disability in young adults. Its prevalence and incidence are increasing, and it has been estimated at over 2.8 million cases worldwide, in addition to recent trends towards a shift in MS prevalence to older ages, with peak prevalence estimates in the sixth decade of life. Although historically the relapsing and progressive phases of the disease have been considered separate clinical entities, recent evidence of progression independent of relapse activity (PIRA) has led to a reconsideration of multiple sclerosis as a continuum, in which relapsing and progressive features variably coexist from the earliest stages of the disease, challenging the traditional view of the disease course. In this Series article, we provide an overview of how the traditional description of the clinical course of MS and epidemiological trends in Europe have evolved. For this purpose, we focus on the concept of PIRA, discussing its potential as the main mechanism by which patients acquire disability, how its definition varies between studies, and ongoing research in this field. We emphasise the importance of incorporating the assessment of hidden clinical manifestations into patient management to help uncover and quantify the PIRA phenomenon and the possible implications for future changes in the clinical classification of the disease. At the same time, we provide insights into overcoming the challenges of identifying and defining PIRA and adopting a new understanding of the clinical course of MS.
Keywords: Multiple sclerosis, Epidemiology, Progression idependent of relapse activity, Clinical classification
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory and neurodegenerative disorder of the central nervous system (CNS), affecting over 2.8 million people worldwide.1 Since every CNS site can harbor disease processes, the clinical picture is characterized by highly intra- and inter-individual variability, encompassing, changes in sensation, mobility, balance, vision, sphincter function, and cognition.1 This variability is mirrored by an highly heterogenous clinical phenotype. On the basis of the initial disease course, MS is traditionally classified as either relapsing-remitting (RR) or primary progressive (PP) onset.1 RRMS is the more common phenotype, affecting 85–90% of patients, while PPMS occurs in 10–15% of patients, and is characterized by insidious, relentless accumulation of neurological disability, usually without relapses. Over time, most people with RRMS may develop a progressive course, known as secondary progressive (SP) MS, characterized by a gradual accumulation of disability with or without relapses. The identification of asymptomatic subjects with magnetic resonance imaging (MRI) lesions suggestive of MS, which is termed radiologically isolated syndrome,1 indicates a preclinical prodromal stage of the disease, that can precede symptom onset for years. In the RR phase of MS, disability accumulation has been traditionally attributed to incomplete recovery from relapses, known as relapse-associated worsening (RAW), while during the progressive courses, disability accumulation is mainly indepedent of relapse activity.2 However, this classical dichotomous view of MS has been recently challenged.3 The last few decades have witnessed great advances in our understanding of MS pathogenesis and clinical courses, leading to a new view of the disease, the definition of which is still under debate and research.4 These advances have been accompanied by significant improvements in the diagnosis and treatment of the disease, particularly for the earliest relapsing phases.
Search strategy and selection criteria.
References for this Series paper were identified through searches of PubMed (https://www.ncbi.nlm.nih.gov/pubmed) with the search term “Prevalence”, “Incidence”, “Healthcare”, “Burden”, “Costs”, “Symptoms”, “Hidden”, “Fatigue”, “Cognitive impairment”, “Emotional”, “Depression”, “Anxiety”, “Pain”, “Multiple Sclerosis”, “Relapse associated worsening”, “Progression independent of relapse activity”, “Course”, “Relapsing-remitting”, “Primary Progressive”, “Progressive”, “Secondary Progressive”, from 1st January 1980 until 26th September 2023. Only papers published in English were reviewed. The final reference list was generated with the consensus of all co-Authors of this review based on originality and relevance to the broad scope of this Review, with a focus on articles published during the past five years.
Key messages.
-
-
Recent epidemiological data indicating increasing disease incidence after the age of 50, combined with improved prognosis and reduced disease-related mortality, have contributed to the shift in MS prevalence towards later ages, with peak-age prevalence estimates in the 6th decade of life.
-
-
One of the major advancements in recent research is the improvement of our knowledge of the disease clinical course and the underlying pathogenetic processes, leading to a reconsideration of MS classification.
-
-
Progression independent of relapse activity (PIRA), which refers to disability accrual in the absence of relapses and “inflammatory activity,” is present even in the early stages of relapsing MS and often goes undetected due to the limitations of clinical and paraclinical measures.
-
-
Lowering the threshold of clinical observation—such as focusing on MS “hidden symptoms”, along with regular evaluations of ambulation and upper limb performance—could enable the early identification of the PIRA phenomenon.
-
-
The concept of PIRA should expand rather than replace earlier definitions of disease activity and the therapeutic target of no evidence of disease activity (NEDA).
-
-
Overall, MS can be viewed as a clinical continuum, where concurrent pathophysiological processes and their clinical phenomenological counterparts vary across individuals and within the same individual over time.
In this Series of paper, we discuss recent updates on epidemiology and public health issues of the disease with a specific focus on Europe. We discuss clinical features, focusing on hidden symptoms often overlooked and particularly relevant to the subtle accumulation of disability. The traditional view of MS course and newer data on progression independent of relapse activity (PIRA) are taken into account, as well as the potential implications of such aspects in the clinical classification of the disease.
Current epidemiological landscape in Europe: changing trends and challenges
MS is a prevalent neurological disorder that significantly impacts public health across Europe. It typically starts between 20 years and 40 years, with a female preponderance and an overall ratio of 3:1 for females to males.1 While up to 10% of patients experience the first clinical disease manifestation before the age of 18 years5 recent epidemiological evidence indicates an increasing incidence of the disease after the age of 50 years.6,7 The latter finding, along with improving prognosis and reduced disease-related mortality,8,9 contribute to a shift of MS prevalence towards older age, with peak-age prevalence estimates in the 6th decade of life.10
MS incidence, prevalence, and consequent health burden vary widely among countries, reflecting differences in genetic, environmental, and healthcare factors.1
The geographical distribution of MS in Europe exhibits a “latitudinal gradient,” wherein people living in regions farther from the equator exhibit a higher risk of developing the disease. Conversely, countries closer to the Mediterranean tend to have lower prevalence rates.11 Interestingly, several studies revealed that migration in early life can affect the risk of developing MS, as individuals acquire the same risks as the host population, whereas individuals who migrate after the age of 15 years retain the disease risk of their native country.12, 13, 14, 15 Although latitude seems to influence prevalence more than incidence,16 this pattern implicates a predominant role of environmental factors such as EBV infection, sunlight exposure and vitamin D levels in the development of MS, in addition to genetic predispositions.17,18
Recent epidemiological trends in MS prevalence and incidence across different regions in Europe are summarized in Table 1. Prevalence reflects a combination of cumulated incidence over many years and survival time, which can change independently. The highest regional prevalence was reported in the Scottish Highlands–376 cases per 100,000 inhabitants -,54 followed by other Nordic countries such as, Denmark–315/100,000–(nationwide data from the Danish MS Registry 2023) and Norway–213.8 (95% CI 196.4–231.1) -.20 Countries in Southern Europe tend to have lower rates, as seen in Greece with a prevalence of 43.6 per 100,000 inhabitants. The prevalence increase is likely a combined result of earlier diagnosis, due to the revisions and improvement of diagnostic criteria, better long-term prognosis related to earlier and more effective treatment, and improvement in the quality of data sources.
Table 1.
Epidemiological trends of multiple sclerosis in Europe.
| Region/country | Time period | Incidence (per 100,000/year) (95% Cl) | Prevalence (per 100,000) (95% Cl) | Female/male ratio |
|---|---|---|---|---|
| Denmark19 | 2010–2019 | 11.5 (10.6–12.4) | 284 | 2.02 |
| Southern Norway20 | 2008–2012 | 13.1 | – | – |
| Nordland County, Norway21 | 2010 | 10.1 | 182.4 | 2.2 |
| Hordaland County, Western Norway22 | 2013 | 8.5 (7.3–9.7) | 211.4 (198.3–224.2) | 1.8 |
| Norway23 | 2013 | 8 | 208 | 2.2 |
| Norway24 | 2012 | – | 203 | – |
| Swedish county of Värmland25 | 1996–2000 | 6.46 (5.14–7.78) | 170.1 (154.5–185.5) | 2.3 |
| Sweden26,27 | 2008 | 10.2 | 188.9 (186.1–191.7) | 2.35 |
| Finland Southwest Noth Karelia28 |
2012–2016 | 12.1 (10.5–13.8) 8.6 (6.4–11.2) |
280 (264–296) 168 (148–190) |
2.24 2.11 |
| Iceland29 | 2002–2007 | 7.6 (6.4–9.0) | – | 3 |
| Scotland30 | 2010–2017 | 8.76 | – | 2.3 |
| Isle of Man31 | 2006–2011 | – | 167.7 (143.1–196.7) | 2.6 |
| Wales32 | 2002–2013 | 9.1 (8.8–9.4) | – | – |
| United Kingdom33 | 1990–2010 | 9.64 | 203.4 | 2,5 |
| Ireland34 | 2014–2015 | 6.0 (5.3–6.6) | – | 2.7 |
| Padua, Italy35 | 2011–2015 | 6.5 (4.8–8.2) | 182 (172.9–191.1) | 2.2 |
| Tuscany, Italy36,37 | 2015 2017 |
6.58 | 208.7 | 2 |
| Italy38 | 2015 | – | 109 | – |
| Lazio, Italy39 | 2011 | – | 119.6 (116.8–122.4) | 1.9 |
| Catania, Sicily, Italy40 | 2004 | – | 127.1 (115.1–140.4) | 1.4 |
| Carbonia-Iglesias, Sardinia, Italy41 | 2007 | – | 210.4 (186.3–234.5) | 2 |
| Region Murcia, Spain42 | 2010 | 6.2/100 | 71.9 (60–85) | 2.6 |
| Santiago de Compostela, Spain43 | 2010–2015 | 8 (6–10) | 152 (127–176) | 1.8 |
| Germany44 | 2012 | 10.1 (9.1–11.3) | – | – |
| France45,46 | 2000–2007 | 6.8 (6.7–6.9) | 68–296.5 | 2.7 |
| Switzerland47 | 2011–2015 | 16 (13–19) | 190 (180–190) | 2.8 |
| Netherlands48 | 2008 | 9 (6–16) | – | – |
| Austria49 | 2010–2013 | 19.5 (14.3–24.7) | 158.9 (141.2–175.9) | 1.6 |
| Hungary50 | 2014 | – | 101.8 | 2.9 |
| Germany51 | 2010 | – | 199.5 | 2.3 |
| Germany (children age 15–17)52 | 2009–2018 | – | 19.6–22.7 | 2.47 |
| Czech Republic53 | 2008 | – | 170 | 11.7 |
Incidence, or the number of new cases diagnosed annually, also exhibits regional disparities. Longitudinal studies have documented an increase in MS incidence, which seems to have stabilized around 2000.8 Higher incidence rates have been reported in Northern Europe, including Scotland and Scandinavia, compared to Southern and Eastern Europe.55 However, variations in case definitions, population size, and follow-up periods complicate direct comparisons.
Increases in incidence are generally higher for RRMS rather than for PPMS.56 Additionally, incidence has risen more in women than in men.10 The relative increase in the Danish MS population, followed for over 60 years, was in late-onset MS, which may reflect increased awareness of disease onset in older individuals.
MS poses significant public health challenges in Europe due to its high prevalence, aging patient population, and consequent strain on healthcare resources. Marziniak et al.57 highlighted disparities in MS care across Europe, with varying access to disease-modifying therapies and rehabilitation services.
The chronic and disabling nature of MS exerts substantial economic burdens on individuals, families, and societies. Costs related to MS management, including medical treatments, supportive care, and productivity loss due to disability, strain healthcare budgets and social welfare systems. A cross-sectional study conducted in 16 European countries by Kobelt et al.,58 reported costs from a societal perspective in adjusted for purchasing power parity (PPP). Mean costs were 22,800€ PPP in mild, 37,100€ PPP in moderate and 57,500€ PPP in severe disease; healthcare costs accounted for 68%, 47% and 26%, respectively. With advancing disease, work capacity declined from 82% to 8%, and utility declined from normal population values to less than zero, showing that loss of employment is still one of the most troubling consequences of MS greatly contributing to the economic burden of the disease on society and at a personal level.
The employment gap between MS patients and the general population ranges from 15 to 20%.59 MS patients typically earn less and receive social benefits more than the general population, as reported in the UK60 and Denmark.61 However, an Australian study reported a reduction in the employment gap over a few years, from 14.3% to 3.5%.62 Changes in the current treatment paradigm, including early treatment, particularly with high efficacy disease-modifying treatments can delay disability development and reduce the risk of disability pension.63
Overall, the continuous update of high-quality epidemiological evidence in MS is crucial to inform clinical practice, healthcare policy, and research initiatives. In this context, national and population-based MS registries play a crucial role providing comprehensive, standardized, and longitudinal data on disease incidence, prevalence, and treatment outcomes, thereby facilitating more robust and reliable analyses.64
Traditional view of MS clinical course
The unpredictable course of MS, with its wide range of neurological symptoms, has been puzzling physicians for years. Relapses are the distinguishing features of the RR phase. A relapse is defined as a single clinical episode with symptoms and objective findings reflecting a focal or multifocal inflammatory demyelinating event in the CNS, which can develop acutely or subacutely, with a duration of at least 24 h, and in the absence of fever or infection. Relapses can present with largely heterogeneous neurological disturbances and can be followed by complete or partial recovery, resulting in permanent loss of function.65 In contrast, the progressive phase almost invariably manifests with rather stereotyped motor manifestations, leading to the relentless accumulation of irreversible ambulation impairment. The clinical boundaries between the RR and SPMS are often indistinct,66 as there is no universally accepted definition for the progressive disability worsening, and even experienced physicians can sometimes find it challenging to describe the clinical phenotype.67
Although the risk of transitioning to the progressive phase increases proportionally with disease duration,68 a small percentage of patients avert the progressive course, even after decades from the disease onset.69 In addition, it remains largely unexplained why some patients (∼10–15%) do not experience RR symptoms, but present with a progressive course since the disease onset (PPMS). Notably, compared to historic natural history studies assessing predominantly untreated patients, recent observational studies demonstrated that over the disease modifying treatment era, the latency from disease onset to SPMS has significantly extended.70, 71, 72
At an individual level, relapse features, including their frequency, type and severity of symptoms, and degree of recovery are extremely variable. The occurrence of inflammatory attacks decreases proportionally to the disease duration73 and occasionally overlaps the progressive stage.74 Compelling evidence indicates that relapses rates tend to be higher among females, compared to males,75 to decrease during pregnancy, and to sharply increase during the first post-partum trimester.76 In addition, environmental factors appear to play an important role in the severity of the disease course, as the incidence of relapses has been shown to be higher among smokers, compared to non-smokers,77 and to follow a seasonal variation, with a peak in spring and summer,78 seemingly resulting from low serum levels of 25-hydroxyvitamin D during the preceding winter months.79 Interestingly, relapse phenotype over time was found to be similar to preceding acute episodes, as, at the individual level, disease flares tend to recur with the same symptoms,73 indicating a predisposition to a certain pattern of anatomic focal damage.80
Observational studies showed a prognostic correlation between a higher frequency of relapses during the early phase and more rapid disability accumulation in the long term,81,82 although this predictive effect tends to decrease over time.83 A larger number of inflammatory attacks within 2–5 years from onset proportionally increases the risk of transitioning to the SP course and of accruing severe physical impairment,81,82 lending support to the notion that early florid biological inflammatory activity predisposes to the late development of more severe degenerative processes.84 In addition, the occurrence of motor, sphincteric or cerebellar symptoms at disease onset was found to be associated with poorer disease long-term outcome,85 while sensory and visual relapses predispose to a more favorable course.73
Overall, epidemiological evidence indicates that age is the strongest factor affecting the clinical phenotype, which, by growing older, gradually shifts from relapsing to progressive. With older age, the probability of experiencing a relapsing course decreases, while the risk of becoming progressive increases proportionally.86 Those younger at clinical onset are more likely to experience a high relapse frequency and longer latency to the SP phase. However, relapsing activity declines with increasing age, irrespective of the disease duration,75,86,87 which is in line with radiological,88 pathological,89 and biological90 evidence of a gradual age-dependent reduction of focal inflammation. In addition, with increasing age the pathological processes underlying the progressive phase gradually emerge clinically, Patients experiencing the disease onset after the age of 50 are three times more likely to develop a progressive course, compared to those with the first clinical symptom at 20 years old.91 Indeed, progressive MS has not been described in the pediatric MS population92 and is only rarely observed among young adults.74
PIRA concept: redefining the disease course
The term “progression independent of relapse activity” (PIRA) was coined for the first time by Kappos and colleagues, who described disability progression in people with RRMS occurring in a period free of relapses and which, consequently, was not influenced by any residual disability resulting from previous relapses.93 This concept of PIRA is strongly related to the concept of silent progression, proposed by Cree and colleagues.94 In 2020, Kappos et al. presented a post-hoc analysis of the OPERA I and II trials which were designed to evaluate the efficacy of an anti-CD20 monoclonal antibody (ocrelizumab) against interferon beta 1 b in patients with relapsing MS. In this analysis, despite patients being in the early RR phase (6 mean years from onset), PIRA was reported to be the main mechanism of disability accumulation.95 Since then, a number of research groups have assessed the PIRA phenomenon in observational and trial cohorts, confirming that, in all MS phenotypes, PIRA appears to be the main mechanism by which patients acquire disability.96, 97, 98
However, its definition greatly varies among the different studies, especially in relation to the definition of the relapse-free period. For instance, while some authors indicate that a relapse-free period should start at least three months after the last acute relapse,97, 98, 99 others suggest that it could start as early as one month after any relapse.95 However, if a high specificity for PIRA is desired, a complete absence of relapses over the observation period should be required.4 Moreover, a reliable identification of PIRA should take into account the occurrence of new MRI signs of acute disease activity (the presence of new/enlarging T2 lesions and/or gadolinium-enhancing T1 lesions) in both the brain and spinal cord. A few studies have adopted different definitions of disability progression independent of both clinical and MRI activity (true PIRA,97 pure PIRA98), substantially confirming the prominent role of PIRA in disability accumulation across all MS phenotypes.
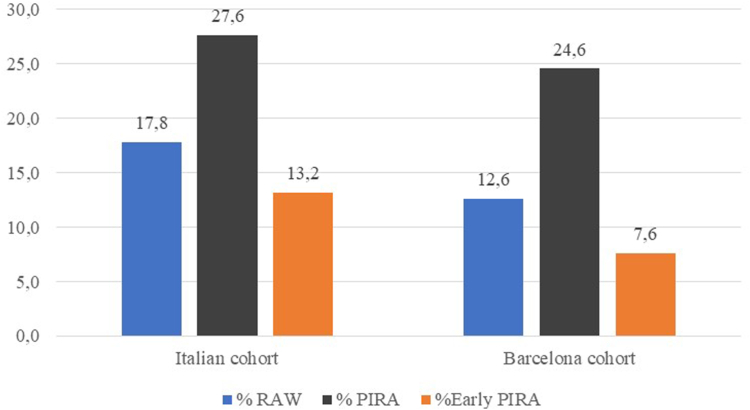
PIRA can occur at any stage of the disease, although, with longer disease duration, its occurrence tends to increase proportionally (Fig. 1).97 Indeed, although its frequency has been globally reported to range between 3% and 4% per each year of follow-up, during the first years of the disease it is probably more sporadic (Fig. 1).98 Overall, PIRA events have been shown to be associated with unfavorable mid- and long-term outcomes, possibly suggesting that it is underpinned by neurodegenerative processes. In addition, PIRA events occurring relatively early after the first attack associate with an even higher risk of unfavorable long-term outcomes.98
Fig. 1.
Percentage of RAW and PIRA events over the follow-up period and early PIRA (within the first 5 years of follow-up) in relapsing MS patients. RAW: Relapse Associated Worsening; PIRA: Progression Independent of Relapse Activity; MS: Multiple Sclerosis.
So far, only two studies have focused on factors predicting PIRA using clinical and demographic characteristics at the first attack or at early stages of the disease.97,98 Interestingly, among the disease features at the first attack, only an older age has been associated with a greater risk of PIRA.97,98 In addition, a longer disease duration97 and the presence of cord lesions100 were also found to be associated with a greater risk of PIRA. Lastly, there are other, less consistent predictors of PIRA across different studies, such as a lower relapse rate prior to PIRA,97 a higher level of disability at study baseline,96,101 or previous exposure to disease-modifying treatments.97 The pathological processes underlying PIRA are yet to be well understood, but several studies indicate that brain and cord atrophy may play a crucial role,94,95,99,101 although other pathological underpinnings may also play a role, such as the accumulation of inflammatory lesions in the brain (and possibly in the cord).95,98 Lastly, studies investigating a possible association between PIRA and other pathological markers which are typically associated with progressive disease, such as slowly expanding lesions (white matter lesions showing linear expansion over time on serial T1-and T2-weighted scans)102,103 and paramagnetic rim lesions (lesions with a paramagnetic hypointense rim on susceptibility-weighted MRI scans, corresponding to peripheral iron-laden microglia/macrophages),103,104 are currently being carried out and will likely shed more light on potential biological mechanisms implicated in relapse-free disease progression.
So far, evidence supporting an effectiveness of the currently available drugs on PIRA is weak, despite the observed treatment effect on some of the PIRA underpinnings in many of their corresponding phase III clinical trials.105,106 Thus, whereas some authors have found a strong treatment effect on PIRA, either considering disease-modifying treatment as a whole97 or focusing on one particular treatment such as ocrelizumab95 or ofatumumab,107 some others have quite clearly shown an absence of such a treatment effect.108 Great expectations are placed on some of the drugs that are currently being tested in randomized phase III trials, providing preliminary encouraging results not only on the anti-inflammatory front but also in counteracting neurodegeneration.109
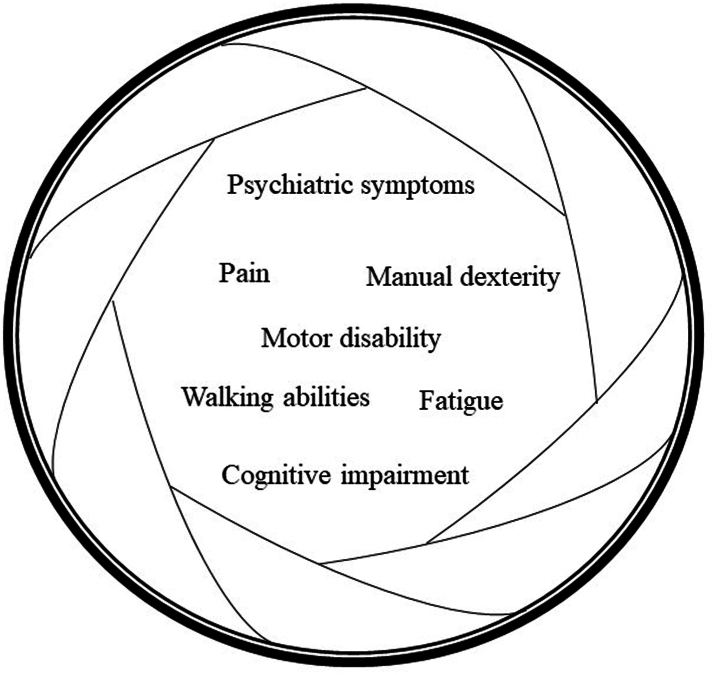
Finally, it must be acknowledged that the concept of PIRA and the fact that it can occur very early in the disease course may trigger some inevitable questions directly related to how we handle these patients in clinical practice. Future efforts should be focused on addressing how the concept of PIRA fits into our current descriptors of the disease course2 and whether these should now be changed, as they may lack sensitivity to capture those patients experiencing physical and cognitive disability accumulation independent of the occurrence of relapses. Timely identification of patients at risk of PIRA may be crucial for more effective clinical management, given the unfavorable prognosis associated with early relapse-free progression. Notably, PIRA may frequently remain undetected due to the low granularity of our clinical measures,4 as its definition is based on changes on the Expanded Disability Status Scale (EDSS) alone,110 which is the most widely accepted measure of clinical disability in MS. The EDSS is a scale that ranges from 0 (a completely normal neurological examination) to 10 (death owing to MS), which is strongly influenced by the assessment of motor abilities, in particular ambulation. On the other hand, changes in other motor and cognitive domains, as well as other less manifest symptoms—known as “hidden” symptoms–such as fatigue, pain and mental health conditions (see below) may also be helpful to define PIRA (Fig. 2). Indeed, in the post-hoc analysis of OPERA I and II trials, approximately 70% of PIRA events were captured by the timed 25-foot walk test,111 a test of walking abilities, and the 9-hole peg test,111 a test of manual dexterity.
Fig. 2.
Widening the focus on hidden symptoms of MS. PIRA may frequently remain undetected due to the low granularity of clinical measures. Widening the focus on clinical features beyond motor disability alone (as measured by the EDSS) could improve the definition and identification of PIRA, as it occurred when assessments of walking ability (using the T25FWT) and manual dexterity (using the 9HPT) were included95 (see text for details). MS: Multiple Sclerosis; EDSS: Expanded Disability Status Scale; 9HPT: 9-Hole Peg Test; T25FWT: Timed 25-foot Walk Test; PIRA: Progression Independent of Relapse Activity.
Towards a new classification of MS clinical course
Recent evidence, partly reviewed in the previous sections, clearly points to the need to revise current definitions of MS courses and progression. The main purpose of the traditional distinction in RR and progressive course of the disease was to standardize terminology and improve homogeneity in clinical trials on the one hand, and to identify patients that are most likely to be responsive to existing disease-modifying treatments on the other. Growing data on PIRA phenomenon render the boundary between RR and SP disease unclear and subtle in most cases, since it is now acknowledged that progression without accompanying relapses takes place early and is difficult to be clinically detected, leading to a delay in the recognition of SP phase.112 Moreover, it has become obvious that the current classification of MS phenotypes does not reflect the biological heterogeneity of the disease. The clinical course of MS should be better considered as a continuum, with concurrent pathophysiological processes that vary across individuals and over time in the same patient.
At the neuropathological level, the dynamics of RAW and PIRA mirror two types of inflammation in MS, as recently outlined by Lassmann and colleagues.113 The focal inflammation is the dominant feature in acute and relapsing MS, and results from focal bulk CNS invasion of T- and B-lymphocytes, causing the classical active demyelinated plaques. The adaptive immune system appears therefore to be particularly important in driving focal inflammation and relapses, which manifest clinically as new episodes of neurological disability, being the pathological substrate of RAW. On the other hand, the second type of inflammation is characterized by both the slow and compartmentalized accumulation of B-cells and T-cells in meningeal lymphoid aggregates, and by the uncontrolled activation of the innate immune system. This type of “smoldering” inflammation is already present in early stages of MS, but gradually increases with disease duration and patient age; it has been preliminarily linked to PIRA events114 and found to be associated with the formation of subpial demyelinated lesions in the cerebral and cerebellar cortex, with the slow expansion of pre-existing lesions in the white matter and with diffuse neurodegeneration in the normal-appearing white or gray matter. The innate immune system is thought to be mainly involved in such chronic pathological processes (the smoldering inflammation), which manifest clinically as a slow, often unnoticed, worsening of neurological deficits. Astrocytes and microglia are indeed recognized elements mediating proinflammatory and neurodegenerative pathological mechanisms in MS. Utilizing MRI-informed, single-nucleus RNA sequencing to profile the chronically inflamed lesion edge of demyelinated lesions at various stages of inflammation, Absinta and colleagues uncovered microglial and astroglial phenotypes demonstrating neurodegenerative programming with transcriptional profiles overlapping with that of microglia in other neurodegenerative diseases.115 Activated microglia and astrocytes become a relevant source of reactive oxygen and nitrogen species, as well as of proinflammatory cytokines and chemokines (such as TNF-α, IL-1β, IL-6, B cell activating factor (BAFF), and CCL2) leading to neurons, oligodendrocytes and endothelial cells alterations, impairments in synaptic transmission and plasticity, mitochondrial failure, eventually reinforcing a positive feedback loop of local CNS inflammation.116 In this scenatio, different radiological and fluid biomarkers of neurodegenerative and smouldering inflammatory processes (reviewed in other papers of this Series) are now available and can help in building up a new mechanism-driven framework to define MS stages and progression.
At the clinical standpoint, it has become clear that the clinical measures currently used in standard clinical practice (such as the EDSS) are not fully capable of capturing the manifestations of the disease and may fail to identify more subtle progression. In this context, searching for hidden symptoms (discussed in the next section) can allow for lowering the threshold of clinical assessment, enabling the detection of earlier and more subtle functional changes.
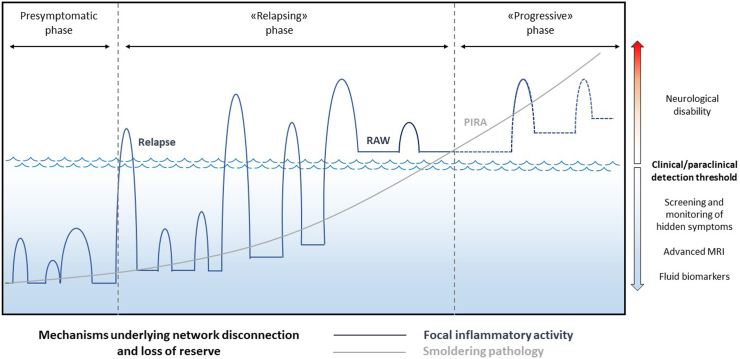
Overall, as recently proposed in the topographical model of MS by Krieger and colleagues,117,118 increasing the granularity of the observations could uncover hidden disease activity; therefore, a “classical” PP or RRMS with later conversion to SP could be reclassified as a disease with early coexistence of relapsing and progressive features (Fig. 3).
Fig. 3.
MS phenoptype may depend on clinical/paraclinical detection threshold. Recent evidence points to an unified view of multiple sclerosis (MS), in which “inflammatory” manifestations (blue line), including clinical relapses and focal magnetic resonance imaging (MRI) activity (new/enlarging T2 lesions, gadolinium-enhancing lesions), mainly driven by adaptive immunity, coexist since the earliest phases of the disease with “neurodegenerative” features (grey line) including disability progression, atrophy, slowly expanding lesions/paramagnetic rim lesions at MRI, mainly driven by innate “smoldering” inflammation. In this scenario, the manifest clinical course of the disease may depend on the type and granularity of the observations. For instance, in assessments relying on less refined clinical measures, such as the Expanded Disability Status Scale, classical primary progressive or relapsing-remitting with later conversion to secondary progression could emerge. However, in the same patient, increasing the granularity of the clinical/paraclinical detection threshold, including advanced MRI measures, composite clincal scales capturing hidden symptoms beyond typical manifestations, patient-reported outcomes, fluid biomarkers (such as neurofilament light chain or glial fibrillary acid protein), could unveil earlier disease acitivty, with inflammatory and neurodegenerative mechanisms largely overlapping since onset. Improving assessments clearly lead to the anticipation of MS diagnosis, pushing back in time the boundary separating the presymptomatic phase from clinically manifest MS, providing the opportunity for early therapeutic interventions. Likewise, the onset of the progressive phase could be identified eralier, becoming coexistent with the initial inflammatory manifestations of the disease.
In this new framework, MS could be precisely characterized at the individual level, based on the presence of specific pathobiological mechanisms that can vary between different patients and in the single patient over time. In this scenario, the combination of different treatments targeting different key pathobiological axes would be crucial for a personalized approach.
Widening the focus on MS clinical features: hidden symptoms
Although the clinical presentation of MS is highly heterogeneous, depending on the site of demyelinating lesions within the central nervous system, some clinical findings are characteristic of the disease. Typical neurological dysfunctions of the initial attack of RRMS are optic neuritis, myelitis, brainstem syndromes, cerebellar syndromes, and cerebral hemispheric syndromes.1 Such clinical events depend on focal inflammatory lesions exerting a disconnecting effect in strategic white matter tracts. Beyond these typical symptoms, there are several frequent clinical manifestations that go often undetected and overlooked, although they account for a significant proportion of the disease burden of people with MS (the so called “hidden symptoms”). These include cognitive impairment (CI), mental health conditions, fatigue, and pain (Fig. 2). Given their significant prevalence even in the early stages of the disease, a better kwnoledge and assessment of these “hidden” clinical manifestations can help to uncover and quantify the PIRA phenomenon, together with a more precise and regular quantification of walking abilities and manual dexterity.
Cognitive impairment
CI can affect up to 75% of patients with MS and occurs in all disease phenotypes (clinically isolated syndrome, RRMS and primary and SPMS).119 The frequency of CI is higher in the progressive forms and in patients with longer disease duration.119, 120, 121, 122 Neuropsychological impairment is believed to be linked to the alteration of nerve conduction in demyelinated or damaged nerve fibres involved in cognitive networks, but also to failure of the compensatory mechanisms associated with the progression of brain damage.123 Focal brain inflammatory lesions, pathological changes of both CNS grey matter and normal-appearing white matter, and inflammation-related dysfunction of synaptic plasticity and neurotransmission can interfere with cognitive functions.124
CI in MS is dominated by a slowdown in information processing speed (IPS), as well as by disturbances of more specific cognitive functions such as attention, episodic memory, working memory, and executive function.125 If a relatively circumscribed alteration in IPS linked to a specific process deficit can occur, changes in IPS can alter other cognitive processes.126 Indeed, cognitive dysfunction can occur independently of IPS alterations, and tends to develop in homogenous phenotypes.122
CI negatively affects health-related quality of life, daily activities such as driving, vocational status, absenteeism, and instrumental activities in persons living with MS.127, 128, 129
Given its prevalence and relevance in people with MS, cognitive dysfunction should be routinely evaluated for a more comprehensive assessment of disease burden, or if specific complaints about difficulties at work or in daily life emerge.
Clinical assessment by a neuropsychologist and the administration of a comprehensive neuropsychological battery are the gold standard for the diagnosis of CI in MS.130 The evaluation should take into account potential confounding factors like fatigue and depression which could influence cognitive performances. However, due to time constraints and limited availability of trained neuropsychologists in most MS centers, this approach is rarely part of the clinical routine evaluation. Therefore, several screening tests or short batteries have been validated and are currently recommended for cognitive screening in MS.
Among those, the Symbol Digit Modalities Test (SDMT) is recognized as the most reliable and sensitive measure of cognition in MS.130,131 Other tests, such as the computerized-speed-cognitive-test,132 less subject to practice effect, are able to identify patients with MS with CI with good accuracy.131,132 Among short batteries, the Brief-International Cognitive Assessment (BICAMS) has been validated in many countries and could be used in clinical practice for detecting cognitive dysfunction in MS133 Whenever a patient tests positive on the initial screening evaluation or reports problems at work or poor performance, a more thorough assessment by a neuropsychologist is recommended.130
Mental health conditions
Depression is the most common psychiatric complaint in MS, affecting 25–50% of the patient population over the course of the illness, a figure which is two to five times higher than that reported in the general population.134 The etiology of depression in patients with MS is associated with pathophysiological changes in the brain, as well as coexistent psychosocial variables.135 In MS patients, a reliable diagnosis of depression can present a potential problem because certain symptoms underpinning the diagnosis of depression may also be caused by MS. A few self-report scales that take this symptom overlap into account have been validated for MS patients (Beck Fast Screen for Medical Patients and the Hospital Anxiety and Depression Scale).134,136 Depression in MS patients is often associated with anxiety.137 MS patients who have both anxiety and depression are more likely to have increased thoughts of self-harm, greater somatic complaints and more extensive social dysfunction than MS patients with depression or anxiety alone.136 Anxiety as a symptom occurs more frequently than depression and conditions such as generalized anxiety, panic disorder, obsessive-compulsive disorder and social phobia are all more frequent in people with MS compared to the general population.136
Overall, depression and anxiety are two potentially treatable factors that affect the psychosocial burden of MS patients. Therefore, they should be systematically screened to enable early identification and prompt introduction of appropriate, personalized interventions.
Fatigue
Fatigue, an overwhelming feeling of tiredness and exhaustion, is a highly prevalent symptom occurring in 50–90% of patients with MS.138 It occurs at all stages of the disease, may precede its clinical onset and may also be associated with relapses.
Fatigue can be classified into primary and secondary fatigue, the latter being related to other MS manifestations (such as overall disability and reduced activity, spasticity, sleep disorders, sphincter disorders, pain), psychological factors, drugs and other medical conditions.139,140 Primary MS fatigue is generally multifactorial, likely linked to brain lesion load, functional changes and disruptions of cortical and subcortical networks,141,142 nerve conduction alterations,143 immune, metabolic and neuroendocrine factors.144
For many patients, fatigue is the most disabling symptom in daily life, yet its nonspecific nature and lack of tight association with disability mean that it is often overlooked by family and caregivers. Fatigue often aggravates other symptoms of the disease, and, in some studies, can occur on a daily basis in up to 40% of cases.
Detection and monitoring during routine visits, as well as under experimental treatments, rely on self-report questionnaires, such as the Fatigue Severity Scale, the Modified Fatigue Impact Scale and the Fatigue Scale for Motor and Cognitive Functions.138 The absence of reliable, objective assessment tools hampers successful measurement and treatment of MS-related fatigue.
Pain
In MS patients, the prevalence of pain, both nociceptive and neuropathic, is around 63%.145 Nociceptive pain in MS includes relapse-associated pain (for example, retro-ocular pain in optic neuritis), spasticity (mainly in progressive MS), low back pain, colic pain, iatrogenic pain. Migraine is also frequently associated with MS.146,147
Neuropathic pain, a type of chronic pain caused by a lesion or disease of the somatosensory nervous system,148 occurs in approximately 27% of people with MS, frequently linked to a neuropathic mechanism secondary to both focal and diffuse CNS lesions (continuous and paroxysmal neuropathic pain).149,150
More than half of neuropathic pain episodes observed in MS are continuous neuropathic pain. They may arise during a myelitis attack and persist as sequelae, or arise insidiously outside any attack. They may persist for many months or even years, and are not improved by corticosteroid treatment once the attack is over. They mainly affect the lower limbs, and sometimes the trunk. Clinically, they have the characteristics of central neuropathic pain. Paroxysmal neuropathic pain is typically electrical discharges or painful paresthesias/dysesthesias. The prototype is trigeminal neuralgia secondary to MS. The young age of the patient and their often bilateral nature distinguish them from essential neuralgia, the semiology of which is very similar. Other paroxysmal phenomena include painful tonic seizures, non-epileptic acute dystonic episodes, which are very characteristic of MS, and are often triggered by movements. These may appear during the recovery phase of a relapse, and they are very painfully sustained for a few minutes.149 Overall, pain significantly contributes to the impairment of health-related quality of life and has an impact on work capabilities.149,151
Correct identification and classification of different pain syndromes can lead to better management strategies for coping with this manifestation of MS.
Conclusions and future perspectives
MS is a chronic disease resulting in neurological impairment and disability in young adults. Due to its increasing prevalence and its substantial economic burden, it represents a significant healthcare challenge. Over the past 20 years, the development of an increasing number of disease-modifying therapies and improvements in treatment strategies have reduced the long-term impact of the disease. However, effective prevention and recovery of disability progression remain largely unmet needs. One of the major advancements in recent research is the improvement of our knowledge of the disease clinical course and the underlying pathogenetic processes, leading to a reconsideration of MS classification. PIRA and neurodegenerative changes, formerly deemed to be confined to the more advanced phases of the disease, have been clearly demonstrated even in the earliest stages, and should be incorporated among the outcomes of MS clinical trials. On the other hand, effective therapeutic suppression of relapses and focal MRI activity with existing immunotherapies remains the cornerstone of current therapeutic management, and has been shown to substantially improve MS long-term prognosis. Indeed, the concept of PIRA should expand rather than replace earlier definitions of disease activity and the therapeutic target of no evidence of disease activity (NEDA). In its classical definition, NEDA includes the concomitant absence of relapses, new focal MRI activity, and disability accrual on the EDSS, and has been shown to be significantly associated with better long-term outcomes.152 We envisage that, combining and refining the traditional NEDA with newer acquisitions on PIRA and both radiological and laboratory biomarkers could improve the definition and identification of disease activity and subsequently optimize the response to old and new disease modifying treatments.
While the debate on the revision of MS phenotypes and the characterization of disease activity is ongoing, there are several points that need to be addressed. Firstly, a more reliable definition of PIRA should be identified,4 as great variability across studies persists, with different baseline assessment based on different clinical measures, various definitions of meaningful change and time interval for its confirmation. Taking into account the measurement of hidden symptoms such as cognition and fatigue, along with evaluations of ambulation and upper limb performance can improve the identification of relapse-free progression. The relevance of MRI to PIRA definition needs to be clarified.4 Moreover, much effort is needed to expand our knowledge of predictors of PIRA. To date, only age has been consistently shown to be the main factor associated with insidious progression, but other clinical, genetic, biological fluid and MRI markers (the latter two addressed in other papers of this Series) should be identified. As for the pathogenetic underpinnings of PIRA, the role of microglia, astroglia, and “smoldering” inflammation is increasingly acknowledged. However, further studies are needed to link different clinical manifestations to specific pathogenetic mechanisms and, in turn, to develop specific treatment interventions. Addressing these challenges will allow an individualized phenotyping of the disease, a personalized pharmacological approach and, hopefully, the prevention of disability accumulation.
Contributors
Concept and design of the review: E. Portaccio, M.P. Amato, M. Di Filippo. Interpretation of data and drafting of the review: E. Portaccio, M. Magyari, E.K. Havrdova, A. Ruet, B. Brochet, A. Scalfari, M. Di Filippo, C. Tur, X. Montalban, M.P. Amato. Critical revision of the manuscript for important intellectual content: E. Portaccio, M. Magyari, E.K. Havrdova, A. Ruet, B. Brochet, A. Scalfari, M. Di Filippo, C. Tur, X. Montalban, M.P. Amato.
Declaration of interests
E. Portaccio received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, participation on a Data Safety Monitoring Board or Advisory Board and support for attending meetings and/or travel from Biogen, Merck Serono, Sanofi, Teva, Roche, BMS Cellgene, Janssen and Novartis.
M. Magyari received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, Merck, Novartis, Roche, Sanofi, Bristol Myers Squibb and for participation on a Data Safety Monitoring Board or Advisory Board from Sanofi, Novartis, Merck, Moderna.
E.K. Havrdova received grants or contracts from Czech Ministry of Education—project Cooperatio LF1, research area Neuroscience and the project National Institute for Neurological Research (Programme EXCELES, ID project No LX22NPO5107), payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Actelion (Janssen/J&J), Biogen, Celgene (BMS), Merck, Novartis, Roche, Sanofi and Teva and for participation on a Data Safety Monitoring Board or Advisory Board from Actelion (Janssen/J&J), Biogen, Celgene (BMS), Merck, Novartis, Roche and Sanofi.
A. Ruet received grants or contracts from BMS Celgene, Roche, Biogen, Sanofi Genzyme, Merck, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, Merck, Sanofi Genzyme and support for attending meetings and/or travel from Alexion, Biogen, Novartis.
B Brochet received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events and support for attending meetings and/or travel from BMS Celgene, Merck Serono, Roche and for participation on a Data Safety Monitoring Board or Advisory Board from BMS Celgene, Merck Serono, Novartis, Roche and Sanofi.
A. Scalfari reports no disclosures relevant to this manuscript.
M. Di Filippo received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, participation on a Data Safety Monitoring Board or Advisory Board and support for attending meetings and/or travel from Alexion, BMS, Bayer, Biogen Idec, Genzyme, Horizon, Janssen, Merck, Mylan, Novartis, Roche, Siemens Healthineers, Teva and Viatris.
Carmen Tur received grants or contract from Junior Leader La Caixa Fellowship (fellowship code is LCF/BQ/PI20/11760008), awarded by “la Caixa” Foundation (ID 100010434), Miguel Servet Contract (CP23/00117), awarded by Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation and FORTALECE grant (FORT23/00034), awarded by Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, Novartis, Bristol Myers Squibb, Johnson and Johnson, Immunic AG, and Merck; support for attending meetings and/or travel, participation on a Data Safety Monitoring Board or Advisory Board from Roche, Novartis, Bristol Myers Squibb, and Merck.
X. Montalban received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, participation on a Data Safety Monitoring Board or Advisory Board and support for attending meetings and/or travel from AbbVie, Actelion, Alexion, Biogen, BMS/Celgene, EMD Serono Research & Development Institute, Inc., Billerica, MA, USA, an affiliate of Merck KGaA, Genzyme, Hoffmann-La Roche, Immunic, Janssen Pharmaceuticals, MedDay, Merck, Mylan, NervGen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, EXCEMED, MSIF and NMSS.
M.P. Amato received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, participation on a Data Safety Monitoring Board or Advisory Board and support for attending meetings and/or travel from Biogen Idec, Merck Serono, Bayer Schering Pharma, and Sanofi Aventis.
References
- 1.Jakimovski D., Bittner S., Zivadinov R., et al. Multiple sclerosis. Lancet. 2023 doi: 10.1016/S0140-6736(23)01473-3. [DOI] [PubMed] [Google Scholar]
- 2.Lublin F.D., Reingold S.C., Cohen J.A., et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhlmann T., Moccia M., Coetzee T., et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 2023;22(1):78–88. doi: 10.1016/S1474-4422(22)00289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller J., Cagol A., Lorscheider J., et al. Harmonizing definitions for progression independent of relapse activity in multiple sclerosis: a systematic review. JAMA Neurol. 2023;80(11):1232–1245. doi: 10.1001/jamaneurol.2023.3331. [DOI] [PubMed] [Google Scholar]
- 5.Iaffaldano P., Simone M., Lucisano G., et al. Prognostic indicators in pediatric clinically isolated syndrome. Ann Neurol. 2017;81(5):729–739. doi: 10.1002/ana.24938. [DOI] [PubMed] [Google Scholar]
- 6.Vaughn C.B., Jakimovski D., Kavak K.S., et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. 2019;15(6):329–342. doi: 10.1038/s41582-019-0183-3. [DOI] [PubMed] [Google Scholar]
- 7.Prosperini L., Lucchini M., Ruggieri S., et al. Shift of multiple sclerosis onset towards older age. J Neurol Neurosurg Psychiatry. 2022 doi: 10.1136/jnnp-2022-329049. [DOI] [PubMed] [Google Scholar]
- 8.Koch-Henriksen N., Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol. 2021;17(11):676–688. doi: 10.1038/s41582-021-00556-y. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen P.S., Sellebjerg F., Hartung H.P., Montalban X., Comi G., Tintoré M. The apparently milder course of multiple sclerosis: changes in the diagnostic criteria, therapy and natural history. Brain. 2020;143(9):2637–2652. doi: 10.1093/brain/awaa145. [DOI] [PubMed] [Google Scholar]
- 10.Koch-Henriksen N., Thygesen L.C., Stenager E., Laursen B., Magyari M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology. 2018;90(22):e1954–e1963. doi: 10.1212/WNL.0000000000005612. [DOI] [PubMed] [Google Scholar]
- 11.Simpson S., Blizzard L., Otahal P., Van der Mei I., Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 12.Alter M., Leibowitz U., Speer J. Risk of multiple sclerosis related to age at immigration to Israel. Arch Neurol. 1966;15(3):234–237. doi: 10.1001/archneur.1966.00470150012002. [DOI] [PubMed] [Google Scholar]
- 13.Dean G., Kurtzke J.F. On the risk of multiple sclerosis according to age at immigration to South Africa. Br Med J. 1971;3(5777):725–729. doi: 10.1136/bmj.3.5777.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean G., Elian M. Age at immigration to England of Asian and Caribbean immigrants and the risk of developing multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997;63(5):565–568. doi: 10.1136/jnnp.63.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotstein D.L., Marrie R.A., Maxwell C., et al. MS risk in immigrants in the McDonald era: a population-based study in Ontario, Canada. Neurology. 2019;93(24):e2203–e2215. doi: 10.1212/WNL.0000000000008611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koch-Henriksen N., Sørensen P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 17.Olsson T., Barcellos L.F., Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13(1):25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 18.Bjornevik K., Cortese M., Healy B.C., et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 19.Magyari M., Joensen H., Laursen B., Koch-Henriksen N. The Danish multiple sclerosis Registry. Brain Behav. 2021;11(1) doi: 10.1002/brb3.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonsen C.S., Edland A., Berg-Hansen P., Celius E.G. High prevalence and increasing incidence of multiple sclerosis in the Norwegian county of Buskerud. Acta Neurol Scand. 2017;135(4):412–418. doi: 10.1111/ane.12615. [DOI] [PubMed] [Google Scholar]
- 21.Benjaminsen E., Olavsen J., Karlberg M., Alstadhaug K.B. Multiple sclerosis in the far north--incidence and prevalence in Nordland County, Norway, 1970-2010. BMC Neurol. 2014;14:226. doi: 10.1186/s12883-014-0226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grytten N., Aarseth J.H., Lunde H.M., Myhr K.M. A 60-year follow-up of the incidence and prevalence of multiple sclerosis in Hordaland County, Western Norway. J Neurol Neurosurg Psychiatry. 2016;87(1):100–105. doi: 10.1136/jnnp-2014-309906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grytten N., Torkildsen Ø., Myhr K.M. Time trends in the incidence and prevalence of multiple sclerosis in Norway during eight decades. Acta Neurol Scand. 2015;132(199):29–36. doi: 10.1111/ane.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg-Hansen P., Moen S.M., Sandvik L., et al. Prevalence of multiple sclerosis among immigrants in Norway. Mult Scler. 2015;21(6):695–702. doi: 10.1177/1352458514554055. [DOI] [PubMed] [Google Scholar]
- 25.Boström I., Stawiarz L., Landtblom A.M. Age-specific sex ratio of multiple sclerosis in the National Swedish MS Register (SMSreg) Mult Scler. 2014;20(4):513–514. doi: 10.1177/1352458513498636. [DOI] [PubMed] [Google Scholar]
- 26.Ahlgren C., Odén A., Lycke J. High nationwide prevalence of multiple sclerosis in Sweden. Mult Scler. 2011;17(8):901–908. doi: 10.1177/1352458511403794. [DOI] [PubMed] [Google Scholar]
- 27.Ahlgren C., Odén A., Lycke J. High nationwide incidence of multiple sclerosis in Sweden. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirttisalo A.L., Soilu-Hänninen M., Sipilä J.O.T. Multiple sclerosis epidemiology in Finland: regional differences and high incidence. Acta Neurol Scand. 2019;139(4):353–359. doi: 10.1111/ane.13057. [DOI] [PubMed] [Google Scholar]
- 29.Eliasdottir O.J., Olafsson E., Kjartansson O. Incidence of multiple sclerosis in Iceland, 2002-2007: a population-based study. Mult Scler. 2011;17(8):909–913. doi: 10.1177/1352458511402112. [DOI] [PubMed] [Google Scholar]
- 30.Kearns P.K.A., Paton M., O'Neill M., et al. Regional variation in the incidence rate and sex ratio of multiple sclerosis in Scotland 2010-2017: findings from the Scottish Multiple Sclerosis Register. J Neurol. 2019;266(10):2376–2386. doi: 10.1007/s00415-019-09413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson S., Mina S., Morris H., Mahendran S., Taylor B., Boggild M. The epidemiology of multiple sclerosis in the Isle of Man: 2006-2011. Acta Neurol Scand. 2015;132(6):381–388. doi: 10.1111/ane.12405. [DOI] [PubMed] [Google Scholar]
- 32.Balbuena L.D., Middleton R.M., Tuite-Dalton K., Pouliou T., Williams K.E., Noble G.J. Sunshine, sea, and season of birth: MS incidence in wales. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie I.S., Morant S.V., Bloomfield G.A., MacDonald T.M., O'Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990-2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry. 2014;85(1):76–84. doi: 10.1136/jnnp-2013-305450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell K., Tubridy N., Hutchinson M., McGuigan C. Incidence of multiple sclerosis in the Republic of Ireland: a prospective population-based study. Mult Scler Relat Disord. 2017;13:75–80. doi: 10.1016/j.msard.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Grassivaro F., Puthenparampil M., Pengo M., et al. Multiple sclerosis incidence and prevalence trends in the province of Padua, Northeast Italy, 1965-2018. Neuroepidemiology. 2019;52(1-2):41–46. doi: 10.1159/000493857. [DOI] [PubMed] [Google Scholar]
- 36.Bezzini D., Policardo L., Profili F., et al. Multiple sclerosis incidence in Tuscany from administrative data. Neurol Sci. 2018;39(11):1881–1885. doi: 10.1007/s10072-018-3513-0. [DOI] [PubMed] [Google Scholar]
- 37.Bezzini D., Ulivelli M., Gualdani E., et al. Increasing prevalence of multiple sclerosis in Tuscany, Italy. Neurol Sci. 2020;41(2):397–402. doi: 10.1007/s10072-019-04090-0. [DOI] [PubMed] [Google Scholar]
- 38.Battaglia M.A., Bezzini D. Estimated prevalence of multiple sclerosis in Italy in 2015. Neurol Sci. 2017;38(3):473–479. doi: 10.1007/s10072-016-2801-9. [DOI] [PubMed] [Google Scholar]
- 39.Bargagli A.M., Colais P., Agabiti N., et al. Prevalence of multiple sclerosis in the Lazio region, Italy: use of an algorithm based on health information systems. J Neurol. 2016;263(4):751–759. doi: 10.1007/s00415-016-8049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoletti A., Patti F., Lo F.S., et al. Increasing frequency of multiple sclerosis in Catania, Sicily: a 30-year survey. Mult Scler. 2011;17(3):273–280. doi: 10.1177/1352458510386995. [DOI] [PubMed] [Google Scholar]
- 41.Cocco E., Sardu C., Massa R., et al. Epidemiology of multiple sclerosis in south-western Sardinia. Mult Scler. 2011;17(11):1282–1289. doi: 10.1177/1352458511408754. [DOI] [PubMed] [Google Scholar]
- 42.Candeliere-Merlicco A., Valero-Delgado F., Martínez-Vidal S., et al. Prevalence of multiple sclerosis in health district III, Murcia, Spain. Mult Scler Relat Disord. 2016;9:31–35. doi: 10.1016/j.msard.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Costa Arpín E., Naveiro Soneira J., Lema Bouzas M., González Quintela A., Prieto González J.M. Epidemiology of multiple sclerosis in Santiago de Compostela (Spain) Acta Neurol Scand. 2020;142(3):267–274. doi: 10.1111/ane.13265. [DOI] [PubMed] [Google Scholar]
- 44.Schmedt N., Khil L., Berger K., Riedel O. Incidence of multiple sclerosis in Germany: a cohort study applying different case definitions based on claims data. Neuroepidemiology. 2017;49(3-4):91–98. doi: 10.1159/000481990. [DOI] [PubMed] [Google Scholar]
- 45.Fromont A., Binquet C., Sauleau E., et al. National estimate of multiple sclerosis incidence in France (2001-2007) Mult Scler. 2012;18(8):1108–1115. doi: 10.1177/1352458511433305. [DOI] [PubMed] [Google Scholar]
- 46.Pivot D., Debouverie M., Grzebyk M., et al. Geographical heterogeneity of multiple sclerosis prevalence in France. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blozik E., Rapold R., Eichler K., Reich O. Epidemiology and costs of multiple sclerosis in Switzerland: an analysis of health-care claims data, 2011-2015. Neuropsychiatr Dis Treat. 2017;13:2737–2745. doi: 10.2147/NDT.S143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer M.A., van der Maas N.A., van Soest E.M., Kemmeren J.M., de Melker H.E., Sturkenboom M.C. Incidence of multiple sclerosis in the general population in the Netherlands, 1996-2008. Neuroepidemiology. 2012;39(2):96–102. doi: 10.1159/000338678. [DOI] [PubMed] [Google Scholar]
- 49.Salhofer-Polanyi S., Cetin H., Leutmezer F., et al. Epidemiology of multiple sclerosis in Austria. Neuroepidemiology. 2017;49(1-2):40–44. doi: 10.1159/000479696. [DOI] [PubMed] [Google Scholar]
- 50.Biernacki T., Sandi D., Fricska-Nagy Z., et al. Epidemiology of multiple sclerosis in Central Europe, update from Hungary. Brain Behav. 2020;10(5) doi: 10.1002/brb3.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen G., Wittmann R., Arndt V., Göpffarth D. [Epidemiology of multiple sclerosis in Germany: regional differences and drug prescription in the claims data of the statutory health insurance] Nervenarzt. 2014;85(8):990–998. doi: 10.1007/s00115-014-4097-4. [DOI] [PubMed] [Google Scholar]
- 52.Frahm N., Peters M., Bätzing J., et al. Prevalence of pediatric multiple sclerosis in Germany: a nationwide population-based analysis. Eur J Neurol. 2021;28(9):3173–3176. doi: 10.1111/ene.15015. [DOI] [PubMed] [Google Scholar]
- 53.Vachová M. Epidemie roztroušené sklerózy ve světě? Cesk Slov Neurol N. 2012;75/108(6):701–706. [Google Scholar]
- 54.Carod-Artal F.J. The epidemiology of multiple sclerosis in the Scottish Highlands: prevalence, incidence and time to confirmed diagnosis and treatment initiation. Mult Scler Relat Disord. 2021;47 doi: 10.1016/j.msard.2020.102657. [DOI] [PubMed] [Google Scholar]
- 55.Lane J., Ng H.S., Poyser C., Lucas R.M., Tremlett H. Multiple sclerosis incidence: a systematic review of change over time by geographical region. Mult Scler Relat Disord. 2022;63 doi: 10.1016/j.msard.2022.103932. [DOI] [PubMed] [Google Scholar]
- 56.Westerlind H., Stawiarz L., Fink K., Hillert J., Manouchehrinia A. A significant decrease in diagnosis of primary progressive multiple sclerosis: a cohort study. Mult Scler. 2016;22(8):1071–1079. doi: 10.1177/1352458516643394. [DOI] [PubMed] [Google Scholar]
- 57.Marziniak M., Ghorab K., Kozubski W., et al. Variations in multiple sclerosis practice within Europe–is it time for a new treatment guideline? Mult Scler Relat Disord. 2016;8:35–44. doi: 10.1016/j.msard.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Kobelt G., Thompson A., Berg J., et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–1136. doi: 10.1177/1352458517694432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kavaliunas A., Danylaite Karrenbauer V., Hillert J. Socioeconomic consequences of multiple sclerosis-A systematic literature review. Acta Neurol Scand. 2021;143(6):587–601. doi: 10.1111/ane.13411. [DOI] [PubMed] [Google Scholar]
- 60.Green G., Todd J., Pevalin D. Biographical disruption associated with multiple sclerosis: using propensity scoring to assess the impact. Soc Sci Med. 2007;65(3):524–535. doi: 10.1016/j.socscimed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Jennum P., Wanscher B., Frederiksen J., Kjellberg J. The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol. 2012;22(1):36–43. doi: 10.1016/j.euroneuro.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Van Dijk P.A., Kirk-Brown A.K., Taylor B., van der Mei I. Closing the gap: longitudinal changes in employment for Australians with multiple sclerosis. Mult Scler. 2017;23(10):1415–1423. doi: 10.1177/1352458516678934. [DOI] [PubMed] [Google Scholar]
- 63.Wandall-Holm M.F., Buron M.D., Kopp T.I., Thielen K., Sellebjerg F., Magyari M. Time to first treatment and risk of disability pension in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(8):858–864. doi: 10.1136/jnnp-2022-329058. [DOI] [PubMed] [Google Scholar]
- 64.Glaser A., Stahmann A., Meissner T., et al. Multiple sclerosis registries in Europe–an updated mapping survey. Mult Scler Relat Disord. 2019;27:171–178. doi: 10.1016/j.msard.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 65.Lublin F.D., Baier M., Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61(11):1528–1532. doi: 10.1212/01.wnl.0000096175.39831.21. [DOI] [PubMed] [Google Scholar]
- 66.Kremenchutzky M., Rice G.P., Baskerville J., Wingerchuk D.M., Ebers G.C. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain. 2006;129(Pt 3):584–594. doi: 10.1093/brain/awh721. [DOI] [PubMed] [Google Scholar]
- 67.Rojas J.I., Patrucco L., Alonso R., et al. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis: multicenter study in Argentina. Mult Scler. 2021;27(4):579–584. doi: 10.1177/1352458520924586. [DOI] [PubMed] [Google Scholar]
- 68.Scalfari A., Neuhaus A., Daumer M., Deluca G.C., Muraro P.A., Ebers G.C. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. 2013;70(2):214–222. doi: 10.1001/jamaneurol.2013.599. [DOI] [PubMed] [Google Scholar]
- 69.Skoog B., Runmarker B., Winblad S., Ekholm S., Andersen O. A representative cohort of patients with non-progressive multiple sclerosis at the age of normal life expectancy. Brain. 2012;135(Pt 3):900–911. doi: 10.1093/brain/awr336. [DOI] [PubMed] [Google Scholar]
- 70.Lorscheider J., Buzzard K., Jokubaitis V., et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–2405. doi: 10.1093/brain/aww173. [DOI] [PubMed] [Google Scholar]
- 71.Brown J.W.L., Coles A., Horakova D., et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175–187. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iaffaldano P., Lucisano G., Patti F., et al. Transition to secondary progression in relapsing-onset multiple sclerosis: definitions and risk factors. Mult Scler. 2021;27(3):430–438. doi: 10.1177/1352458520974366. [DOI] [PubMed] [Google Scholar]
- 73.Kalincik T., Buzzard K., Jokubaitis V., et al. Risk of relapse phenotype recurrence in multiple sclerosis. Mult Scler. 2014;20(11):1511–1522. doi: 10.1177/1352458514528762. [DOI] [PubMed] [Google Scholar]
- 74.Confavreux C., Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- 75.Kalincik T., Vivek V., Jokubaitis V., et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609–3617. doi: 10.1093/brain/awt281. [DOI] [PubMed] [Google Scholar]
- 76.Vukusic S., Hutchinson M., Hours M., et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(Pt 6):1353–1360. doi: 10.1093/brain/awh152. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka E., Watanabe M., Fukumoto S., et al. Effect of smoking on disease activity in multiple sclerosis patients treated with dimethyl fumarate or fingolimod. Mult Scler Relat Disord. 2023;70 doi: 10.1016/j.msard.2023.104513. [DOI] [PubMed] [Google Scholar]
- 78.Spelman T., Gray O., Trojano M., et al. Seasonal variation of relapse rate in multiple sclerosis is latitude dependent. Ann Neurol. 2014;76(6):880–890. doi: 10.1002/ana.24287. [DOI] [PubMed] [Google Scholar]
- 79.Runia T.F., Hop W.C., de Rijke Y.B., Buljevac D., Hintzen R.Q. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79(3):261–266. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- 80.Mowry E.M., Carey R.F., Blasco M.R., et al. Association of multiple sclerosis susceptibility variants and early attack location in the CNS. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scalfari A., Neuhaus A., Degenhardt A., et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914–1929. doi: 10.1093/brain/awq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Confavreux C., Vukusic S., Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126(Pt 4):770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 83.Tremlett H., Yousefi M., Devonshire V., Rieckmann P., Zhao Y., Neurologists U. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology. 2009;73(20):1616–1623. doi: 10.1212/WNL.0b013e3181c1e44f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coles A.J., Cox A., Le Page E., et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253(1):98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 85.Bergamaschi R., Berzuini C., Romani A., Cosi V. Predicting secondary progression in relapsing-remitting multiple sclerosis: a Bayesian analysis. J Neurol Sci. 2001;189(1-2):13–21. doi: 10.1016/s0022-510x(01)00572-x. [DOI] [PubMed] [Google Scholar]
- 86.Scalfari A., Lederer C., Daumer M., Nicholas R., Ebers G.C., Muraro P.A. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler. 2016;22(13):1750–1758. doi: 10.1177/1352458516630396. [DOI] [PubMed] [Google Scholar]
- 87.Tremlett H., Zhao Y., Joseph J., Devonshire V., Neurologists U.C. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368–1374. doi: 10.1136/jnnp.2008.145805. [DOI] [PubMed] [Google Scholar]
- 88.Koch M.W., Mostert J., Zhang Y., et al. Association of age with contrast-enhancing lesions across the multiple sclerosis disease spectrum. Neurology. 2021;97(13):e1334–e1342. doi: 10.1212/WNL.0000000000012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frischer J.M., Bramow S., Dal-Bianco A., et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khademi M., Dring A.M., Gilthorpe J.D., et al. Intense inflammation and nerve damage in early multiple sclerosis subsides at older age: a reflection by cerebrospinal fluid biomarkers. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scalfari A., Neuhaus A., Daumer M., Ebers G.C., Muraro P.A. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77(13):1246–1252. doi: 10.1212/WNL.0b013e318230a17d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harding K.E., Liang K., Cossburn M.D., et al. Long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2013;84(2):141–147. doi: 10.1136/jnnp-2012-303996. [DOI] [PubMed] [Google Scholar]
- 93.Kappos L., Butzkueven H., Wiendl H., et al. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963–973. doi: 10.1177/1352458517709619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cree B.A.C., Hollenbach J.A., Bove R., et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kappos L., Wolinsky J.S., Giovannoni G., et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. doi: 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lublin F.D., Häring D.A., Ganjgahi H., et al. How patients with multiple sclerosis acquire disability. Brain. 2022 doi: 10.1093/brain/awac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Portaccio E., Bellinvia A., Fonderico M., et al. Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain. 2022 doi: 10.1093/brain/awac111. [DOI] [PubMed] [Google Scholar]
- 98.Tur C., Carbonell-Mirabent P., Cobo-Calvo Á., et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80(2):151–160. doi: 10.1001/jamaneurol.2022.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cagol A., Schaedelin S., Barakovic M., et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 2022;79(7):682–692. doi: 10.1001/jamaneurol.2022.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prosperini L., Ruggieri S., Haggiag S., Tortorella C., Pozzilli C., Gasperini C. Prognostic accuracy of NEDA-3 in long-term outcomes of multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6) doi: 10.1212/NXI.0000000000001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bischof A., Papinutto N., Keshavan A., et al. Spinal cord atrophy predicts progressive disease in relapsing multiple sclerosis. Ann Neurol. 2022;91(2):268–281. doi: 10.1002/ana.26281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elliott C., Wolinsky J.S., Hauser S.L., et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler. 2019;25(14):1915–1925. doi: 10.1177/1352458518814117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Calvi A., Clarke M.A., Prados F., et al. Relationship between paramagnetic rim lesions and slowly expanding lesions in multiple sclerosis. Mult Scler. 2023;29(3):352–362. doi: 10.1177/13524585221141964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elliott C., Rudko D.A., Arnold D.L., et al. Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis. Mult Scler. 2023;29(6):680–690. doi: 10.1177/13524585231162262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tur C., Moccia M., Barkhof F., et al. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat Rev Neurol. 2018;14(2):75–93. doi: 10.1038/nrneurol.2017.171. [DOI] [PubMed] [Google Scholar]
- 106.Sastre-Garriga J., Pareto D., Battaglini M., et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat Rev Neurol. 2020;16(3):171–182. doi: 10.1038/s41582-020-0314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gärtner J., Hauser S.L., Bar-Or A., et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Mult Scler. 2022;28(10):1562–1575. doi: 10.1177/13524585221078825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Graf J., Leussink V.I., Soncin G., et al. Relapse-independent multiple sclerosis progression under natalizumab. Brain Commun. 2021;3(4):fcab229. doi: 10.1093/braincomms/fcab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnold D.L., Elliott C., Martin E.C., Hyvert Y., Tomic D., Montalban X. Effect of evobrutinib on slowly expanding lesion volume in relapsing multiple sclerosis: a post hoc analysis of a phase 2 trial. Neurology. 2024;102(5) doi: 10.1212/WNL.0000000000208058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 111.Cutter G.R., Baier M.L., Rudick R.A., et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(Pt 5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 112.Cree B.A.C., Arnold D.L., Chataway J., et al. Secondary progressive multiple sclerosis: new insights. Neurology. 2021;97(8):378–388. doi: 10.1212/WNL.0000000000012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2018;9:3116. doi: 10.3389/fimmu.2018.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ransohoff R.M. Multiple sclerosis: role of meningeal lymphoid aggregates in progression independent of relapse activity. Trends Immunol. 2023;44(4):266–275. doi: 10.1016/j.it.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Absinta M., Maric D., Gharagozloo M., et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597(7878):709–714. doi: 10.1038/s41586-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Husseini L., Geladaris A., Weber M.S. Toward identifying key mechanisms of progression in multiple sclerosis. Trends Neurosci. 2024;47(1):58–70. doi: 10.1016/j.tins.2023.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Krieger S.C., Cook K., De Nino S., Fletcher M. The topographical model of multiple sclerosis: a dynamic visualization of disease course. Neurol Neuroimmunol Neuroinflamm. 2016;3(5) doi: 10.1212/NXI.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krieger S., Cook K., Hersh C.M. Understanding multiple sclerosis as a disease spectrum: above and below the clinical threshold. Curr Opin Neurol. 2024;37(3):189–201. doi: 10.1097/WCO.0000000000001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brochet B., Ruet A. Cognitive impairment in multiple sclerosis with regards to disease duration and clinical phenotypes. Front Neurol. 2019;10:261. doi: 10.3389/fneur.2019.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruet A., Deloire M., Charré-Morin J., Hamel D., Brochet B. Cognitive impairment differs between primary progressive and relapsing-remitting MS. Neurology. 2013;80(16):1501–1508. doi: 10.1212/WNL.0b013e31828cf82f. [DOI] [PubMed] [Google Scholar]
- 121.Brochet B., Clavelou P., Defer G., et al. Cognitive impairment in secondary progressive multiple sclerosis: effect of disease duration, age, and progressive phenotype. Brain Sci. 2022;12(2) doi: 10.3390/brainsci12020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Meo E., Portaccio E., Giorgio A., et al. Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol. 2021;78(4):414–425. doi: 10.1001/jamaneurol.2020.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schoonheim M.M., Broeders T.A.A., Geurts J.J.G. The network collapse in multiple sclerosis: an overview of novel concepts to address disease dynamics. Neuroimage Clin. 2022;35 doi: 10.1016/j.nicl.2022.103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Filippo M., Portaccio E., Mancini A., Calabresi P. Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci. 2018;19(10):599–609. doi: 10.1038/s41583-018-0053-9. [DOI] [PubMed] [Google Scholar]
- 125.Benedict R.H.B., Amato M.P., DeLuca J., Geurts J.J.G. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19(10):860–871. doi: 10.1016/S1474-4422(20)30277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Costa S.L., Genova H.M., DeLuca J., Chiaravalloti N.D. Information processing speed in multiple sclerosis: past, present, and future. Mult Scler. 2017;23(6):772–789. doi: 10.1177/1352458516645869. [DOI] [PubMed] [Google Scholar]
- 127.Morrow S.A., Baldwin C., Alkabie S. Importance of identifying cognitive impairment in multiple sclerosis. Can J Neurol Sci. 2023;50(6):813–819. doi: 10.1017/cjn.2022.334. [DOI] [PubMed] [Google Scholar]
- 128.Ruet A., Deloire M., Hamel D., Ouallet J.C., Petry K., Brochet B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. 2013;260(3):776–784. doi: 10.1007/s00415-012-6705-1. [DOI] [PubMed] [Google Scholar]
- 129.Deloire M., Ruet A., Hamel D., Bonnet M., Brochet B. Early cognitive impairment in multiple sclerosis predicts disability outcome several years later. Mult Scler. 2010;16(5):581–587. doi: 10.1177/1352458510362819. [DOI] [PubMed] [Google Scholar]
- 130.Kalb R., Beier M., Benedict R.H., et al. Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler. 2018;24(13):1665–1680. doi: 10.1177/1352458518803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Deloire M.S., Bonnet M.C., Salort E., et al. How to detect cognitive dysfunction at early stages of multiple sclerosis? Mult Scler. 2006;12(4):445–452. doi: 10.1191/1352458506ms1289oa. [DOI] [PubMed] [Google Scholar]
- 132.Ruet A., Deloire M.S., Charré-Morin J., Hamel D., Brochet B. A new computerised cognitive test for the detection of information processing speed impairment in multiple sclerosis. Mult Scler. 2013;19(12):1665–1672. doi: 10.1177/1352458513480251. [DOI] [PubMed] [Google Scholar]
- 133.Langdon D.W., Amato M.P., Boringa J., et al. Recommendations for a Brief international cognitive assessment for multiple sclerosis (BICAMS) Mult Scler. 2012;18(6):891–898. doi: 10.1177/1352458511431076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feinstein A. Multiple sclerosis and depression. Mult Scler. 2011;17(11):1276–1281. doi: 10.1177/1352458511417835. [DOI] [PubMed] [Google Scholar]
- 135.Menculini G., Mancini A., Gaetani L., et al. Psychiatric symptoms in multiple sclerosis: a biological perspective on synaptic and network dysfunction. J Neurol Neurosurg Psychiatry. 2023;94(5):389–395. doi: 10.1136/jnnp-2022-329806. [DOI] [PubMed] [Google Scholar]
- 136.Filser M., Buchner A., Fink G.R., Gold S.M., Penner I.K. The manifestation of affective symptoms in multiple sclerosis and discussion of the currently available diagnostic assessment tools. J Neurol. 2023;270(1):171–207. doi: 10.1007/s00415-022-11359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Menculini G., Gentili L., Gaetani L., et al. Clinical correlates of state and trait anxiety in multiple sclerosis. Mult Scler Relat Disord. 2023;69 doi: 10.1016/j.msard.2022.104431. [DOI] [PubMed] [Google Scholar]
- 138.Penner I.K., Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol. 2017;13(11):662–675. doi: 10.1038/nrneurol.2017.117. [DOI] [PubMed] [Google Scholar]
- 139.Kos D., Kerckhofs E., Nagels G., D'Hooghe M.B., Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair. 2008;22(1):91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- 140.Bol Y., Duits A.A., Hupperts R.M., Vlaeyen J.W., Verhey F.R. The psychology of fatigue in patients with multiple sclerosis: a review. J Psychosom Res. 2009;66(1):3–11. doi: 10.1016/j.jpsychores.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 141.Filippi M., Rocca M.A. Toward a definition of structural and functional MRI substrates of fatigue in multiple sclerosis. J Neurol Sci. 2007;263(1-2):1–2. doi: 10.1016/j.jns.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 142.Sepulcre J., Masdeu J.C., Goñi J., et al. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler. 2009;15(3):337–344. doi: 10.1177/1352458508098373. [DOI] [PubMed] [Google Scholar]
- 143.Liepert J., Mingers D., Heesen C., Bäumer T., Weiller C. Motor cortex excitability and fatigue in multiple sclerosis: a transcranial magnetic stimulation study. Mult Scler. 2005;11(3):316–321. doi: 10.1191/1352458505ms1163oa. [DOI] [PubMed] [Google Scholar]
- 144.Krupp L.B., Serafin D.J., Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437–1447. doi: 10.1586/ern.10.99. [DOI] [PubMed] [Google Scholar]
- 145.Foley P.L., Vesterinen H.M., Laird B.J., et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154(5):632–642. doi: 10.1016/j.pain.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 146.Wang L., Zhang J., Deng Z.R., Zu M.D., Wang Y. The epidemiology of primary headaches in patients with multiple sclerosis. Brain Behav. 2021;11(1) doi: 10.1002/brb3.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mirmosayyeb O., Barzegar M., Nehzat N., Shaygannejad V., Sahraian M.A., Ghajarzadeh M. The prevalence of migraine in multiple sclerosis (MS): a systematic review and meta-analysis. J Clin Neurosci. 2020;79:33–38. doi: 10.1016/j.jocn.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 148.Scholz J., Finnerup N.B., Attal N., et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi: 10.1097/j.pain.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Racke M.K., Frohman E.M., Frohman T. Pain in multiple sclerosis: understanding pathophysiology, diagnosis, and management through clinical vignettes. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.799698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodrigues P., da Silva B., Trevisan G. A systematic review and meta-analysis of neuropathic pain in multiple sclerosis: prevalence, clinical types, sex dimorphism, and increased depression and anxiety symptoms. Neurosci Biobehav Rev. 2023;154 doi: 10.1016/j.neubiorev.2023.105401. [DOI] [PubMed] [Google Scholar]
- 151.Brochet B., Deloire M.S., Ouallet J.C., et al. Pain and quality of life in the early stages after multiple sclerosis diagnosis: a 2-year longitudinal study. Clin J Pain. 2009;25(3):211–217. doi: 10.1097/AJP.0b013e3181891347. [DOI] [PubMed] [Google Scholar]
- 152.Rotstein D., Solomon J.M., Sormani M.P., et al. Association of No evidence of disease activity with No long-term disability progression in multiple sclerosis: a systematic review and meta-analysis. Neurology. 2022;99(2):e209–e220. doi: 10.1212/WNL.0000000000200549. [DOI] [PubMed] [Google Scholar]