Summary
Multiple sclerosis (MS) is an immune-mediated inflammatory and degenerative disorder of the central nervous system (CNS) with heterogeneous clinical manifestations. In the last decade, the landscape of cerebrospinal fluid (CSF) and blood biomarkers as potential key tools for MS diagnosis, prognosis and treatment monitoring has evolved considerably, alongside magnetic resonance imaging (MRI). CSF analysis has the potential not only to provide information on the underlying immunopathology of the disease and exclude differential diagnoses, but also to predict the risk of future relapses and disability accrual, guide therapeutic decisions and thus improve patient outcomes.
This Series article overviews the biological framework and current applicability of fluid biomarkers for MS, exploring their potential role in the molecular characterisation of the disease. We discuss recent advances in the field of neurochemistry that enabled the detection of brain-derived proteins in blood, opening the door to much more efficient longitudinal disease monitoring. Furthermore, we identify the current challenges in the application of fluid biomarkers for MS in a real-world setting, while offering recommendations for harnessing their full potential as key paraclinical tools to improve patient management and personalise treatment.
Keywords: Multiple sclerosis, Biomarkers, Cerebrospinal fluid, Blood
Introduction
Multiple sclerosis (MS) is an immune-mediated inflammatory disorder affecting the central nervous system (CNS), characterized by the accumulation of focal lesions and more diffuse damage in different brain and spinal cord areas over time.1 From a clinical perspective, MS is thus highly heterogeneous, with symptoms reflecting the impairment of several functional neurological systems and a clinical phenotype that may range from a relapsing disease with recurrent episodes of neurological dysfunction to an insidious and irreversible accumulation of neurological disability (an updated overview on the clinical features and phenotype of MS is available in the “Multiple sclerosis: emerging epidemiological trends and redefining the clinical course” article in this Series2).
The diagnosis of MS relies on the combination of different clinical and paraclinical findings, as no single diagnostic test is available.3 To establish an MS diagnosis, it is essential to demonstrate inflammatory immune-mediated damage that has affected at least two distinct regions (referred to as dissemination in space) of the CNS at different time points (dissemination in time). Additionally, it is crucial to exclude alternative diagnoses, including neuromyelitis optica spectrum disorders and other systemic inflammatory and infectious diseases.3 Magnetic resonance imaging (MRI) of the brain and spinal cord plays a pivotal role in this diagnostic process, as discussed in another paper in this Series on Current and future role of MRI in the diagnosis and prognosis of MS.4 However, while conventional MRI provides structural insights, it lacks a comprehensive pathophysiological characterization of the disease. Although advanced imaging techniques can address this limitation, their widespread application remains challenging.
Key messages.
-
•
Fluid biomarkers play a key potential role in the clinical management of multiple sclerosis (MS), aiding in diagnosis, prognosis, and treatment decision-making.
-
•
Intrathecal Ig synthesis markers (IgG oligoclonal bands and κ-free light chain index) by means of CSF analysis support the diagnosis of MS and help to differentiate it from other conditions.
-
•
Biomarkers of intrathecal Ig synthesis and axonal damage (neurofilament light chain) can aid in defining disease prognosis, predicting the risk of future relapses, and guiding treatment decisions.
-
•
Biomarkers of astrocytic and microglial activation can help in tracking the chronic smouldering pathology associated with the progression of disability in MS.
-
•
Integrated biomarker approaches, combining markers reflecting different pathophysiological mechanisms, show promise in the characterization of MS with diagnostic, prognostic and treatment monitoring implications.
-
•
Despite significant progress, challenges persist, including standardization, integration into routine clinical practice, and validation of novel biomarkers.
-
•
Future research should focus on validating novel biomarkers, enhancing interdisciplinary collaboration, and addressing challenges to fully integrate fluid biomarkers into MS management.
Search strategy and selection criteria.
References for this Series article were identified through searches of PubMed (https://www.ncbi.nlm.nih.gov/pubmed) with the search term “Biomarker”, “Blood”, “Cerebrospinal fluid”, “CHI3L1”, “CXCL13”, “Diagnosis”, “Diagnostic Criteria”, “Differential Diagnosis”, “GFAP”, “Guidelines”, “IgG index”, “kappa index”, “McDonald criteria”, “Multiple Sclerosis”, “NfL”, “oligoclonal bands”, “Plasma”, “Prognosis”, “Primary Progressive”, “Progressive”, “Secondary Progressive”, “Serum”, sTREM2”, from 1st January 1980 until 26th September 2023. Only papers published in English were reviewed. The final reference list was generated with the consensus of all co-authors of this Series article based on originality and relevance to the broad scope of this Series article, with a focus on articles published during the past five years.
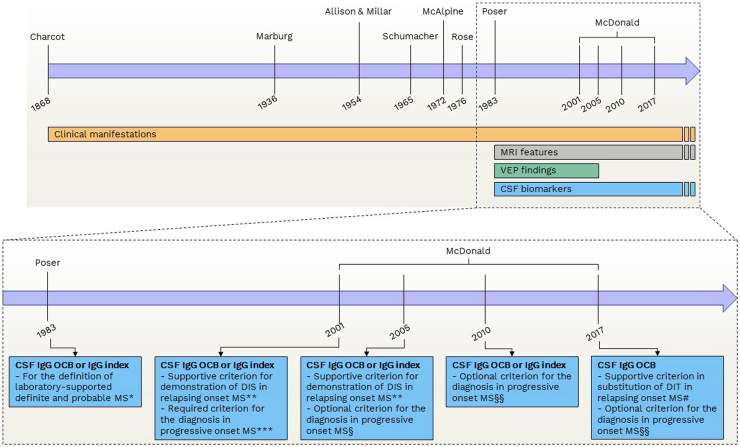
To complement neuroimaging and offer additional insights in clinical practice, fluid biomarkers have been largely investigated and some of them are already part of the clinically useful investigations to be performed in the suspicion of the disease. Among the various body fluids suitable for biomarker measurement, cerebrospinal fluid (CSF) stands out due to its close association with the CNS. Obtaining CSF involves a lumbar puncture, a procedure that, although invasive,5 when performed according to appropriate protocols, it is safe and straightforward6 and allows for the collection of adequate volumes of CSF with minimal side effects.7 The study of CSF can provide clinicians with crucial information about CNS-related events beyond sensitivity and specificity achieved in serum. Typically, the biochemical analysis and cell count in people with MS (pwMS) appear normal or exhibit slight lympho-monocytic pleocytosis at most. A cell count exceeding approximately 50 cells/μL should raise suspicion of an alternative CNS disease, necessitating further investigation.8,9 CSF analysis is therefore relevant to rule out differential diagnoses, especially when the clinical picture and the MRI findings are atypical. Beyond routine biochemical analyses, several biomarkers that are helpful in improving the diagnostic accuracy and in the assessment of prognosis can be measured in the CSF. CSF analysis has played an important role in the evaluation of patients with suspected MS since its incorporation in 1983 in the diagnostic criteria (Fig. 1).10 The last update of the diagnostic criteria of MS in 2017 has confirmed the value of demonstrating intrathecal immunoglobulin G (IgG) synthesis to make an MS diagnosis,3 and markers of other specific immunopathological processes are rapidly emerging. Furthermore, the development of assays targeting neuronal proteins, such as neurofilament light chain (NfL), has led to a surge in studies over recent years investigating the presence and intensity of neuro-axonal damage in MS. Recent advancements in the field of neurochemistry have also made it possible to detect brain-derived proteins at remarkably low concentrations in blood.11 This advancement has paved the way for the exploration of blood-based biomarkers in neurological diseases, including MS, since blood sampling is better suited for longitudinal follow-up measurements. In this Series article, we will summarize the pathophysiological meaning and the role of current and upcoming CSF and blood biomarkers in MS, discussing the unmet needs and future perspectives (Panel 1) in this rapidly evolving field.
Fig. 1.
The evolution of diagnostic criteria for multiple sclerosis. ∗In the 1983 Poser criteria, the inclusion of CSF IgG OCB or IgG index was necessary to establish the definition of laboratory supported definite MS. This designation was considered a more conservative level of diagnosis when compared to clinically definite MS. For the latter, fulfilment of the criteria required either two distinct attacks with clinical evidence of two separate lesions or two attacks with clinical evidence of one lesion and paraclinical evidence of another separate lesion. In contrast, the former necessitated any of the following combinations: i) two attacks with either clinical or paraclinical evidence of one lesion along with CSF OCB/IgG index; ii) one attack with clinical evidence of two separate lesions and CSF OCB/IgG index; iii) one attack with clinical evidence of one lesion and paraclinical evidence of another separate lesion, along with CSF OCB/IgG index. In cases where two attacks and CSF OCB or IgG index were present, a diagnosis of laboratory-supported probable MS was permissible, even in the absence of MRI data. ∗∗ According to the 2001 and 2005 McDonald criteria, abnormalities in CSF analysis could offer supportive evidence of the immune and inflammatory nature of lesion(s). This became particularly valuable when imaging criteria proved insufficient, lacked specificity (as in the case of older patients), or when the clinical presentation was atypical. DIS had to be demonstrated by the presence of three of the following: i) One gadolinium-enhancing lesion or nine T2-hyperintense lesions if no gadolinium-enhancing lesion was present; ii) At least one infratentorial lesion; iii) At least one juxtacortical lesion; iv) At least three periventricular lesions. Alternatively, dissemination in space could also be established by two MRI lesions consistent with the suspicion of MS and CSF IgG OCB or IgG index. ∗∗∗ According to the 2001 McDonald criteria, the presence of abnormalities in CSF analysis was mandatory to diagnose MS in cases of insidious neurological progression suggestive of MS. § According to the 2005 revision of the McDonald criteria, in cases of insidious neurological progression suggestive of MS, MS could be diagnosed if there was one year of disease progression (retrospectively or prospectively determined) and two of the following: i) positive brain MRI (nine T2 lesions or four or more T2 lesions with positive VEP); ii) positive spinal cord MRI (two focal T2 lesions); iii) CSF IgG OCB or IgG index. CSF was therefore not necessary for the diagnosis of progressive MS. §§ According to the 2010 and 2017 revisions of the McDonald criteria, in cases of insidious neurological progression suggestive of MS, MS could be diagnosed it there was one year of disease progression (retrospectively or prospectively determined) and two of the following: i) evidence for DIS in the brain based on ≥ 1 T2 lesions in at least 1 area characteristic for MS (periventricular, juxtacortical, or infratentorial); ii) evidence for DIS in the spinal cord based on ≥ 1 T2 lesions in the cord; iii) positive CSF (isoelectric focusing evidence of IgG OCB and/or elevated IgG index). CSF was therefore not necessary for the diagnosis of progressive MS. # According to the 2017 revision of the McDonald criteria, the evidence of CSF IgG OCB and/or elevated IgG index could substitute for the evidence of DIT. Abbreviations. CSF: Cerebrospinal fluid. DIS: Dissemination in space. DIT: Dissemination in time. IgG: Immunoglobulin G. MRI: Magnetic resonance imaging. MS: Multiple sclerosis. OCB: Oligoclonal bands. VEP: Visual evoked potentials.
Panel 1. Recommendations to facilitate implementation of fluid biomarkers for MS-related processes in clinical practice.
Standardization and reference values
-
-
To promote consistency across methodologies, it is essential to advocate for and support standardization efforts.
-
-
Establishing universally accepted cut-off values for biomarkers would provide clear benchmarks for clinical interpretation.
Accessibility and training
-
-
Developing user-friendly assays would enhance accessibility, enabling a broader range of healthcare professionals to utilize these biomarkers effectively.
-
-
Comprehensive training programs are necessary to ensure that healthcare professionals have the necessary skills to incorporate fluid biomarkers into clinical practice.
Blood-based measurement advancements
-
-
Further research and cross-validation studies should be encouraged to advance the understanding and reliability of blood-based measurements.
-
-
The potential of blood-based markers in routine clinical practice should be further investigated to define their prognostic and monitoring capabilities.
Collaboration and data sharing
-
-
Interdisciplinary collaboration should be promoted to leverage diverse expertise and perspectives in biomarker research.
-
-
Initiatives to encourage data sharing are crucial for fostering a comprehensive understanding of fluid biomarkers and their clinical application.
Clinical integration
-
-
Advocating for the inclusion of fluid biomarkers in clinical guidelines would formalize their role in MS management and encourage their widespread adoption.
-
-
Investigating the complementary nature of fluid biomarkers alongside MRI in monitoring disease activity would better define their value in comprehensive patient care.
Monitoring treatment efficacy
-
-
Recognizing the role of blood NfL and potentially GFAP in evaluating treatment effectiveness would underscore their importance as endpoints in clinical trials.
-
-
Proposing these markers as potential endpoints could facilitate more precise assessment of treatment outcomes and inform therapeutic decisions.
Addressing unmet needs
-
-
Encouraging research to address unmet needs in fluid biomarker utilization is essential for enhancing their clinical utility.
-
-
Exploring ways to expand biomarker portfolios would provide a more comprehensive understanding of MS pathophysiology and improve patient care strategies.
Pathophysiological bases of fluid biomarkers in multiple sclerosis
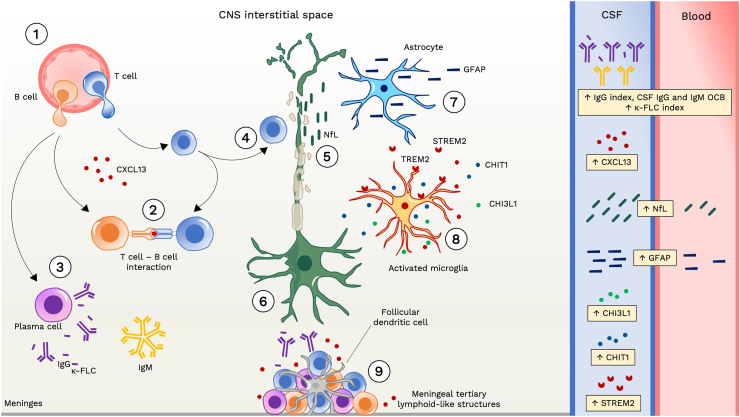
MS is a chronic neurological disease of autoimmune origin that is driven by the recurrent invasion of T and B cells in the brain and spinal cord, leading to a cascade of pathophysiological processes taking place in the CNS.12 The complex interaction between these mechanisms contributes to the diverse clinical manifestations observed in the disease.13 In this scenario, several fluid biomarkers have emerged as effective indicators of the key underlying processes in MS pathophysiology (Fig. 2).14 Especially in the earliest phases of the disease, symptoms arise due to episodes of acute focal inflammation, demyelination and axonal damage, driven by invading adaptive immune cells, which may cause functional and structural disconnection of CNS areas. This phenomenon can typically be detected by conventional MRI, showing the appearance of new lesions in T2-weighted and/or of gadolinium enhancing lesions in T1-weighted sequences.4 Clinical disability due to such acute manifestations of disease activity tends to ameliorate or completely resolve. During this relapsing phase of the disease, episodes of focal inflammation spreading in time and space within the CNS recur, as a consequence of the infiltration of peripheral immune cells into the CNS.15 Inflammatory infiltrating cells, including macrophages, CD8+ T cells, CD4+ T cells and B cells release several immune mediators, such as cytokines and chemokines, into the CNS tissue, CSF and blood.16 Unfortunately, most of T cell-derived chemokines and cytokines that can be measured in biofluids have not shown a strong potential as disease biomarkers.17 In contrast, the immune pathway that involves the activation of B cells historically provided the most robust fluid biomarkers for MS. Activated B cells are indeed transformed in the CNS into plasma cells that produce intrathecal immunoglobulins, both immunoglobulins G (IgG) and M (IgM). These intrathecally synthesized immunoglobulins appear to target specific antigens which are currently not yet completely known and vary between patients and studies.18 Intrathecal IgG and IgM can be detected, with different methods, in the CSF. Immunoglobulins are formed by two heavy chains, which determine their class (IgG, IgM, IgE, IgD and IgA) and by two light chains (either kappa or lambda). Free forms of light chains are released and can be quantified due to the 10–40% excess synthesis of light over heavy chains by plasma cells. Immunoglobulin free light chains might also act as potential biomarkers for diagnosis and prognosis in MS.19,20
Fig. 2.
Pathophysiological basis of fluid biomarkers in multiple sclerosis. (1) In the early stages of the disease, immune cells infiltrate the central nervous system (CNS) through the blood–brain barrier. This includes macrophages, CD8+ T cells, CD4+ T cells, B cells, and plasma cells. T and B cells are primed in the periphery and attracted to the CNS by chemotactic factors, like chemokine (C-X-C motif) ligand 13 (CXCL13) for B cells. (2) Within the CNS, T cells and B cells interact closely, with B cells serving as antigen-presenting cells. (3) Activated B cells can mature into plasma cells, secreting IgG and IgM antibodies into the intrathecal space. This process also results in the release of free light chains (FLC) due to a mismatch between immunoglobulin light and heavy chains synthesis. (4–5) The inflammatory process leads to axonal damage, and the release of neuronal markers like neurofilament light chain (NfL) into the interstitial space, cerebrospinal fluid (CSF), and bloodstream. (6) Focal axonal injury induces axonal die-back or retrograde degeneration as well as Wallerian or anterograde degeneration contributing to neuronal loss. (7–8) CNS resident immune cells such as microglia and astrocytes become activated, impacting axon and synaptic integrity and function. Activated microglia and astrocytes release various mediators into the CSF, including soluble triggering receptor expressed on myeloid cells 2 (sTREM2), chitinase 1 (CHIT1) and chitinase-3-like protein 1 (CHI3L1). Additionally, astrocytic injury results in the release of structural proteins like glial fibrillary acidic protein (GFAP) into both the CSF and bloodstream. (9) Failure to resolve inflammation adequately over time leads to sustained immune response, resulting in persistent meningeal inflammation with formation of lymphoid structures. Abbreviations. CHI3L1: chitinase-3-like protein 1. CHIT1: chitinase 1. CNS: central nervous system. CSF: cerebrospinal fluid. CXCL13: chemokine (C-X-C motif) ligand 13. FLC: free light chain. GFAP: glial fibrillary acidic protein. NfL: neurofilament light chain. sTREM2: soluble triggering receptor expressed on myeloid cells 2. TREM2: cell surface triggering receptor expressed on myeloid cells 2.
Another interesting molecule linked to B cell activity is the chemokine (C-X-C motif) ligand 13 (CXCL13), which is present at increased concentrations in the CSF and in peripheral fluids of individuals with MS.21,22 It plays a pivotal role in attracting B cells that express its ligand CXCR5 to the CNS.23 CXCL13 protein and RNA levels are increased in MS lesions, while levels are barely detectable outside active inflamed lesions.24 Along the disease course, the inadequate resolution of inflammation is accompanied by different chronic inflammatory changes, one of them being the development of ectopic germinal centers in the meninges.25 CXCL13 has been found in ectopic germinal centers in the inflamed meninges of individuals with progressive MS, mainly in follicular dendritic cells.25 The increased levels of CXCL13 may enhance B cell accumulation in the meninges,23 causing the gradual clustering of lymphoid cells. The worsening of chronic meningeal inflammation, in turn, leads to subpial demyelination, notable neuronal loss, and a cortical cell pathology gradient from the surface to the deeper layers of the cortex.26
Over time, neuro-axonal damage accumulates, functional reserve diminishes and neurological symptoms persist and progressively deteriorate.12 The worsening of disability and accumulation of neurological deficits in MS in the absence of concurrent relapses is defined as “progression independent of relapse activity” (PIRA).27 From a clinical point of view, PIRA characterizes the progressive disease phase, but the underlying mechanisms are increasingly recognized as part of a single pathophysiological continuum with the early “relapsing” phase and it is thought to be driven by a chronic, “smouldering” inflammatory process that is compartmentalized within the CNS since disease onset and primarily involves innate immune system cells and astrocytes.28 Intriguingly, recent positron emission tomography studies employing radioligands for assessing innate immunity activation have revealed an unexpectedly high prevalence of MS lesions with a smouldering component.29
Chronic active lesions in MS can be detected as slowly expanding lesions that tend to gradually enlarge over time or as paramagnetic rim lesions, usually detected on susceptibility-based MRI sequences. Chronic active lesions have been indeed found to express a dense network of activated iron-laden microglia/macrophages forming a glial barrier at the lesion boundary.30 In the chronically inflamed lesion edge, a specific phenotype of microglial cells has been identified and defined as “microglia inflamed in MS”, showing a transcriptomic profile similar to that of microglia in neurodegenerative diseases.31 Microglia inflamed in MS may hinder neuronal remyelination by oligodendrocytes,30 and lead to neuronal dysfunction, damage and loss.32 Among CSF biomarkers of such chronic inflammatory processes, chitinase-3-like protein 1 (CHI3L1), which is expressed by different cells, including microglia, astrocytes, and also cells in the brain vasculature,33 indicates microglial activation and chronic tissue damage.34,35 Another microglial marker is chitinase 1 (CHIT1), also known as chitotriosidase, which is more cell-specific than CHI3L1,36 and can be measured in the CSF. Different microglial functions, including the phagocytosis of damaged axons and myelin, and the resolution of inflammation, are regulated by the cell surface triggering receptor expressed on myeloid cells 2 (TREM2). Its soluble form (sTREM2) can be measured in the CSF and arises after shedding of the external domain of the receptor.37 In the chronic immunological processes characterizing MS, transcriptomics and neuropathological data not only point to microglial cells, but also to “astrocytes inflamed in MS”. These reactive astrocytes can be found in demyelinating lesions and adjacent normal appearing white matter. Glial fibrillary acidic protein (GFAP), the main intermediate filament in human astrocytes, serves as a biomarker for astrocytic dysfunction, activation, and damage. In response to brain injury, GFAP is released into the interstitial/extracellular fluid, CSF, and the bloodstream.38,39
As introduced above, many of the described pathophysiological events taking place in the MS brain and spinal cord along the disease course (recurrent focal inflammation, diffuse activation of glial cells, formation of meningeal infiltrates and slowly expanding lesions) lead since the early disease phases to axonal injury, neuronal loss and CNS network failure that drive disability progression.40 Accordingly, the demonstration, quantification and monitoring of acute and chronic neuro-axonal injury could represent relevant information for the MS specialist. Such demonstration can be achieved by measuring structural proteins of neurons. One of the major components of the axonal cytoskeleton is neurofilament, a 10-nm filament that confers tensile strength to dendrites and axons. Neurofilaments are composed of three major polypeptides with molecular masses of 200, 150 and 68 kDa (kD), respectively, with the latter being NfL.41 NfL is released in the interstitial space of CNS upon axonal injury and it can be measured in both CSF and blood, using a range of immunoassays.42 Since NfL is highly sensitive to large caliber axonal damage, it has shown to accurately reflect the axonal damage within the white matter occurring in MS, thus becoming one of the most promising markers among those emerging.
Toolbox of fluid biomarkers for multiple sclerosis
In the past decade, the landscape of biomarkers in MS has undergone a remarkable evolution, with a concentrated effort in studies aimed at unravelling molecules that can offer insights into the pathogenic processes of the disease, alongside notable advancements in assay technologies. Among the hallmarks of adaptive immunity, markers reflecting B cell activity stand out as prototypical, encompassing IgM and IgG oligoclonal bands (OCB), immunoglobulin free light chains, and CXCL13. These markers capture only a fraction of the intricate immunopathology of MS. At the same time, biomarkers associated with innate immunity and glial cells, although not as extensively explored as those for B cell activity, represent a promising frontier for future advancements. Particularly noteworthy are emerging data on biomarkers indicative of astrocyte and microglial activation, with notable attention directed towards GFAP, CHI3L1, CHIT1, and sTREM2. Further, the ability to measure and quantify axonal damage through NfL provides a clear depiction of the status of axonal injury and disease intensity.
In the following sections, we describe each of these markers individually. It is worth noting that while IgG OCB and immunoglobulin free light chains are used in the specific context of diagnosing MS, the remaining markers lack specificity for MS and thus are not diagnostically useful in isolation. Further, none of the discussed biomarkers can serve as differential diagnostic tool to discriminate between MS and other immune-mediated disease of the CNS, such as neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease, acute disseminated encephalomyelitis, or autoimmune encephalitis more in general. Consequently, our focus is the exploration of their potential role in molecular characterization and monitoring of the disease.
IgG index and cerebrospinal fluid IgG oligoclonal bands
Over 90% of pwMS have an increase in intrathecal IgG production, which can be quantitatively measured through heightened local IgG synthesis, and/or qualitatively detected by the presence of IgG OCB.43 The local production of IgG in the CNS is calculated using different formulas that distinguish the component produced within the CNS from that derived from the serum. The most commonly used measure is the Tibbling and Link index (i.e., IgG index), obtained by dividing the CSF IgG/serum IgG by the CSF albumin/serum albumin.44 The CSF albumin/serum albumin is an established marker of the blood–CSF–barrier function and the CSF IgG/serum IgG corrects for the absolute serum IgG concentration. A value above 0⋅7 indicates the existence of increased intrathecal IgG synthesis and it is considered to be pathological.45 The IgG index was incorporated in earlier versions of the McDonald MS diagnostic criteria, but its use as a diagnostic tool is limited due to low sensitivity. While an association between IgG index and future disability worsening has been documented,46 its correlation with the severity of MS remains inconclusive.47 Consequently, its utility as a prognostic indicator is somewhat limited.
An alternative method for evaluating intrathecal Ig synthesis involves the utilization of Reibergrams. These graphical representations typically depict the concentration of CSF IgG, IgA and IgM plotted against its corresponding concentration in serum, accounting for blood-CSF barrier dysfunction. Reibergrams present various ranges, from normal to intrathecal Ig synthesis with or without blood-CSF barrier dysfunction.48
The qualitative determination of intrathecal IgG synthesis is based on the identification of IgG OCB, whereby isoelectrofocusing is the most appropriate method of detection, with subsequent immunospecific staining for IgG molecules.49 Any protein found in the CSF that is absent in the serum is assumed to be produced in the CNS. Isoelectrofocusing followed by IgG staining showing IgG bands in the CSF and not in serum implies an intrathecal synthesis of IgG. An oligoclonal pattern can be identified for specific bands. When there are two or more IgG bands present in the CSF that are not found in the serum, it is regarded as suggestive of intrathecal IgG synthesis. The OCB pattern remains constant in the same individual and is unaffected by treatment with corticosteroids.50 The presence of OCB displays high sensitivity, and its absence shows a high negative predictive value for MS.51 The identification of IgG OCB at the first clinical episode of MS, i.e., the clinically isolated syndrome has been linked to a higher likelihood (odds ratio of 9·88) of future conversion to MS and an increased probability (odds ratio of 1·96) of reaching disability outcomes.52 Due to this clear prognostic effect, the presence of intrathecal IgG synthesis documented by OCB offers an alternative to the evidence of dissemination in time in the current 2017 diagnostic criteria of MS.3,53
The primary limitations of OCB include their lack of specificity since they may also present in other inflammatory or infectious neurological disorders, their qualitative nature, and their time-consuming determination process. Furthermore, correct interpretation relies on the rater, which restricts their use to specialised centres.54
Cerebrospinal fluid immunoglobulin free light chains
Determination of the intrathecal kappa free light chain (κ-FLC) fraction in the CSF can overcome some of the limitations of the IgG index and IgG OCB. κ-FLC can be easily measured by nephelometry or turbidimetry, which are automated, less costly, and less time-consuming methods compared to CSF OCB detection, and the read-out is quantitative and not operator-dependent. Of all reported methods to capture an intrathecal release of κ-FLC, the κ-FLC index holds strongest evidence. κ-FLC index is calculated by dividing the CSF κ-FLC/serum κ-FLC by the CSF albumin/serum albumin.55 A recent systematic review and meta-analysis indicated that an elevated κ-FLC index has a diagnostic sensitivity and specificity of around 90% to differentiate pwMS from those with other neurological diseases, similar to OCB.19 Furthermore, κ-FLC index yields a numerical result within a range of approximately 1–500, diverging from the binary nature of OCB status, which merely indicates a positive or negative outcome.55 Due to this favourable profile, a panel of experts in CSF diagnostics and MS recommended adding the detection of intrathecal κ-FLC synthesis in the MS diagnostic process and considering it in further editions of the McDonald criteria.56
When κ-FLC index is used as marker for intrathecal inflammation instead of OCB, certain principal differences must still be considered. OCB solely indicate intrathecal IgG synthesis, while κ-FLC index is also elevated in cases of intrathecal IgA and/or IgM synthesis.20 Several studies have demonstrated a prognostic value of κ-FLC index in early stages of MS. Multivariable analyses including patients with a first typical CNS demyelinating event demonstrated that a high κ-FLC index can predict early relapse independently of other known risk factors such as baseline MRI lesion load.54,57, 58, 59 Although OCB can also have some prognostic value as mentioned above,60 the determination of κ-FLC index offers distinct advantages. Therefore, κ-FLC index enables a further stratification of MS disease activity risk in OCB positive patients.58
Cerebrospinal fluid IgM oligoclonal bands
Intrathecal IgM synthesis can be detected using semi-quantitative and qualitative methods. While semi-quantitative methods like the IgM index or non-linear formulae (e.g. Reiber, or Auer & Hegen formula) have low sensitivity, qualitative methods such as the exploration of IgM OCB are more accurate.61, 62, 63, 64, 65 There is less data on the value of IgM OCB compared with IgG OCB in MS diagnostics, mainly due to technical issues, since the high molecular weight of IgM pentamers made their isoelectric focusing difficult. The issue can be resolved by agarose electrophoresis with immunoblotting or (and maybe preferably) by breaking down IgM pentamers into their monomeric form and examining the IgM pattern with isoelectric focusing and immunodetection.66 The existence of intrathecal IgM synthesis—indicated by IgM OCB present in CSF but not in matched serum samples–is observed in over 40% of pwMS and is predictive of a highly inflammatory disease.67 PwMS with CSF IgM OCB exhibit a shorter time to a subsequent relapse and a higher relapse rate.68 Moreover, they associate with a higher risk of disability progression, of developing a secondary progressive disease,61 and of cognitive impairment.62 These findings were confirmed in some studies, which showed that the detection of IgM OCB can be a dependable prognostic marker in MS.63,69,70 An association between IgM OCB and increased CSF levels of CHI3L1, a marker of glial cells activation has been demonstrated.71 Intrathecal IgM antibodies also associate with a more aggressive course of primary progressive MS.72 CSF IgM in pwMS shows a high degree of somatic hypermutations primarily located in the complementarity determining regions of the IgM, which indicates antigen-driven affinity maturation.73 In most cases, these antibodies identify lipids that are considerably present in the CNS, mainly phosphatidylcholine. Anti-lipid IgM antibodies are more strongly associated with aggressive MS than total IgM bands.68 It has been observed that anti-lipid IgM antibodies may decrease in pwMS who have an optimal response to disease-modifying therapies like natalizumab.74
Cerebrospinal fluid CXCL13
CSF CXCL13 concentration is elevated in MS, with median levels up to 4 times higher than controls, similar to other cytokines such as IFN-gamma and TNF-alpha.25 The greatest increases in CSF CXCL13 levels are observed in early active disease, but levels are also elevated in progressive MS.75 CSF CXCL13 concentration correlates with the number of gadolinium enhancing lesions, B cell counts, intrathecal IgG concentrations, κ-FLC index, relapse rate and disease activity, supporting a role in active inflammatory disease.75, 76, 77, 78, 79 Levels are generally undetectable in CSF from controls with non-inflammatory diseases. CSF levels of this marker, alone or as a CXCL13 index (CSF/serum or plasma concentration), have prognostic value,80 such as for conversion from clinically isolated syndrome to MS in several independent studies.80, 81, 82 A value for monitoring is suggested by the decrease early after (<3 weeks) corticosteroid infusion, but also after the use of disease-modifying therapies in MS.75,83 In addition, a similar treatment response, i.e., reaching undetectable levels after one year of treatment, has been observed after autologous haematopoietic stem cell transplantation.84
Despite it has been suggested to calculate a CXCL13 index to quantify intrathecally synthesized amounts and accommodate for blood–brain barrier dysfunction, recent evidence indicates that CXCL13 likely does not enter the CSF from the bloodstream, even in the presence of blood–brain barrier alterations, despite its small molecular weight.85 Therefore, elevated CXCL13 levels in CSF may be derived from the CNS only, and indicative of neuroinflammatory processes. On the other side, serum CXCL13 levels are elevated in various systemic autoimmune, inflammatory, infectious, and neoplastic diseases, thereby limiting its utility as a biomarker of intrathecal immune activity when measured in serum.85
Microglia and astrocytes activation markers
CHI3L1
CHI3L1, or YKL40, has been consistently proposed as a potential biomarker for MS in CSF, while replication of results in blood has so far been difficult.86 In a meta-analysis in CSF, CHI3L1 levels were increased in pwMS versus healthy controls and, to a lesser extent, in individuals with clinically isolated syndrome.87 In those individuals who convert from clinically isolated syndrome to MS, CHI3L1 levels were higher,88 similar to increases observed in people with primary progressive MS compared with people with relapsing and secondary progressive MS.87 CHI3L1 levels were lower during acute relapses compared to the remission phase and showed no association with gadolinium-enhancing lesions on MRI.87 The combined evidence therefore indicates that CSF CHI3L1 is a promising marker in the progressive MS phases. CHI3L1 levels were not associated with GFAP levels,89 even though both markers are expressed by astrocytes, suggesting that CHI3L1 captures a distinct pathological process. On the other hand, serum levels of CHI3L1 are in general not significantly different between MS and healthy controls,90 suggesting a lack of correlation between CSF and serum measures.87 This might be attributed to the extensive expression of the protein beyond the CNS, including chondrocytes, vascular smooth muscle cells, mesothelial cells, alveolar cells, and basal respiratory cells.91
CHIT1
Compared with CHI3L1, CHIT1 is more specific for microglial cells.92 In pwMS, CSF CHIT1 levels at diagnosis have been demonstrated to correlate with markers of neuronal injury, such as NfL, and with disease activity measures at follow-up up to 6 years later independently from other disease severity measures.92 Of interest, the levels of CHIT1 RNA are increased in the white matter of post-mortem brain tissue from pwMS.92 Specifically, CHIT1 is upregulated (up to 10-fold) in the rim of chronic active lesions versus the rim of chronic inactive lesions.92 CHIT1 RNA expression discriminated well between chronic active lesions and chronic inactive lesions, the latter not different from control white matter tissue.92 Taken together, this evidence supports the rationale for studying CSF CHIT1 as a marker of chronic active lesions in MS.
sTREM2
Despite the evidence of a role of TREM2 in animal models for demyelination and its elevation in foamy macrophages in MS lesions,93, 94, 95 only few studies have investigated sTREM2 as biomarker for relevant contexts of use (diagnosis, prognosis, monitoring or treatment response) in MS. Increased CSF concentration of sTREM2 in MS, as well as increased expression on CSF monocytes, have been described in all MS subtypes, compared with noninflammatory neuronal controls, in a study in which the soluble form was described for the first time.95 CSF sTREM2 levels did not correlate with EDSS scores, or IgG index,96 while another study showed a moderate correlation with EDSS and the Multiple Sclerosis Severity Score.97 The levels in patients with other inflammatory neurological conditions were highest. Levels normalised upon treatment with natalizumab and decreased partly after treatment with mitoxantrone.98 However, the lack of correlation with clinical and biological outcomes suggests limited clinical value of this marker in MS and claims for further studies. Unfortunately, although sTREM2 concentration can be readily measured in serum and plasma, there is no correlation with CSF, TREM2 genetic variation or diagnosis in neurodegenerative dementias, speaking against its usefulness as a blood biomarker for microglial activation.99
GFAP
Among astrocyte-specific markers, GFAP is the most promising, and could be instrumental in delineating the intricate involvement of astrocytes in neuroinflammation and neurodegeneration. GFAP in CSF has been associated with disease progression in MS since decades, but its potential as biomarker of progression in MS strongly evolved over the last years, with the emergence of technologies that allowed accurate detection of GFAP in blood. Early studies showed that CSF GFAP levels correlated with increased neurological disability, were higher in progressive patients, and associated less with the acute clinical phase of the disease.100,101 Also, higher serum and CSF GFAP levels were found to prognosticate worse outcomes of progression as well as brain atrophy, especially in the grey matter and independent of serum NfL, which further support a role in the pathophysiology of MS disease progression.102,103 Interestingly, GFAP is labile in CSF and very sensitive to freeze-thawing, whilst the protein is stable in blood.104
It is noteworthy that GFAP has also been studied in NMOSD, a neuroinflammatory condition that is clinically relevant as a differential diagnosis of MS.105 NMOSD is characterized by antibody-mediated astrocytopathy within the CNS, wherein GFAP levels have been observed to elevate in both CSF and serum during acute exacerbations. Notably, during remission phases, elevated serum GFAP levels have demonstrated predictive value for future disease activity in NMOSD, suggesting its potential as a prognostic marker.106
In MS, GFAP appears to reflect a composite of tissue damage caused by inflammation, potentially explaining its correlation with NfL levels (as discussed below), as well as disease progression likely stemming from structural effects such as astrogliosis, glial scar formation, or astrocytic damage. Specifically, serum GFAP levels tend to rise during relapses in relapsing-remitting MS compared to periods of remission.107 However, it is noteworthy that serum GFAP has demonstrated the ability to predict confirmed disability progression even in non-active MS cases, indicating an association with MS pathophysiological mechanisms beyond acute focal inflammation.108
To assess the independent value of serum GFAP, studies endeavoured to isolate these factors, for example by investigating progression in pwMS under highly effective disease modifying therapies. In a study which monitored 88 pwMS commencing natalizumab treatment, a notable decrease in serum GFAP after treatment initiation was observed, thus implying an association between GFAP levels and inflammatory activity of MS.109 Further, serum GFAP levels did not increase in pwMS who experienced progression compared to those who remained stable twelve months after treatment initiation.109 In contrast, another study found increased serum GFAP levels in progressing versus stable patients and in individuals receiving B cell depleting treatment and identified a connection between increased GFAP levels (taken one year after starting treatment) were significantly prognostic of time to disease progression.103 Age is associated with serum GFAP concentrations, potentially influencing the variance in biomarker levels when comparing individuals with progressive and relapsing phases of MS.110 Notably, the association between GFAP serum levels and the risk of disability progression in non-active pwMS has persisted even in age-adjusted models.108 These promising findings underline the need for cross-validation and large, longitudinal studies examining different aspects of GFAP levels, such as baseline levels, effects of age and sex, longitudinal change after different treatments in different settings.
Cerebrospinal fluid and blood NfL
In 1987, fractions of neurofilaments from bovine brain were isolated,111 forming the foundation for developing polyclonal rabbit antisera targeting specific NF polypeptides.112 This lead to the development of the first enzyme-linked immunosorbent assay (ELISA) for NfL.113 Rosengren et al. (1996) demonstrated elevated levels of NfL in the CSF of various neurodegenerative disorders,113 indicating its potential as a biomarker of neuro-axonal injury. Subsequently, monoclonal antibodies were developed against NfL,114 and a new NfL ELISA was established.115 This assay was used in most studies on CSF NfL in MS during the following decade. There are now several commercially available assays for the marker, including those compatible with fully automated random access clinical chemistry instruments.
As NfL exhibits high expression in large-calibre myelinated axons, research into MS quickly followed. In 1998, research indicated a significant increase in CSF NfL concentration among subjects with relapsing-remitting MS during active inflammatory bouts of the disease, compared to healthy controls.116 This finding highlighted the potential value of CSF NfL as a biomarker to monitor disease activity. Over time, several studies have confirmed that CSF NfL increases in both relapsing-remitting and primary progressive MS. CSF NfL concentration indicates ongoing axonal injury and reflects its intensity. In pwMS, following clinically effective treatment, the concentration of CSF NfL normalizes within 6–12 months.117 Thus, CSF NfL holds promise as a biomarker for disease intensity, progression, and response to treatment.118
The development of high-throughput ultrasensitive immunoassay technologies (Single-molecule array (Simoa), Ella® microfluidics,119,120 Meso Scale Discovery electrochemiluminescence,121 and fully automated enzyme-linked immunosorbent assays Lumipulse®,122 Elecsys® and Atellica®123) has led to a leap in our understanding of NfL as a biomarker in MS by allowing its accurate quantification in the pg/ml range in blood (both serum and plasma).124 Serum NfL levels are elevated in pwMS already before the onset of clinical symptoms,125 which suggests a potential future clinical use in monitoring individuals at high risk of developing the disease. Higher NfL levels are predictive of worse outcomes, such as a higher risk of conversion from clinically isolated syndrome to MS,59,126 (more) future relapses, lesion activity, EDSS worsening and brain atrophy.127, 128, 129, 130 Highly effective disease modifying therapies normalise serum NfL levels to those of healthy controls, while dose-dependent smaller reductions are observed with oral and platform compounds.131,132 In pwMS without active inflammation, such as progressive MS or relapsing remitting MS on highly effective therapies, retrospective analyses of phase 2/3 clinical trials showed evidence of increased serum NfL levels predictive of whole brain and thalamic atrophy, expansion of slowly expanding lesions and clinical progression.103,133, 134, 135 In a combined analysis of two large observational MS cohorts, serum NfL concentrations were significantly elevated 1–2 years before, but not at the time of, disease progression, compared with stable MS cases, and proved to be highly predictive of future progression independent of relapse activity events when taking this time lag into account.136
The evaluation of serum or plasma NfL as a response biomarker for early clinical trials in progressive MS has received a letter of support from the US Food and Drug Administration in 2021 (https://www.fda.gov/media/149608/download). For paediatric cases, serum NfL has received support from the European Medicines Agency in 2022 as a biomarker to monitor disease activity related to axonal damage and to assess treatment response (https://www.ema.europa.eu/en/documents/other/letter-support-neurofilament-light-childhood-neurological-diseases_en.pdf).137 One of the limitations of serum NfL is that it is specific for pathology (i.e., neuronal damage) but not for a specific disease. Elevated levels have been reported in acute conditions such as traumatic brain injury or stroke, and in chronic neurodegenerative diseases such as Alzheimer's disease and frontotemporal dementia. Therefore, individual values can only be interpreted within the clinical context. In contrast, the physiological variation of serum NfL levels with age and body weight has been successfully addressed by the establishment of normative values for both adults and children.138, 139, 140, 141 These tools facilitate the interpretation of serum NfL levels in clinical practice, providing solutions as age-dependent reference ranges, either as percentiles or as Z-scores, which allow to quantify the deviation of pwMS serum NfL concentration compared to that observed in a control person with similar age and weight and relative to other MS subtypes.
Altogether, serum or plasma NfL is about to become an essential tool for personalised medicine in MS clinical practice and may as well be an endpoint to accelerate successful drug development in clinical trials. Pressing open clinical questions to be addressed in prospective studies include the definition of threshold values to indicate ‘successful treatment’ or ‘reactivation of disease’.
Moving from current clinical applications to future perspectives
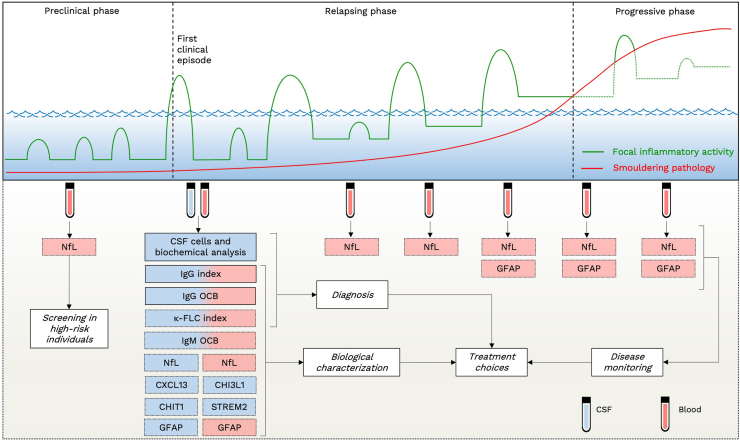
In the clinical management of MS, fluid biomarkers can play distinct roles across various stages of the disease (Fig. 3). During the initial diagnostic evaluation, CSF analysis emerges as a recommended tool, facilitating a more precise differentiation from MS mimics.8 If standard CSF analysis yields normal results or only reveals mild lymphocytic pleocytosis alongside intrathecal IgG synthesis, the need for additional tests can be diminished. Conversely, when both the clinical-MRI presentation and CSF analysis appear atypical, it should raise suspicion of alternative diagnoses, prompting further detailed investigations.
Fig. 3.
Applications of fluid biomarkers in the clinical management of multiple sclerosis. Upper Panel: schematic representation of the multiple sclerosis (MS) clinical course and underlying events. The vertical axis depicts the level of clinical disability, while the horizontal axis represents time. The “sea level” corresponds to the threshold of clinical detection. Green lines represent episodes of acute focal inflammation in the central nervous system (CNS). Acute inflammation can manifest as asymptomatic (below the clinical threshold) or symptomatic (above the clinical threshold). During the preclinical phase of the disease, asymptomatic acute inflammations occur. The first episode of acute inflammation crossing the clinical threshold represents the clinical onset of the disease. Over time, clinical relapses may be followed by incomplete recovery. In parallel with recurring events of acute focal inflammation in the CNS, since the early phase of the disease a “smouldering” pathological process of persistent low-grade inflammation occur (red line), eventually leading to clinically detectable continuous worsening of disability. Lower Panel: MS biomarkers and their potential role in different disease phases. Biomarkers within bars with a solid black frame are those currently used on a large scale in clinical practice. Abbreviations. CHI3L1: chitinase-3-like protein 1. CHIT1: chitinase 1. CIS: clinically isolated syndrome. CNS: central nervous system. CSF: cerebrospinal fluid. CXCL13: chemokine (C-X-C motif) ligand 13. DMT: disease-modifying treatment. FLC: free light chain. GFAP: glial fibrillary acidic protein. NfL: neurofilament light chain. sTREM2: soluble triggering receptor expressed on myeloid cells 2.
The assessment of intrathecal IgG synthesis is an integral component of the standard CSF examination during the diagnostic work-up of MS.53 While the IgG index calculation is straightforward, it lacks optimal sensitivity and specificity. To address this limitation, documenting IgG OCB or measuring κ-FLC index proved to be superior. Illustratively, in the context of discriminating between MS and white matter lesions associated with migraine and vascular lesions, the absence of intrathecal Ig synthesis stands out as the most robust independent predictor of a non-MS diagnosis (odds ratio 18·1 for IgG OCB).142 However, it must be kept in mind that the informativeness of intrathecal Ig synthesis in distinguishing MS from other autoimmune and infectious disorders of the CNS might be comparatively limited.143
In capturing intrathecal Ig synthesis, the agreement between OCB and k-FLC index is high (approximately 90%).144 It is noteworthy that the determination of CSF IgG OCB demands time and specialized personnel, while FLC measurements are performed through automated processes, saving time.20 Consequently, in the future, centers with comprehensive capabilities may adopt both analyses to achieve optimal accuracy encompassing qualitative and quantitative assessments. In centers with limited resources, κ-FLC index could serve as an initial screening tool, with CSF IgG OCB reserved as a confirmatory test.20
Both CSF IgG OCB and κ-FLC index can aid clinicians also in defining disease prognosis, particularly in relapsing forms of MS, by predicting the risk of future relapses.52,55 Notably, CSF IgG OCB has gained recognition as a marker substituting clinical and MRI evidence of dissemination in time in the latest MS diagnostic criteria,3 allowing to diagnose MS after the first clinical manifestation, even in the absence of a positive history of previous relapses or non-simultaneous CNS lesions on MRI. As CSF proves highly informative in the diagnostic work-up, its sampling can facilitate the measurement of additional biomarkers, with the aim to provide a better biological characterization of the disease, to define its prognosis and to guide treatment decisions. CSF IgM OCB,68 Reiber IgM index61, 62, 63 and CXCL13 might offers additional information but their determination is currently limited to research purposes. Beyond such markers of B cell activity, research on other molecules deserve to be implemented to define and quantify the other facets of MS immunopathology at disease onset and follow-up. In this scenario, particular relevance is assumed by markers of microglial and astrocytes activation. CHI3L1, CHIT1 and GFAP may indeed offer insights into disease progression and its underlying pathogenesis.92 Elevated levels of CHI3L1, CHIT1 and GFAP could for instance in the future influence the choice of therapies directly targeting microglia and astrocytes.
This comprehensive panel of markers, when measured during the diagnostic process, would substantially benefit from the inclusion of CSF NfL as a robust prognostic marker, complementing established clinical and MRI measures.42 Its sensitivity in quantifying ongoing axonal injury resulting from inflammatory focal lesions in MS holds significant implications for predicting the future risk of disease inflammatory activity. However, it is important to note that CSF NfL should not be employed for diagnostic purposes due to its lack of specificity across various neurological diseases.42
The potential of these biomarkers to offer complementary insights has led to the exploration of an integrated biomarker approach, similarly to strategies employed in other neurological disorders. In Alzheimer's disease, for instance, the integration of biomarkers reflecting diverse pathophysiological mechanisms has demonstrated to be very useful.145 Likewise, in MS, integrated scoring systems have been proposed, notably the “Glia score”.146 This score, derived from the calculation of the ratio of glial to axonal markers (CHI3L1∗GFAP/NfL), has been found higher in the CSF of individuals with MS in the progressive phase compared to those in the relapsing phase.146 These findings underscore the potential utility of these integrated biomarker profiles for distinguishing between MS subtypes. Whether this combination might also predict and correlate with PIRA still deserves to be demonstrated.
This enriched CSF characterization should also align with blood-based measures, particularly for NfL and GFAP. The measurement of these markers in plasma or serum samples is already feasible through fully automated analytical platforms, marking a significant advancement for widespread utilization. Monitoring these markers provides clinicians with a combined measure of neuro-axonal damage in the CNS (NfL) and astrocytosis/astrocytic damage potentially more associated with MS disease progression (GFAP). The “Glia score” mentioned above, when assessed in serum, has demonstrated a correlation with disability levels, thereby showing its potential utility in monitoring disease progression in MS.146
These integrated measures hold relevance in evaluating the effectiveness of current and future disease modifying therapies aiming to tackle the different facets of MS pathology. Several therapies now exist to mitigate the risk of new lesions and clinical relapses in MS. Monitoring the efficacy of these treatments heavily relies on repetitive brain and spinal cord MRI scans, which pose financial burdens on healthcare systems and MRI services. Moreover, concerns arise regarding the safety of monitoring through repeated MRI scans with gadolinium injection to detect recent lesions.147 While fluid biomarkers will not entirely replace MRI-based monitoring, they might offer a complementary approach in the future. For example, frequent blood NfL measurements could motivate an MRI request in the event of pathologically increased NfL concentrations (despite a lack of new clinical symptoms). Additionally, blood NfL may aid in discerning whether a neurological symptom stems from ongoing neuro-axonal injury or potentially a transient functional network dysfunction, as it occurs in MS pseudo-relapses.
Furthermore, as treatments that slow disability progression become available and more are coming, assessing treatment efficacy based solely on clinical data becomes challenging. Many treated individuals with MS, even when benefiting, still experience disability progression. Conventional MRI measures have limited utility in monitoring the underlying progression phenomena, and advanced MRI measures face limitations in large-scale applicability. Therefore, having an objective marker of the pathogenic events contributing to disease progression, such as potentially GFAP, could significantly impact treatment response evaluation and the periodic assessment of the benefit-risk ratio of continuing a drug, aligning with emerging experiences in other neurodegenerative diseases and disease-modifying therapies.
Toward a biomarker-based approach to multiple sclerosis: unmet needs
In the ongoing use and exploration of fluid biomarkers in the context of MS, several unmet needs for clinical implementation come into focus (Panel 1). Many of the discussed markers, particularly those associated with microglia and astrocyte activation, have demonstrated promising outcomes in group-level studies. However, their significance at the individual level remains to be investigated. Only CSF IgG OCB are currently and diffusely tested across different countries during the processes of MS diagnosis and prognostication. While significant progresses in identifying potential biomarkers have been made, challenges indeed persist. One challenge is the need for standardization and harmonization across laboratories and clinical settings. For certain biomarkers, such as κ-FLC index, GFAP and NfL, the absence of universally accepted cut-off values and various technologies leading to variation in absolute concentrations complicate interpretation and hinder consistent clinical decision-making. The standardization of methodologies and the establishment of robust reference standards are essential steps to ensure the reliability and comparability of measures. Another critical aspect is the integration of these biomarkers into routine clinical practice. Currently, the utilization of many of them is primarily limited to specialized centers. Bridging this gap necessitates the development of user-friendly assays and comprehensive training programs for healthcare professionals. The aim is to make these biomarkers more widely accessible, regardless of geographical location or institutional resources. Advancements in blood-based measurements, facilitated by fully automated analytical platforms, offer convenience and real-time monitoring capabilities. Markers like NfL and GFAP, when assessed in plasma or serum samples, might indeed provide a dynamic measure of disease activity and progression. However, there is a need for large, longitudinal, cross-validation studies examining different aspects of these markers, such as baseline levels, longitudinal changes, and modifications after disease modifying therapies. Furthermore, it is imperative to elucidate the comparative relevance of each biomarker in relation to other clinical and MRI metrics, alongside exploring the synergistic benefits of integrating diverse biomarker measurements within diagnostic and prognostic frameworks. For instance, recent studies have underscored the capacity of fluid biomarkers like blood NfL to mirror the spectrum of MS-related global and regional brain dysconnectivity, as evidenced by advanced high-field brain MRI-derived disconnectome mapping.148 This highlights the potential of integrating fluid biomarkers with structural assessments, such as retinal optical coherence tomography measures, to potentially amplify the prognostic efficacy beyond that achievable by fluid biomarkers alone.149 This prompts a critical inquiry into whether the advantages conferred by such integrated multimodal biomarker approaches outweigh the inherent sustainability challenges.
In the effort of enhancing our understanding and management of MS, several promising biomarkers are emerging. Neuronal markers such as parvalbumin shows promise in reflecting cortical neurodegeneration and atrophy in MS.150 Extracellular vesicles, microscopic particles released by cells, could serve as windows into the CNS from peripheral fluids, potentially reflecting MS-related pathophysiology.151 Lastly, immune phenotyping using flow cytometry offers a sophisticated approach to dissecting the intricate immune system dysregulation characteristic of MS, allowing to distinguish the disease from other immune-mediated disorders affecting the CNS.152 Future studies must focus on validating the utility of these novel biomarkers in large-scale investigations, ultimately paving the way for their translation into clinical use.
The road to full integration of fluid biomarkers into clinical practice is complex (Panel 1). It requires interdisciplinary collaboration, standardization initiatives, data sharing, and validation efforts. Fluid biomarkers in MS represent a promising frontier with ongoing challenges. Addressing standardization, accessibility, and expanding biomarker portfolios is essential.
Contributors
Massimiliano Di Filippo, Lorenzo Gaetani, and Lucilla Parnetti conceived and designed the Series article. Massimiliano Di Filippo, Lorenzo Gaetani, Diego Centonze, Harald Hegen, Jens Kuhle, Charlotte E. Teunissen, Mar Tintoré, Luisa Maria Villar, Eline Willemse, Henrik Zetterberg, and Lucilla Parnetti drafted the Series article. All the authors critically revised the Series article for significant intellectual content.
Declaration of interests
Massimiliano Di Filippo participated on advisory boards and steering committees for and received speaker or writing honoraria, research support and funding for travelling from Alexion, BMS, Bayer, Biogen Idec, Genzyme, Horizon, Janssen, Merck, Mylan, Novartis, Roche, Siemens Healthineers, Teva and Viatris.
Lorenzo Gaetani participated on advisory boards for, and received writing honoraria and travel grants from Almirall, Biogen, Euroimmun, Fujirebio, Lilly, Merck, Mylan, Novartis, Roche, Sanofi, Siemens Healthineers and Teva.
Diego Centonze is an advisory board member of Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva, and received honoraria for speaking or consultation fees from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. He is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Mitsubishi, Novartis, Roche, Sanofi-Genzyme, and Teva. His preclinical and clinical research was supported by grants from Bayer Schering, Biogen Idec, Celgene, Merck Serono, Novartis, Roche, Sanofi-Genzyme and Teva.
Harald Hegen has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Bristol Myers Squibb, Horizon, Janssen, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva, and received honoraria for acting as consultant for Biogen, Bristol Myers Squibb, Novartis, Roche, Sanofi-Genzyme and Teva. He is associate editor of Frontiers in Neurology.
Jens Kuhle received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Alnylam, Bayer, Biogen, Bristol Myers Squibb, Celgene, Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Quanterix, Roche, Sanofi, Stata DX.
Charlotte E. Teunissen performed contract research for Acumen, ADx Neurosciences, AC-Immune, Alamar, Aribio, Axon Neurosciences, Beckman–Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Toyama, Vivoryon. She is editor in chief of Alzheimer Research and Therapy, and serves on editorial boards of Medidact Neurologie/Springer, and Neurology: Neuroimmunology & Neuroinflammation. She had speaker contracts for Eli Lilly, Grifols, Novo Nordisk, Olink and Roche.
Mar Tintoré has received compensation for consulting services, speaking honoraria and research support from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela Bio and Teva Pharmaceuticals. Data Safety Monitoring Board for Parexel and UCB Biopharma, Relapse Adjudication Committee for IMCYSE SA.
Luisa Maria Villar participated on advisory boards and for and received speaker or writing honoraria, research support and funding for travelling from BMS, Bayer, Binding Site, Biogen, Horizon, Janssen, Merck, Novartis, Roche, and Sanofi.
Eline Willemse declared no competing interests.
Henrik Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Lucilla Parnetti declared no competing interests.
Acknowledgements
Massimiliano Di Filippo receives support from the Ministero della Salute—Ricerca Finalizzata (RF-2021-12373319) and from Fondazione Italiana Sclerosi Multipla (FISM; project code 2023/PR-Single/017).
Research of Charlotte E. Teunissen is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 (MIRIADE), Innovative Medicines Initiatives 3 TR (Horizon 2020, grant no 831434) EPND (IMI 2 Joint Undertaking (JU), grant No. 101034344) and JPND (bPRIDE), National MS Society (Progressive MS alliance), Alzheimer's Drug Discovery Foundation, Alzheimer's Association, Health Holland, the Dutch Research Council (ZonMW), Alzheimer's Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Nederland. CT is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMw (#73305095007) and Health∼Holland, Top Sector Life Sciences & Health (PPP-allowance; #LSHM20106). Charlotte E. Teunissen is recipient of TAP-dementia, a ZonMw funded project (#10510032120003) in the context of the Dutch National Dementia Strategy.
Luisa M Villar receives support from the Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER; “Otra manera de hacer Europa”) (Grants RD21/0002/0053 and grant PI21/00828) and, integrated in the Plan Estatal I + D + i, cofunded by Instituto de Salud Carlos III (ISCIII). She also receives funding from the European Commission (Innovative Medicines Initiatives 3 TR. Horizon 2020, grant no 831434).
Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2023-00356; #2022-01018 and #2019–02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer's Drug Discovery Foundation (ADDF), USA (#201809–2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C), the Bluefield Project, Cure Alzheimer's Fund, the Olav Thon Foundation, the Erling-Persson Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003).
Role of the funding source: No specific grant from any funding agency in the public, commercial, or not-for-profit sectors were involved in the preparation of this paper.
References
- 1.Jakimovski D., Bittner S., Zivadinov R., et al. Multiple sclerosis. Lancet (London, England) 2023 doi: 10.1016/S0140-6736(23)01473-3. [DOI] [PubMed] [Google Scholar]
- 2.Portaccio E., Magyari M., Havrdova E.K., et al. Multiple sclerosis: emerging epidemiological trends and redefining the clinical course. Lancet Reg Health Eur. 2024;44:100977. doi: 10.1016/j.lanepe.2024.100977. [DOI] [Google Scholar]
- 3.Thompson A.J., Banwell B.L., Barkhof F., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 4.Rocca M.A., Preziosa P., Barkhof F., et al. MRI in the diagnosis and prognosis of multiple sclerosis. Lancet Reg Heal Eur. 2024;44:100978. doi: 10.1016/j.lanepe.2024.100978. [DOI] [Google Scholar]
- 5.Judge D., Roberts J., Khandker R.K., Ambegaonkar B., Black C.M. Physician practice patterns associated with diagnostic evaluation of patients with suspected mild cognitive impairment and Alzheimer's disease. Int J Alzheimer's Dis. 2019;2019 doi: 10.1155/2019/4942562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelborghs S., Niemantsverdriet E., Struyfs H., et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer's Dementia. 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monserrate A.E., Ryman D.C., Ma S., et al. Factors associated with the onset and persistence of post-lumbar puncture headache. JAMA Neurol. 2015;72:325–332. doi: 10.1001/jamaneurol.2014.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stangel M., Fredrikson S., Meinl E., Petzold A., Stüve O., Tumani H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol. 2013;9:267–276. doi: 10.1038/nrneurol.2013.41. [DOI] [PubMed] [Google Scholar]
- 9.Berek K., Bsteh G., Auer M., et al. Cerebrospinal fluid findings in 541 patients with clinically isolated syndrome and multiple sclerosis: a Monocentric study. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.675307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poser C.M., Paty D.W., Scheinberg L., et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 11.Teunissen C.E., Kimble L., Bayoumy S., et al. Methods to discover and validate biofluid-based biomarkers in neurodegenerative dementias. Mol Cell Proteomics. 2023;22 doi: 10.1016/j.mcpro.2023.100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi M., Bar-Or A., Piehl F., et al. Multiple sclerosis. Nat Rev Dis Prim. 2018;4:43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt B., Comabella M., Chan A. Multiple sclerosis: immunopathological heterogeneity and its implications. Eur J Immunol. 2022;52:869–881. doi: 10.1002/eji.202149757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teunissen C.E., Malekzadeh A., Leurs C., Bridel C., Killestein J. Body fluid biomarkers for multiple sclerosis—the long road to clinical application. Nat Rev Neurol. 2015;11:585–596. doi: 10.1038/nrneurol.2015.173. [DOI] [PubMed] [Google Scholar]
- 15.Lassmann H. The contribution of neuropathology to multiple sclerosis research. Eur J Neurol. 2022;29:2869–2877. doi: 10.1111/ene.15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dendrou C.A., Fugger L., Friese M.A. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 17.Bai Z., Chen D., Wang L., et al. Cerebrospinal fluid and blood cytokines as biomarkers for multiple sclerosis: a systematic review and meta-analysis of 226 studies with 13,526 multiple sclerosis patients. Front Neurosci. 2019;13:1026. doi: 10.3389/fnins.2019.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brändle S.M., Obermeier B., Senel M., et al. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci U S A. 2016;113:7864–7869. doi: 10.1073/pnas.1522730113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegen H., Walde J., Berek K., et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: a systematic review and meta-analysis. Mult Scler J. 2023;29:169–181. doi: 10.1177/13524585221134213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegen H., Arrambide G., Gnanapavan S., et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: a consensus statement. Mult Scler. 2023;29:182–195. doi: 10.1177/13524585221134217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magliozzi R., Howell O.W., Nicholas R., et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol. 2018;83:739. doi: 10.1002/ana.25197. [DOI] [PubMed] [Google Scholar]
- 22.Novakova L., Axelsson M., Malmeström C., et al. NFL and CXCL13 may reveal disease activity in clinically and radiologically stable MS. Mult Scler Relat Disord. 2020;46 doi: 10.1016/j.msard.2020.102463. [DOI] [PubMed] [Google Scholar]
- 23.Pan Z., Zhu T., Liu Y., Zhang N. Role of the CXCL13/CXCR5 Axis in autoimmune diseases. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.850998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krumbholz M., Theil D., Cepok S., et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 25.Serafini B., Rosicarelli B., Magliozzi R., Stigliano E., Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magliozzi R., Howell O.W., Calabrese M., Reynolds R. Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis. Nat Rev Neurol. 2023;19:461–476. doi: 10.1038/s41582-023-00838-7. [DOI] [PubMed] [Google Scholar]
- 27.Kappos L., Wolinsky J.S., Giovannoni G., et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132. doi: 10.1001/jamaneurol.2020.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tur C., Carbonell-Mirabent P., Cobo-Calvo Á., et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80:151–160. doi: 10.1001/jamaneurol.2022.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamzaoui M., Garcia J., Boffa G., et al. Positron emission tomography with [18 F]-DPA-714 unveils a smoldering component in most multiple sclerosis lesions which drives disease progression. Ann Neurol. 2023;94:366–383. doi: 10.1002/ana.26657. [DOI] [PubMed] [Google Scholar]
- 30.Jäckle K., Zeis T., Schaeren-Wiemers N., et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain. 2020;143:2073–2088. doi: 10.1093/brain/awaa158. [DOI] [PubMed] [Google Scholar]
- 31.Absinta M., Maric D., Gharagozloo M., et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597:709–714. doi: 10.1038/s41586-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Filippo M., de Iure A., Giampà C., et al. Persistent activation of microglia and NADPH drive hippocampal dysfunction in experimental multiple sclerosis. Sci Rep. 2016;6 doi: 10.1038/srep20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hok-A-Hin Y.S., Hoozemans J.J.M., Hu W.T., et al. YKL-40 changes are not detected in post-mortem brain of patients with Alzheimer's disease and frontotemporal lobar degeneration. Alzheimer's Res Ther. 2022;14:100. doi: 10.1186/s13195-022-01039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinsinger G., Galéotti N., Nabholz N., et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult Scler. 2015;21:1251–1261. doi: 10.1177/1352458514561906. [DOI] [PubMed] [Google Scholar]
- 35.Cantó E., Tintoré M., Villar L.M., et al. Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes. Brain. 2015;138:918–931. doi: 10.1093/brain/awv017. [DOI] [PubMed] [Google Scholar]
- 36.Steinacker P., Verde F., Fang L., et al. Chitotriosidase (CHIT1) is increased in microglia and macrophages in spinal cord of amyotrophic lateral sclerosis and cerebrospinal fluid levels correlate with disease severity and progression. J Neurol Neurosurg Psychiatry. 2018;89:239–247. doi: 10.1136/jnnp-2017-317138. [DOI] [PubMed] [Google Scholar]
- 37.Filipello F., Goldsbury C., You S.F., Locca A., Karch C.M., Piccio L. Soluble TREM2: innocent bystander or active player in neurological diseases? Neurobiol Dis. 2022;165 doi: 10.1016/j.nbd.2022.105630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Högel H., Rissanen E., Barro C., et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler J. 2020;26:210–219. doi: 10.1177/1352458518819380. [DOI] [PubMed] [Google Scholar]
- 39.Abdelhak A., Foschi M., Abu-Rumeileh S., et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat Rev Neurol. 2022;18:158–172. doi: 10.1038/s41582-021-00616-3. [DOI] [PubMed] [Google Scholar]
- 40.Teunissen C.E., Dijkstra P.C., Polman C. Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 2005;4:32–41. doi: 10.1016/S1474-4422(04)00964-0. [DOI] [PubMed] [Google Scholar]
- 41.Petzold A. The 2022 lady estelle wolfson lectureship on neurofilaments. J Neurochem. 2022;163:179–219. doi: 10.1111/jnc.15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaetani L., Blennow K., Calabresi P., Di Filippo M., Parnetti L., Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90:870–881. doi: 10.1136/jnnp-2018-320106. [DOI] [PubMed] [Google Scholar]
- 43.Freedman M.S., Thompson E.J., Deisenhammer F., et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. 2005;62:865–870. doi: 10.1001/archneur.62.6.865. [DOI] [PubMed] [Google Scholar]
- 44.Link H., Tibbling G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand J Clin Lab Invest. 1977;37:397–401. doi: 10.1080/00365517709091498. [DOI] [PubMed] [Google Scholar]
- 45.Giles P.D., Heath J.P., Wroe S.J. Oligoclonal bands and the IgG index in multiple sclerosis: uses and limitations. Ann Clin Biochem. 1989;26:317–323. [PubMed] [Google Scholar]
- 46.Gasperi C., Salmen A., Antony G., et al. Association of intrathecal immunoglobulin G synthesis with disability worsening in multiple sclerosis. JAMA Neurol. 2019;76:1–9. doi: 10.1001/jamaneurol.2019.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker M., Latarche C., Roman E., Debouverie M., Malaplate-Armand C., Guillemin F. No prognostic value of routine cerebrospinal fluid biomarkers in a population-based cohort of 407 multiple sclerosis patients. BMC Neurol. 2015;15:1–8. doi: 10.1186/s12883-015-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiber H. Cerebrospinal fluid–physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler J. 1998;4:99–107. doi: 10.1177/135245859800400302. [DOI] [PubMed] [Google Scholar]
- 49.Link H., Huang Y.-M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol. 2006;180:17–28. doi: 10.1016/j.jneuroim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Lourenco P., Shirani A., Saeedi J., Oger J., Schreiber W.E., Tremlett H. Oligoclonal bands and cerebrospinal fluid markers in multiple sclerosis: associations with disease course and progression. Mult Scler J. 2013;19:577–584. doi: 10.1177/1352458512459684. [DOI] [PubMed] [Google Scholar]
- 51.McLean B.N., Luxton R.W., Thompson E.J. A study of immunoglobulin G in the cerebrospinal fluid of 1007 patients with suspected neurological disease using isoelectric focusing and the Log IgG-Index. A comparison and diagnostic applications. Brain. 1990;113(Pt 5):1269–1289. doi: 10.1093/brain/113.5.1269. [DOI] [PubMed] [Google Scholar]
- 52.Dobson R., Ramagopalan S., Davis A., Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry. 2013;84:909–914. doi: 10.1136/jnnp-2012-304695. [DOI] [PubMed] [Google Scholar]
- 53.Arrambide G., Tintore M., Espejo C., et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain. 2018;141:1075–1084. doi: 10.1093/brain/awy006. [DOI] [PubMed] [Google Scholar]
- 54.Arrambide G., Espejo C., Carbonell-Mirabent P., et al. The kappa free light chain index and oligoclonal bands have a similar role in the McDonald criteria. Brain. 2022;145:3931–3942. doi: 10.1093/brain/awac220. [DOI] [PubMed] [Google Scholar]
- 55.Hegen H., Berek K., Deisenhammer F. Cerebrospinal fluid kappa free light chains as biomarker in multiple sclerosis-from diagnosis to prediction of disease activity. Wien Med Wochenschr. 2022;172:337–345. doi: 10.1007/s10354-022-00912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levraut M., Laurent-Chabalier S., Ayrignac X., et al. Kappa free light chain biomarkers are efficient for the diagnosis of multiple sclerosis. Neurol Neuroimmunol Neuroinflamm–. 2023;10 doi: 10.1212/NXI.0000000000200049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenstein I., Axelsson M., Novakova L., et al. Intrathecal kappa free light chain synthesis is associated with worse prognosis in relapsing-remitting multiple sclerosis. J Neurol. 2023 doi: 10.1007/s00415-023-11817-9. published online June 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berek K., Bsteh G., Auer M., et al. Kappa-free light chains in CSF predict early multiple sclerosis disease activity. Neurol Neuroimmunol Neuroinflamm. 2021;8 doi: 10.1212/NXI.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hegen H., Berek K., Bsteh G., et al. Kappa free light chain and neurofilament light independently predict early multiple sclerosis disease activity-a cohort study. EBioMedicine. 2023;91 doi: 10.1016/j.ebiom.2023.104573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tintore M., Rovira À., Río J., et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;138:1863–1874. doi: 10.1093/brain/awv105. [DOI] [PubMed] [Google Scholar]
- 61.Monreal E., Sainz de la Maza S., Costa-Frossard L., et al. Predicting aggressive multiple sclerosis with intrathecal IgM synthesis among patients with a clinically isolated syndrome. Neurol Neuroimmunol Neuroinflamm. 2021;8 doi: 10.1212/NXI.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coll-Martinez C., Quintana E., Buxó M., et al. Oligoclonal IgM bands are a promising biomarker for long-term cognitive outcomes in multiple sclerosis. Mult Scler Relat Disord. 2022;68 doi: 10.1016/j.msard.2022.104397. [DOI] [PubMed] [Google Scholar]
- 63.Sola P., Mandrioli J., Simone A.M., et al. Primary progressive versus relapsing-onset multiple sclerosis: presence and prognostic value of cerebrospinal fluid oligoclonal IgM. Mult Scler. 2011;17:303–311. doi: 10.1177/1352458510386996. [DOI] [PubMed] [Google Scholar]
- 64.Pfuhl C., Grittner U., Gieß R.M., et al. Intrathecal IgM production is a strong risk factor for early conversion to multiple sclerosis. Neurology. 2019;93:e1439–e1451. doi: 10.1212/WNL.0000000000008237. [DOI] [PubMed] [Google Scholar]
- 65.Auer M., Hegen H., Zeileis A., Deisenhammer F. Quantitation of intrathecal immunoglobulin synthesis–a new empirical formula. Eur J Neurol. 2016;23:713–721. doi: 10.1111/ene.12924. [DOI] [PubMed] [Google Scholar]
- 66.Villar L.M., González-Porqué P., Masjuán J., Alvarez-Cermeño J.C., Bootello A., Keir G. A sensitive and reproducible method for the detection of oligoclonal IgM bands. J Immunol Methods. 2001;258:151–155. doi: 10.1016/s0022-1759(01)00492-6. [DOI] [PubMed] [Google Scholar]
- 67.Villar L.M., Masjuan J., González-Porqué P., et al. Intrathecal IgM synthesis in neurologic diseases: relationship with disability in MS. Neurology. 2002;58:824–826. doi: 10.1212/wnl.58.5.824. [DOI] [PubMed] [Google Scholar]
- 68.Villar L.M., Sádaba M.C., Roldán E., et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. 2005;115:187–194. doi: 10.1172/JCI22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Capuano R., Zubizarreta I., Alba-Arbalat S., et al. Oligoclonal IgM bands in the cerebrospinal fluid of patients with relapsing MS to inform long-term MS disability. Mult Scler. 2021;27:1706–1716. doi: 10.1177/1352458520981910. [DOI] [PubMed] [Google Scholar]
- 70.Alcalá Vicente C., Lacruz L., Gascón F., et al. Oligoclonal M bands and cervical spinal cord lesions predict early secondary progressive multiple sclerosis. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.991596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Picón C., Tejeda-Velarde A., Fernández-Velasco J.I., et al. Identification of the immunological changes appearing in the CSF during the early immunosenescence process occurring in multiple sclerosis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.685139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Villar L.M., Casanova B., Ouamara N., et al. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann Neurol. 2014;76:231–240. doi: 10.1002/ana.24190. [DOI] [PubMed] [Google Scholar]
- 73.Beltrán E., Obermeier B., Moser M., et al. Intrathecal somatic hypermutation of IgM in multiple sclerosis and neuroinflammation. Brain. 2014;137:2703–2714. doi: 10.1093/brain/awu205. [DOI] [PubMed] [Google Scholar]
- 74.Villar L.M., García-Sánchez M.I., Costa-Frossard L., et al. Immunological markers of optimal response to natalizumab in multiple sclerosis. Arch Neurol. 2012;69:191–197. doi: 10.1001/archneurol.2011.971. [DOI] [PubMed] [Google Scholar]
- 75.Sellebjerg F., Bornsen L., Khademi M., et al. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73:2003–2010. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- 76.Magliozzi R., Scalfari A., Pisani A.I., et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann Neurol. 2020;88:562–573. doi: 10.1002/ana.25786. [DOI] [PubMed] [Google Scholar]
- 77.Sellebjerg F., Börnsen L., Ammitzbøll C., et al. Defining active progressive multiple sclerosis. Mult Scler. 2017;23:1727–1735. doi: 10.1177/1352458517726592. [DOI] [PubMed] [Google Scholar]
- 78.Lamancová P., Urban P., Mašlanková J., Rabajdová M., Mareková M. Correlation of selected serum protein levels with the degree of disability and NEDA-3 status in multiple sclerosis phenotypes. Eur Rev Med Pharmacol Sci. 2022;26:3933–3941. doi: 10.26355/eurrev_202206_28962. [DOI] [PubMed] [Google Scholar]
- 79.Feki S., Damak M., Sakka S., et al. Intrathecal B cell-related markers for an optimized biological investigation of multiple sclerosis patients. Sci Rep. 2022;12 doi: 10.1038/s41598-022-19811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pike S.C., Gilli F., Pachner A.R. The CXCL13 index as a predictive biomarker for activity in clinically isolated syndrome. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241311050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khademi M., Kockum I., Andersson M.L., et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. 2011;17:335–343. doi: 10.1177/1352458510389102. [DOI] [PubMed] [Google Scholar]
- 82.Modvig S., Degn M., Roed H., et al. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult Scler. 2015;21:1761–1770. doi: 10.1177/1352458515574148. [DOI] [PubMed] [Google Scholar]
- 83.Fissolo N., Pappolla A., Rio J., et al. Serum levels of CXCL13 are associated with teriflunomide response in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10 doi: 10.1212/NXI.0000000000200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lundblad K., Zjukovskaja C., Larsson A., Cherif H., Kultima K., Burman J. CSF concentrations of CXCL13 and sCD27 before and after autologous hematopoietic stem cell transplantation for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10 doi: 10.1212/NXI.0000000000200135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pilz G., Sakic I., Wipfler P., et al. Chemokine CXCL13 in serum, CSF and blood–CSF barrier function: evidence of compartment restriction. Fluids Barriers CNS. 2020;17:7. doi: 10.1186/s12987-020-0170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fissolo N., Benkert P., Sastre-Garriga J., et al. Serum biomarker levels predict disability progression in patients with primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2023;95:410. doi: 10.1136/jnnp-2023-332251. [DOI] [PubMed] [Google Scholar]
- 87.Floro S., Carandini T., Pietroboni A.M., De Riz M.A., Scarpini E., Galimberti D. Role of chitinase 3-like 1 as a biomarker in multiple sclerosis: a systematic review and meta-analysis. Neurol Neuroimmunol Neuroinflamm. 2022;9 doi: 10.1212/NXI.0000000000001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lucchini M., De Arcangelis V., Piro G., et al. CSF CXCL13 and chitinase 3-like-1 levels predict disease course in relapsing multiple sclerosis. Mol Neurobiol. 2023;60:36–50. doi: 10.1007/s12035-022-03060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Comabella M., Clarke M.A., Schaedelin S., et al. CSF chitinase 3-like 1 is associated with iron rims in patients with a first demyelinating event. Mult Scler. 2022;28:71–81. doi: 10.1177/13524585211010082. [DOI] [PubMed] [Google Scholar]
- 90.Cantó E., Reverter F., Morcillo-Suárez C., et al. Chitinase 3-like 1 plasma levels are increased in patients with progressive forms of multiple sclerosis. Mult Scler. 2012;18:983–990. doi: 10.1177/1352458511433063. [DOI] [PubMed] [Google Scholar]
- 91.The human protein atlas. https://www.proteinatlas.org/ENSG00000133048-CHI3L1/single+cell+type
- 92.Oldoni E., Smets I., Mallants K., et al. CHIT1 at diagnosis reflects long-term multiple sclerosis disease activity. Ann Neurol. 2020;87:633–645. doi: 10.1002/ana.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takahashi K., Prinz M., Stagi M., Chechneva O., Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cignarella F., Filipello F., Bollman B., et al. TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathol. 2020;140:513–534. doi: 10.1007/s00401-020-02193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piccio L., Buonsanti C., Cella M., et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. 2008;131:3081–3091. doi: 10.1093/brain/awn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abdelhak A., Hottenrott T., Morenas-Rodríguez E., et al. Glial activation markers in CSF and serum from patients with primary progressive multiple sclerosis: potential of serum GFAP as disease severity marker? Front Neurol. 2019;10:280. doi: 10.3389/fneur.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ioannides Z.A., Csurhes P.A., Swayne A., Foubert P., Aftab B.T., Pender M.P. Correlations between macrophage/microglial activation marker sTREM-2 and measures of T-cell activation, neuroaxonal damage and disease severity in multiple sclerosis. Mult Scler J Exp Transl Clin–. 2021;7 doi: 10.1177/20552173211019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Öhrfelt A., Axelsson M., Malmeström C., et al. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult Scler. 2016;22:1587–1595. doi: 10.1177/1352458515624558. [DOI] [PubMed] [Google Scholar]
- 99.Ashton N.J., Suárez-Calvet M., Heslegrave A., et al. Plasma levels of soluble TREM2 and neurofilament light chain in TREM2 rare variant carriers. Alzheimer's Res Ther. 2019;11:94. doi: 10.1186/s13195-019-0545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Norgren N., Sundström P., Svenningsson A., Rosengren L., Stigbrand T., Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63:1586–1590. doi: 10.1212/01.wnl.0000142988.49341.d1. [DOI] [PubMed] [Google Scholar]
- 101.Rosengren L.E., Lycke J., Andersen O. Glial fibrillary acidic protein in CSF of multiple sclerosis patients: relation to neurological deficit. J Neurol Sci. 1995;133:61–65. doi: 10.1016/0022-510x(95)00152-r. [DOI] [PubMed] [Google Scholar]
- 102.Martínez M.A.M., Olsson B., Bau L., et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler. 2015;21:550–561. doi: 10.1177/1352458514549397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meier S., Willemse E.A.J., Schaedelin S., et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol. 2023;80:287–297. doi: 10.1001/jamaneurol.2022.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simrén J., Weninger H., Brum W.S., et al. Differences between blood and cerebrospinal fluid glial fibrillary Acidic protein levels: the effect of sample stability. Alzheimers Dement. 2022;18:1988–1992. doi: 10.1002/alz.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wingerchuk D.M., Banwell B., Bennett J.L., et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schindler P., Aktas O., Ringelstein M., et al. Glial fibrillary acidic protein as a biomarker in neuromyelitis optica spectrum disorder: a current review. Expert Rev Clin Immunol. 2023;19:71–91. doi: 10.1080/1744666X.2023.2148657. [DOI] [PubMed] [Google Scholar]
- 107.Watanabe M., Nakamura Y., Michalak Z., et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology. 2019;93 doi: 10.1212/WNL.0000000000008160. [DOI] [PubMed] [Google Scholar]
- 108.Barro C., Healy B.C., Liu Y., et al. Serum GFAP and NfL levels differentiate subsequent progression and disease activity in patients with progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflamm–. 2023;10 doi: 10.1212/NXI.0000000000200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wessels M.H., Van Lierop Z.Y., Noteboom S., et al. Serum glial fibrillary acidic protein in natalizumab-treated relapsing-remitting multiple sclerosis: an alternative to neurofilament light. Mult Scler. 2023;29:1229–1239. doi: 10.1177/13524585231188625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abdelhak A., Huss A., Kassubek J., Tumani H., Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karlsson J.E., Rosengren L.E., Haglid K.G. A rapid HPLC method to separate the triplet proteins of neurofilament. J Neurochem. 1987;49:1375–1378. doi: 10.1111/j.1471-4159.1987.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 112.Karlsson J.E., Rosengren L.E., Haglid K.G. Polyclonal antisera to the individual neurofilament triplet proteins: a characterization using ELISA and immunoblotting. J Neurochem. 1989;53:759–765. doi: 10.1111/j.1471-4159.1989.tb11770.x. [DOI] [PubMed] [Google Scholar]
- 113.Rosengren L.E., Karlsson J.E., Karlsson J.O., Persson L.I., Wikkelsø C. Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem. 1996;67:2013–2018. doi: 10.1046/j.1471-4159.1996.67052013.x. [DOI] [PubMed] [Google Scholar]
- 114.Norgren N., Karlsson J.-E., Rosengren L., Stigbrand T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics. 2002;21:53–59. doi: 10.1089/15368590252917647. [DOI] [PubMed] [Google Scholar]
- 115.Norgren N., Rosengren L., Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987:25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- 116.Lycke J.N., Karlsson J.-E., Andersen O., Rosengren L.E. Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1998;64:402–404. doi: 10.1136/jnnp.64.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gunnarsson M., Malmeström C., Axelsson M., et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69:83–89. doi: 10.1002/ana.22247. [DOI] [PubMed] [Google Scholar]
- 118.Khalil M., Teunissen C.E., Otto M., et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018 doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 119.Nötzel M., Werder L.I., Ziemssen T., Akgün K. Ella versus simoa serum neurofilament assessment to monitor treatment response in highly active multiple sclerosis patients. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232012361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gauthier A., Viel S., Perret M., et al. Comparison of SimoaTM and EllaTM to assess serum neurofilament-light chain in multiple sclerosis. Ann Clin Transl Neurol. 2021;8:1141–1150. doi: 10.1002/acn3.51355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ulndreaj A., Sohaei D., Thebault S., et al. Quantitation of neurofilament light chain protein in serum and cerebrospinal fluid from patients with multiple sclerosis using the MSD R-PLEX NfL assay. Diagnosis. 2023;10:275–280. doi: 10.1515/dx-2022-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ladang A., Mersch G., Dauwe M., et al. AD/PD 2023. 2023. Blood sample matrix validation, impact of sample freezing and method comparison analysis using the Lumipulse G NfL blood prototype assay. [Google Scholar]
- 123.Lee S., Plavina T., Singh C.M., et al. Development of a highly sensitive neurofilament light chain assay on an automated immunoassay platform. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.935382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kuhle J., Barro C., Andreasson U., et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54:1655–1661. doi: 10.1515/cclm-2015-1195. [DOI] [PubMed] [Google Scholar]
- 125.Bjornevik K., Munger K.L., Cortese M., et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2019;13 doi: 10.1001/jamaneurol.2019.3238. published online Sept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dalla Costa G., Martinelli V., Sangalli F., et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology. 2019;92:1–10. doi: 10.1212/WNL.0000000000006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Disanto G., Barro C., Benkert P., et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barro C., Benkert P., Disanto G., et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;95:1–10. doi: 10.1093/brain/awy154. [DOI] [PubMed] [Google Scholar]
- 129.Kuhle J., Kropshofer H., Haering D.A., et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92:e1007–e1015. doi: 10.1212/WNL.0000000000007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Siller N., Kuhle J., Muthuraman M., et al. Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler. 2018;25 doi: 10.1177/1352458518765666. [DOI] [PubMed] [Google Scholar]
- 131.Benkert P., Meier S., Schaedelin S., et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21:246–257. doi: 10.1016/S1474-4422(22)00009-6. [DOI] [PubMed] [Google Scholar]
- 132.Delcoigne B., Manouchehrinia A., Barro C., et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology. 2020;94:e1201–e1212. doi: 10.1212/WNL.0000000000009097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bar-Or A., Thanei G.-A., Harp C., et al. Blood neurofilament light levels predict non-relapsing progression following anti-CD20 therapy in relapsing and primary progressive multiple sclerosis: findings from the ocrelizumab randomised, double-blind phase 3 clinical trials. EBioMedicine. 2023;93 doi: 10.1016/j.ebiom.2023.104662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sormani M.P., Haering D.A., Kropshofer H., et al. Blood neurofilament light as a potential endpoint in Phase 2 studies in MS. Ann Clin Transl Neurol. 2019;6:1081–1089. doi: 10.1002/acn3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ziemssen T., Arnold D.L., Alvarez E., et al. Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.852563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abdelhak A., Benkert P., Schaedelin S., et al. Neurofilament light chain elevation and disability progression in multiple sclerosis. JAMA Neurol. 2023;80:1317–1325. doi: 10.1001/jamaneurol.2023.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bayoumy S., Verberk I.M.W., Vermunt L., et al. Neurofilament light protein as a biomarker for spinal muscular atrophy: a review and reference ranges. Clin Chem Lab Med. 2024 doi: 10.1515/cclm-2023-1311. [DOI] [PubMed] [Google Scholar]
- 138.Khalil M., Pirpamer L., Hofer E., et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11:1–9. doi: 10.1038/s41467-020-14612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vermunt L., Otte M., Verberk I.M.W., et al. Age- and disease-specific reference values for neurofilament light presented in an online interactive support interface. Ann Clin Transl Neurol. 2022;9:1832–1837. doi: 10.1002/acn3.51676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simrén J., Andreasson U., Gobom J., et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5-90 years. Brain Commun. 2022;4 doi: 10.1093/braincomms/fcac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abdelhak A., Petermeier F., Benkert P., et al. Serum neurofilament light chain reference database for individual application in paediatric care: a retrospective modelling and validation study. Lancet Neurol. 2023;22:826–833. doi: 10.1016/S1474-4422(23)00210-7. [DOI] [PubMed] [Google Scholar]
- 142.Calabrese M., Gasperini C., Tortorella C., et al. ‘Better explanations' in multiple sclerosis diagnostic workup: a 3-year longitudinal study. Neurology. 2019;92:e2527–e2537. doi: 10.1212/WNL.0000000000007573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Deisenhammer F., Zetterberg H., Fitzner B., Zettl U.K. The cerebrospinal fluid in multiple sclerosis. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hegen H., Berek K., Cavalla P., et al. Diagnostic value of kappa free light chain index in patients with primary progressive multiple sclerosis–a multicentre study. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1327947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jack C.R., Bennett D.A., Blennow K., et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huss A., Otto M., Senel M., Ludolph A.C., Abdelhak A., Tumani H. A score based on NfL and glial markers may differentiate between relapsing-remitting and progressive MS course. Front Neurol. 2020;11:608. doi: 10.3389/fneur.2020.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Forslin Y., Shams S., Hashim F., et al. Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. Am J Neuroradiol. 2017;38:1311–1316. doi: 10.3174/ajnr.A5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rise H.H., Brune S., Chien C., et al. Brain disconnectome mapping derived from white matter lesions and serum neurofilament light levels in multiple sclerosis: a longitudinal multicenter study. NeuroImage Clin. 2022;35 doi: 10.1016/j.nicl.2022.103099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin T.-Y., Vitkova V., Asseyer S., et al. Increased serum neurofilament light and thin ganglion cell-inner plexiform layer are additive risk factors for disease activity in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8 doi: 10.1212/NXI.0000000000001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Magliozzi R., Pitteri M., Ziccardi S., et al. CSF parvalbumin levels reflect interneuron loss linked with cortical pathology in multiple sclerosis. Ann Clin Transl Neurol. 2021;8:534–547. doi: 10.1002/acn3.51298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mazzucco M., Mannheim W., Shetty S.V., Linden J.R. CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis. Fluids Barriers CNS. 2022;19:13. doi: 10.1186/s12987-021-00299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gross C.C., Schulte-Mecklenbeck A., Madireddy L., et al. Classification of neurological diseases using multi-dimensional CSF analysis. Brain. 2021;144:2625–2634. doi: 10.1093/brain/awab147. [DOI] [PMC free article] [PubMed] [Google Scholar]