Abstract
Dental implants have been widely used with success, but long-term usage sometimes leads to implant loss. The purpose of this review was to summarize the etiology of early and late failure requiring dental implant removal and the treatment strategies for the removal of failed implants and reimplantation. Early failures are often caused by patient-related factors, such as smoking, diabetes, radiotherapy, bone quality, and periodontitis of the remaining natural teeth. The most common cause of late failure is peri-implantitis, followed by implant fracture and implant malpositioning. Implants should be removed if they are mobile or if their superstructure cannot be maintained (e.g., implant fracture). For peri-implantitis, implant removal should be determined based on the patient’s age and esthetic needs, the implant site, and the severity of bone loss. Many reports have been published on implant removal techniques. The reverse torque technique should always be the first choice because of its low invasiveness. The weighted survival rate for the replacement of failed implants is 86.3%, with a much lower survival rate after the second or subsequent implantations. Therefore, patient-specific problems, such as smoking habits and bruxism, should be checked before reimplantation and controlled to the greatest extent possible.
Keywords: Dental implant, Removal, Risk factors, Reimplantation, Literature review
1. Introduction
Dental implants are likely to function for an extremely long period [1]. The success of dental implantation hinges not only on the initial surgical and prosthetic aspects, but also on the ability of dental professionals to address challenges that may arise during long-term maintenance [2]. Therefore, dentists involved in implantation should have the knowledge and skills to cope with variances and problems during long-term maintenance, as well as the ability to perform the surgical procedure and prosthetic design. When an implant has been removed because of exacerbation of peri-implantitis, which results in a loss of bone support during maintenance, or implant fracture, which occurs infrequently, retreatment should be considered. Several methods for removing implants have been developed [3], and each method should be applied appropriately according to the circumstances of the implant failure. Background factors relating to the implant failure should also be considered before retreatment. However, unified treatment strategies for dental implant removal and replacement have not yet been established. This paper narratively reviews and discusses the etiology of implant failure requiring implant removal, including classification into early and late failure. The techniques of implant removal and replacement after removal are also discussed.
2. Early failure
Early failure can be characterized as failure that occurs within the first few weeks or months after implant placement or before functional loading with a prosthetic superstructure [4], [5]. Failed or inadequate osseointegration achievement causes early failure [4], [6]. The reported prevalence of early failure (implant level) ranges from 0.5% to 5.2% [4], [7], [8], [9], [10]. The wide range of prevalence may reflect differences in the implant type (turned / rough surface, length, diameter), procedure (graftless/graft), and characteristic of included samples.
2.1. Etiology
Early failure occurs when osseointegration is inadequate or nonexistent [4], [6]. Patient-related factors such as systemic and local diseases, health-compromising behaviors, and operator-related factors are outlined below.
2.1.1. Smoking
Smoking is a well-known risk factor in implant treatment [11]. Smoking harms the immune system and impedes wound healing; consequently, it adversely affects almost all outcomes of surgical procedures performed in the oral cavity, including implant placement. Smoking also negatively affects bone metabolism [12]. One systematic review that focused on early failure also indicated that smoking increased the risk of early failure [13]. Dentists should consider counseling their patients in smoking cessation, which has been shown to improve success rates for osseointegration in smokers [14]. However, not all patients follow smoking cessation counseling; in fact, only 15–25% of subjects provided with smoking cessation counseling by a dentist or dental hygienist stopped smoking [15], [16].
2.1.2. Diabetes mellitus
Diabetes mellitus is a common metabolic disorder characterized by hyperglycemia caused by a defect in insulin secretion or insulin action, or both [17]. Diabetes mellitus causes many symptoms such as delayed wound healing [18], immune dysfunction [19], and abnormal bone metabolism [20]. Poorly controlled diabetes negatively affects implant treatment [21], [22], although some systematic reviews concluded that well-controlled diabetes mellitus was not a risk factor in implant treatment [22], [23]. Focusing on early failure, one systematic review indicated that patients with diabetes mellitus showed an increasing trend of implant failure during the period of osseointegration [24], suggesting that diabetes mellitus is a risk factor for early implant failure. It is notable that 6 out of the 7 studies covered in the systematic review included patients with well-controlled diabetes mellitus [24], which suggests that even well-controlled diabetes mellitus might increase the risk of implant failure up to the end of the early period.
2.1.3. Radiotherapy
It is known that the adverse effects of radiotherapy negatively influence the outcome of implant treatment [25]. Although a retrospective study revealed that radiotherapy was a local factor for early implant failure [7], radiotherapy is rarely cited as a significant factor in systematic reviews focusing on early implant failure [4], [5], [13]. This may be because implant treatment is rarely conducted on patients undergoing radiation treatment. Although there is no clear upper limit of radiation dose for the success of implant treatment, a recent study suggested that patients receiving less than 38 Gy radiation therapy for head and neck cancer can safely undergo implant treatment [26].
2.1.4. Bone quality
Bone quality is possibly related to early failure. Nicolielo et al. revealed that a particular trabecular bone pattern of very sparse and very dense bone is a cause of early implant failure [27]. It is speculated that very sparse bone causes low implant insertional torque, which is known to be associated with early implant failure. Bone quality is further determined by factors such as turnover, damage accumulation, and mineralization [28]; these factors warrant further study. An animal study indicated that vitamin K2 enabled bone microstructural and mechanical recovery in an ovariectomized model, suggesting that nutritional guidance may be an important factor in bone turnover during implant treatment in postmenopausal patients [29].
2.1.5. Periodontitis
Some systematic reviews indicated that periodontitis is a risk factor associated with early implant failure [5], [30]. The microbiota of diseased implants and that of teeth are similar, indicating that periodontal pathogens may extend into the peri-implant tissue [31]. Furthermore, periodontitis activates the immune inflammatory response [32], which may negatively affect osseointegration. Periodontal disease should be treated prior to implant placement to decrease the risk of peri-implantitis [33].
2.1.6. Allergic reactions
Titanium is the main material used for implants because it has long been regarded as a biocompatible material with high corrosion resistance as a result of its thin protective oxide layer [34]. However, recent studies indicated that mechanical stress can cause wearing of titanium [35], [36]. Additionally, high serum fluoride has been shown to increase corrosion susceptibility and accelerate titanium ion release, especially in an acidic environment [37]. Released titanium particles can have adverse effects on local soft and hard tissues surrounding implants via activation of the immune system [38]. Previous studies have estimated that the prevalence of titanium allergy is 0.6–2.7% [39], [40]. Although patch tests may not always show up as positive [41], titanium allergy may present as rash, urticaria, pruritus, redness, swelling, dermatitis, pain, stomatitis, lichen planus, pustulosis, and gingival hyperplasia, necessitating implant removal [42]. Consequently, it should be noted that implants can provoke allergic reactions that result in implant failure.
2.1.7. Poor surgical skills
The expertise of the surgeon is a significant factor in the success of implants [43]; thus, poor surgical skills may induce early implant failure. Implant surgery requires skills in maintaining sterility, preventing bone overheating, and placing implants in areas with adequate bone, as well as the use of correct flap technique and insertion with a steady hand [44].
3. Late failure
Late failure is defined as failure that occurs after osseointegration and functional loading with a prosthetic superstructure; in other words, failure after osseointegration has been achieved, and thereby need to be removed [45], [46]. The previously reported prevalence of late failure (implant level) was 0.5–7.8% [4], [8], [9], [10]. As in early failure, the range of variation in the prevalence may be related to implant type (turned / rough surface, length, diameter), procedure (graftless/graft), and characteristic of included samples. Furthermore, the length of the observation period could affect the prevalence of late failure.
3.1. Etiology
Late failure could occur as a result of either biological or mechanical complications. Representative complications related to late failure are outlined below.
3.1.1. Peri-implantitis
Peri-implantitis is a biological complication characterized by inflammation around the implant tissue including the surrounding bone. It has been reported that peri-implantitis occurs in approximately 28% to 77% of patients and in 12% to 43% of implants [47]. The prevalence differed among studies because diagnosis was not standardized; however, peri-implantitis is a common clinical complication. Diabetes mellitus and smoking are often cited as risk factors for peri-implantitis. Some systematic reviews concluded that uncontrolled or poorly controlled diabetes mellitus was associated with a greater risk of peri-implantitis [21], [48]. Although the study by Alasqah et al. investigating the effect of well-controlled diabetes mellitus on peri-implantitis is not conclusive, they found that crestal bone around implants could remain stable in type 2 diabetic patients in a manner similar to non-diabetic patients if glycemic levels are strictly controlled [49]. A recent systematic review of the impact of smoking on peri-implantitis cited evidence of moderate certainty that smoking is associated with peri-implantitis [50]. However, it is unknown how the frequency of smoking affects peri-implantitis, because most studies did not report this. Another systematic review focusing on the effect of smoking on implant failure indicated that implant failure increased in line with the number of cigarettes smoked per day: more than 20 cigarettes per day was a risk factor for implant failure [11].
3.1.2. Implant fracture
Fractured implants should be removed because they cannot effectively support the superstructure. Several studies have reported incident rates for implant fracture: 0.49% of several types of implants over 6.9 years [51], 0.2% of external implants over 5 years [52], 0.92% of internal connection implants over 4.95 years [53], and 3.5% of internal connection implants over 14 years [54]. An in vivo study reported that the type of implant fracture varied depending on the implant diameter: narrow (3.3 mm) implants fractured at the second or third thread of the implant, regular (3.75 mm) diameter implants fractured at the implant neck or second thread, and wide-diameter (5 mm) implants rarely fractured, but the abutment or screw fractured [55]. Narrow diameter, [51], [53], [56], implant location (posterior region) [53], higher grades of titanium [56], direct adjacency to a cantilever [56], and bruxism have been proposed as potential risk factors for implant fracture [56], [57]. Chrcanovic et al. reported the detailed effects of each factor on implant fracture (increase/decrease in fracture probability) as follows: use of higher grades of titanium (decrease 72.9%), bruxism (increase 1819.5%), direct adjacency to a cantilever (increase 247.6%), every 1 mm increase in implant length (increase 22.3%), and every 1 mm increase in implant diameter (decrease 96.9%) [56]. Nightguards are commonly recommended for bruxism patients to decrease mechanical implant complications; however, there is no evidence that nightguards prevent implant facture, so further study is required.
3.1.3. Implant positioning errors
Even if osseointegration has been established, incorrectly positioned implants can cause functional or esthetic complications, which might lead to implant removal. When the implant is placed too close to an adjacent implant or tooth, resorption of the surrounding bone may occur, which reduces the height of the implant–implant or tooth–implant papilla. Implant positioning that is too shallow, too far facially, or too far inclined axially may cause exposure of the implant shoulder, which causes esthetic compromise and poor cleanability [58], [59]. To prevent implant positioning errors, correct diagnosis and planning are required, taking into account the width of the edentulous space, the gingival phenotype, and the bone anatomy [58]. During the implant surgery, computer guidance is useful to reduce positioning errors [60], [61]. However, clinicians should be aware that errors can still occur even when using a computer-guided system [62].
3.1.4. Medication
Some medications, including antiresorptive agents such as bisphosphonates and RANK ligand inhibitors, are known to be related to implant failure. Some systematic reviews suggest that low-dose antiresorptive therapy for osteoporosis does not cause a significant increase in implant failure [63], [64], [65], [66] while others could not conclude whether it negatively affected implant treatment or not [67], [68]. However, many studies have reported medication-related osteonecrosis of the jaw (MRONJ) associated with dental implants [69], [70], [71], [72]. Therefore, patients receiving antiresorptive therapy must be informed about the possible risk of developing MRONJ as a complication related to implant treatment. Additionally, as described in the American Association of Oral and Maxillofacial Surgeons’ position paper, implant placement should be avoided in oncology patients receiving high-dose antiresorptive therapy [73].
Medications such as selective serotonin reuptake inhibitors (SSRIs), proton pump inhibitors (PPIs), β-blockers and non-steroidal anti-inflammatory drugs modulate bone metabolism [74], [75], [76], [77]. A recent systematic review reported that SSRIs and PPIs significantly increase implant failure [65]. However, the number of included studies was small (two studies for each medication); therefore, further study is required.
4. Management of peri-implantitis
A gold standard method for treating peri-implantitis has not yet been established, although the protocol proposed by Heitz-Mayfield and Mombelli consisting of nonsurgical and surgical treatment has become widely accepted [78]. Their nonsurgical treatment includes oral hygiene instruction, counseling for smoking cessation, assessment of the prosthesis for plaque control, removal and adjustment of the prosthesis, nonsurgical debridement, and antimicrobial therapy. Surgical treatment, such as decontamination of the implant surface with a full-thickness mucoperiosteal flap, and regenerative therapy are recommended when resolution is not achieved by nonsurgical treatment. Recent studies have reported the efficacy of other treatment methods such as laser or photodynamic therapy [48], [79]. Implant removal may be chosen in cases in which bone resorption is severe or at the request of the patient. There is probably no reason not to remove an implant that is mobile or is causing uncontrolled pain; however, it is debatable whether implants in which supporting bone is resorbed should be removed. The clinician should take into account the patient’s symptoms, rate of disease progression, age, preferences, and systemic conditions.
5. Removal
As in early implant failures in which osseointegration could not be achieved, late failures resulting from peri-implantitis with loss of most of the supporting bone and implant fixture fracture require implant removal and retreatment. Nontraumatic implant explantation should be selected wherever possible to preserve the surrounding bone, followed by implant replacement.
5.1. Criteria for implant removal
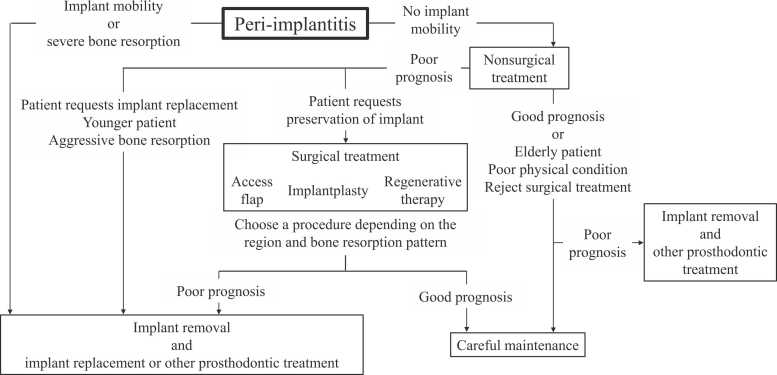
Mechanical incidents can generally be treated by repair or refabrication of the superstructure or replacement of screws. However, implant removal is required for implant fractures. For peri-implantitis, no criteria have been established as to whether peri-implantitis should be aggressively treated to preserve the implant or whether reimplantation should be performed after implant removal. Misch et al. recommended the removal of nonmobile implants with 50% bone loss as failures [80]. Greenstein et al. recommended the removal of implants with ≥ 75% bone resorption or ≤ 3-mm apical bone around the implants [81]. Surgical therapy for peri-implantitis with ≥ 50% or ≥ 5 mm bone loss has a poor prognosis [82], [83], [84]. Therefore, for implants with ≥ 50% bone loss, dentists should actively discuss implant removal with patients. Even for moderate bone resorption, surgical management for peri-implantitis may be required, depending on the patient’s age and wishes. For mild bone resorption, implant removal may be actively performed for reimplantation after comprehensive assessment of the situation. Fig. 1 shows a flowchart for the treatment of peri-implantitis, including the removal of implants. In cases in which dental problems such as gingival recession, poor plaque control, and bone resorption are caused by implant malpositioning, proper reimplantation should be performed after implant removal.
Fig. 1.
Flow chart for treatment of peri-implantitis including implant removal.
5.2. Techniques of implant removal
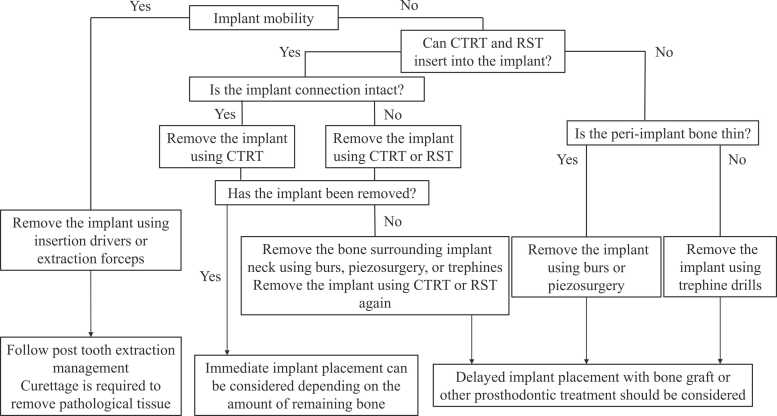
Implant removal is performed using reverse torque, trephines, burs, piezosurgery, laser-assisted explantation, and combinations of these tools [3], [85], [86], [87], [88]. A systematic review of explantation techniques for osseointegrated dental implants revealed that the reverse torque technique is most commonly selected, with a success rate of 87.7% for 284 implants, followed by burs (100% for 49 implants), trephines (94% for 35 implants), and piezosurgery (100% for 11 implants) [87]. Fig. 2 shows some clinical recommendations for the removal of dental implants.
Fig. 2.
Flow chart of clinical recommendations for removal of dental implants. CTRT: counter torque ratchet technique; RST: reverse screw technique.
5.2.1. The reverse torque technique
For nonmobile implant fixtures, the minimally invasive reverse torque technique is the first choice for implant removal. Insertion drivers from various manufacturers can be used in reverse torque removal. A strong torque of ≥ 100 Ncm is generally required for implant fixture removal, so the insertion drivers should be carefully selected to avoid deformation. Various techniques, such as the counter torque ratchet technique (CTRT) (Neo Fixture Remover Kit, Neobiotech, Korea) and the reverse screw technique (RST) (BTI Implant Extraction System, Biotechnology Institute S.L., Spain and implant retrieval tool, Nobel Biocare, Switzerland), have been developed for implant removal. These instruments allow removal while minimizing damage to the surrounding bones. CTRT is recommended for intact connection with the implant without any implant fracture; in cases where the implant connection is damaged, RST should be adopted [85]. However, care should be exercised when using RST because, unlike CTRT, RST may cause tool fractures upon application of a strong torque with the insertion direction of the implant retrieval tool deviated from the axial direction of the implant fixture.
The reverse torque technique allows removal with minimal damage to the surrounding bone, thereby allowing implantation immediately after removal, depending on the amount of remaining bone. With > 200 Ncm removal torque, the surrounding coronal 3–4 mm of bone should be removed using burs, piezosurgery, or trephines, followed by removal in conjunction with the reverse torque technique [89], [90]. Anitua et al. removed 139 of 158 implants using only CTRT with 146 ± 5 Ncm removal torque, and the remaining 19 using CTRT after cutting into the first 3–4 mm using a trephine bur with 161 ± 13 Ncm removal torque [90]. For zirconia implants, CTRT may cause fractures and therefore can be selected only for cases with extensive bone resorption, although relevant data are limited [91].
5.2.2. Burs and piezosurgery
If removal cannot be achieved by the reverse torque technique, the surrounding bone should be removed. In such cases, cone-beam computed tomography (CBCT) should be employed to determine the width of the surrounding bone, especially the buccolingual bone. Then, the side with sufficient bone (usually the mesiodistal bone) is gradually removed using burs and piezosurgery to move the implant fixture using elevators and forceps until it becomes unstable. Advantageously, piezosurgery allows bone cutting while preventing soft tissue damage. Additionally, bone healing can be improved in comparison to using a bur [92], [93]. However, piezosurgery is inefficient for significant bone cutting. Intermittent injection with physiological saline is recommended to prevent overheating of the piezo tip. The implant fixture may sustain surface damage, resulting in the entry of titanium particles into the surrounding tissues.
5.2.3. Trephines
Trephine drills are often used to remove fractured dental implants [94], [95], [96]. They should only be selected when other removal tools cannot be used because of the large bone defect remaining after removal, requiring extensive bone grafting for reimplantation. Trephine drills of various sizes are available. The smallest effective size should be selected to reduce bone loss. The recommended rotation speed is 1200–1500 rpm, although this varies with the manufacturer. Caution should be exercised when using trephine burs to avoid complications such as fatigue fracture of the mandible and osteomyelitis [85]. Applying a trephine bur to thin peri-implant bone may increase the risk of a significant loss of surrounding bone. Therefore, a diagnosis should be made by CBCT before removal.
5.2.4. Patients’ perception of implant removal
Although implant removal after dental implant failure may negatively affect the patient’s perception of implantation, a cross-sectional study of patient’s satisfaction after dental implant removal using a self-reported questionnaire showed that 83.3% of patients were satisfied with the new implants placed after implant removal [97]. Implant removal seemingly does not affect the patient's satisfaction or quality of life. However, the presence of signs of infection before implant removal may have a negative impact on the quality of life score after implantation [97]. Patients who desire reimplantation after implant removal are characterized by younger age, root form implant type, and prosthetic-related complications as a reason for removal [98].
5.3. Hard tissue dimensional changes following implant removal
A retrospective study to examine the dimensional changes of hard tissue after implant removal caused by peri-implantitis demonstrated that the mean decreases in the ridge width at 1 and 3 mm below the crest were 11.3% and 4.4%, respectively [99]. The buccal and lingual ridge heights significantly decreased to 2.2% and 6.3%, respectively. Bone regeneration along with implant removal can minimize dimensional changes both vertically and horizontally. Use of a reverse torque removal kit was effective in reducing dimensional changes [99]. Additionally, preclinical research demonstrated that, after implant removal by the reverse torque technique, osteocytes existed in the lacunae on the bone surface adjacent to the site of the implant removal. These cells had normal morphology without any damage [100]. Although bone healing mechanisms after implant removal remain unclear, nontraumatic implant explantation should be effective in minimizing dimensional changes after implant removal. Covani et al. reported that approximately half of implant removal cases did not require regenerative procedures for reimplantation, while the other half required bone grafts and a resorbable membrane [101]. The use of biologics such as autologous blood-derived products for ridge preservation after implant removal may also be effective in enhancing the healing process [102].
6. Replacement in failed sites
There are advantages and disadvantages related to the timing of reimplantation after implant removal, as observed for implantation after tooth extraction. Immediate placement after removal shortens the treatment duration and eliminates the need for additional surgical procedures, providing a great benefit to patients, although removal tools are required to prevent the loss of the surrounding bone during the removal. Additionally, the implant site should be checked for peri-implant infection and inflammatory bone sclerosis before removal [88]. Even if the implant fixture can be removed with minimal damage to the surrounding bone, an implant of the same size as the previously placed implant cannot be used. Therefore, the implant diameter or length should be increased [103], and adequate bone width and height should be ensured by CBCT before implant removal. In cases in which there is inadequate surrounding bone, bone healing after removal should be confirmed before reimplantation.
Delayed placement after removal has the advantages of reducing the risk of infection and more easily obtaining primary implant stability, but has the disadvantage of a greater patient burden as a result of the prolonged treatment duration and the need for bone grafting. If removal and reimplantation are needed because of malpositioning of the implants, early implantation can be safely performed after soft tissue and bone healing following removal, instead of immediate reimplantation, because of the differences in the implant’s explantation sockets and reimplantation sites.
In cases of implant fracture or peri-implant bone resorption caused by overloading, reimplantation should be accompanied by additional measures such as an increase in the number of implants, use of an implant with a larger diameter, and use of a night guard.
6.1. Survival rate after reimplantation
A systematic review of replacement after implant removal revealed that the timing of reimplantation was generally 4–6 months after removal and the weighted survival rate at 1–5 years after reimplantation was 86.3% [104]. Furthermore, rough-surfaced implants had a higher survival rate than smooth-surfaced implants (90% vs 68.7%). Kim et al. found no significant difference in the failure rate after implant removal between delayed and immediate reimplantation [105]. Delayed placement seemingly has no advantage if there is adequate bone to use an implant with a greater length and diameter than those of the initial implant to achieve primary stability. Another systematic review of reimplantation revealed a lower survival rate of 67.1% for second reimplantations, relative to 88.7% for the first reimplantation, with 91.8% for reimplantation into a site with a previous early failure [106]. Machtei et al. reported that during reimplantation, implants with a larger diameter tend to have a slightly higher survival rate than those with a smaller diameter [103].
6.2. Risk factors and patient selection for reimplantation
Risk factors for reimplantation include patient-related factors (e.g., general health status, smoking habits, uncontrolled diabetes, periodontal disease, and oral hygiene maintenance), implant characteristics (e.g., dimensions, surface characteristics, and loading), and site characteristics (e.g., bone quality and density, vertical and horizontal dimensions, and peri-implant soft tissue) [107]. Occasionally, “cluster effects” (i.e., multiple implant failures in one patient) have been reported as a patient-related factor [108], [109], [110]. Park et al. reported that reimplantation failures are more frequently caused by patient factors than by implant factors [111]. Of note, smokers had more frequent reimplantation failures than non-smokers (hazard ratio, 4.79). Patient-specific problems, such as smoking habits and bruxism, should be checked before reimplantation [81]. Reimplantation should ideally only be considered in cases with no patient-related risk factors or if such risk factors have already been resolved.
7. Conclusion
Various tools have been developed to facilitate implant removal, bringing great benefit to both patients and dentists. Because implant removal with a trephine bur results in a large defect in the surrounding bone, minimally invasive procedures for implant removal using the reverse torque technique are the first treatment choice. Implant problems can be solved temporarily by implant removal. However, for subsequent prosthetic treatment, the patient's age and general condition and retreatment costs should be considered, and a new treatment plan should be established after the causes of the problems are determined. Therefore, we should evaluate the cause of the problem, such as diabetes, smoking, or overloading, to see if it can be resolved before proceeding with reimplantation or considering a change to a removable denture. In this review, we have summarized the current research regarding implant removal and reimplantation; however, it is clear that insufficient evidence is available and further research is required in this field.
Conflict of interest
The authors declare that there are no conflicts of interest related to this study.
Acknowledgements
We thank Helen Jeays, BDSc AE, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Moraschini V., Poubel L.A., Ferreira V.F., Barboza Edos S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: a systematic review. Int J Oral Maxillofac Surg. 2015;44:377–388. doi: 10.1016/j.ijom.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Pjetursson B.E., Thoma D., Jung R., Zwahlen M., Zembic A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin Oral Implants Res. 2012;23(Suppl 6):22–38. doi: 10.1111/j.1600-0501.2012.02546.x. [DOI] [PubMed] [Google Scholar]
- 3.Stajcic Z., Stojcev Stajcic L.J., Kalanovic M., Dinic A., Divekar N., Rodic M. Removal of dental implants: review of five different techniques. Int J Oral Maxillofac Surg. 2016;45:641–648. doi: 10.1016/j.ijom.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Chrcanovic B.R., Kisch J., Albrektsson T., Wennerberg A. Factors influencing early dental implant failures. J Dent Res. 2016;95:995–1002. doi: 10.1177/0022034516646098. [DOI] [PubMed] [Google Scholar]
- 5.Buhara O., Pehlivan S. Estimating the importance of significant risk factors for early dental implant failure: a monte carlo simulation. Int J Oral Maxillofac Implants. 2018;33:161–168. doi: 10.11607/jomi.5802. [DOI] [PubMed] [Google Scholar]
- 6.Krisam J., Ott L., Schmitz S., Klotz A.L., Seyidaliyeva A., Rammelsberg P., et al. Factors affecting the early failure of implants placed in a dental practice with a specialization in implantology - a retrospective study. BMC Oral Health. 2019;19:208. doi: 10.1186/s12903-019-0900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Steenberghe D., Jacobs R., Desnyder M., Maffei G., Quirynen M. The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin Oral Implants Res. 2002;13:617–622. doi: 10.1034/j.1600-0501.2002.130607.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Jung U.W., Cho K.S., Lee J.S. Retrospective radiographic observational study of 1692 Straumann tissue-level dental implants over 10 years: I. Implant survival and loss pattern. Clin Implant Dent Relat Res. 2018;20:860–866. doi: 10.1111/cid.12659. [DOI] [PubMed] [Google Scholar]
- 9.Takamoli J., Pascual A., Martinez-Amargant J., Garcia-Mur B., Nart J., Valles C. Implant failure and associated risk indicators: a retrospective study. Clin Oral Implants Res. 2021;32:619–628. doi: 10.1111/clr.13732. [DOI] [PubMed] [Google Scholar]
- 10.Lin G., Ye S., Liu F., He F. A retrospective study of 30,959 implants: Risk factors associated with early and late implant loss. J Clin Periodo. 2018;45:733–743. doi: 10.1111/jcpe.12898. [DOI] [PubMed] [Google Scholar]
- 11.Naseri R., Yaghini J., Feizi A. Levels of smoking and dental implants failure: a systematic review and meta-analysis. J Clin Periodo. 2020;47:518–528. doi: 10.1111/jcpe.13257. [DOI] [PubMed] [Google Scholar]
- 12.Yoon V., Maalouf N.M., Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int. 2012;23:2081–2092. doi: 10.1007/s00198-012-1940-y. [DOI] [PubMed] [Google Scholar]
- 13.Manzano G., Montero J., Martin-Vallejo J., Del Fabbro M., Bravo M., Testori T. Risk factors in early implant failure: a meta-analysis. Implant Dent. 2016;25:272–280. doi: 10.1097/ID.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 14.Bain C.A. Smoking and implant failure-benefits of a smoking cessation protocol. Int J Oral Maxillofac Implants. 1996;11:756–759. [PubMed] [Google Scholar]
- 15.Nasry H.A., Preshaw P.M., Stacey F., Heasman L., Swan M., Heasman P.A. Smoking cessation advice for patients with chronic periodontitis. Br Dent J. 2006;200:272–275. doi: 10.1038/sj.bdj.4813307. discussion 65. [DOI] [PubMed] [Google Scholar]
- 16.Gonseth S., Abarca M., Madrid C., Cornuz J. A pilot study combining individual-based smoking cessation counseling, pharmacotherapy, and dental hygiene intervention. BMC Public Health. 2010;10:348. doi: 10.1186/1471-2458-10-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 18.Abiko Y., Selimovic D. The mechanism of protracted wound healing on oral mucosa in diabetes. Review. Bosn J Basic Med Sci. 2010;10:186–191. doi: 10.17305/bjbms.2010.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geerlings S.E., Hoepelman A.I. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 20.Murray C.E., Coleman C.M. Impact of diabetes mellitus on bone health. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner J., Spille J.H., Wiltfang J., Naujokat H. Systematic review on diabetes mellitus and dental implants: an update. Int J Implant Dent. 2022;8(1) doi: 10.1186/s40729-021-00399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naujokat H., Kunzendorf B., Wiltfang J. Dental implants and diabetes mellitus-a systematic review. Int J Implant Dent. 2016;2:5. doi: 10.1186/s40729-016-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javed F., Romanos G.E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodo. 2009;80:1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- 24.Annibali S., Pranno N., Cristalli M.P., La Monaca G., Polimeni A. Survival analysis of implant in patients with diabetes mellitus: a systematic review. Implant Dent. 2016;25:663–674. doi: 10.1097/ID.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 25.Schiegnitz E., Reinicke K., Sagheb K., Konig J., Al-Nawas B., Grotz K.A. Dental implants in patients with head and neck cancer-a systematic review and meta-analysis of the influence of radiotherapy on implant survival. Clin Oral Implants Res. 2022;33:967–999. doi: 10.1111/clr.13976. [DOI] [PubMed] [Google Scholar]
- 26.Lee J., Lee J.J.B., Cha I.H., Park K.R., Lee C.G. Risk factor analysis of dental implants in patients with irradiated head and neck cancer. Head Neck. 2022;44:1816–1824. doi: 10.1002/hed.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolielo L.F.P., Van Dessel J., Jacobs R., Quirino Silveira Soares M., Collaert B. Relationship between trabecular bone architecture and early dental implant failure in the posterior region of the mandible. Clin Oral Implants Res. 2020;31:153–161. doi: 10.1111/clr.13551. [DOI] [PubMed] [Google Scholar]
- 28.NIH Consensus Development panel on osteoporosis prevention diagnosis, and therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 29.Iwamoto D., Masaki C., Shibata Y., Watanabe C., Nodai T., Munemasa T., et al. Microstructural and mechanical recovery of bone in ovariectomized rats: the effects of menaquinone-7. J Mech Behav Biomed Mater. 2021;120 doi: 10.1016/j.jmbbm.2021.104571. [DOI] [PubMed] [Google Scholar]
- 30.Buhara O., Pehlivan S. Monte carlo simulation of reasons for early failure of implants: effects of two risk factors. Br J Oral Maxillofac Surg. 2019;57:12–20. doi: 10.1016/j.bjoms.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Mombelli A. Aging and the periodontal and peri-implant microbiota. Periodontol 2000. 1998;16:44–52. doi: 10.1111/j.1600-0757.1998.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 32.Herrero E.R., Fernandes S., Verspecht T., Ugarte-Berzal E., Boon N., Proost P., et al. Dysbiotic biofilms deregulate the periodontal inflammatory response. J Dent Res. 2018;97:547–555. doi: 10.1177/0022034517752675. [DOI] [PubMed] [Google Scholar]
- 33.Renvert S., Quirynen M. Risk indicators for peri-implantitis. A narrative review. Clin Oral Implants Res. 2015;11:15–44. doi: 10.1111/clr.12636. [DOI] [PubMed] [Google Scholar]
- 34.Sidambe A.T. Biocompatibility of advanced manufactured titanium implants-a review. Mater (Basel) 2014;7:8168–8188. doi: 10.3390/ma7128168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tawse-Smith A., Ma S., Duncan W.J., Gray A., Reid M.R., Rich A.M. Implications of wear at the titanium-zirconia implant-abutment interface on the health of peri-implant tissues. Int J Oral Maxillofac Implants. 2017;32:599–609. doi: 10.11607/jomi.5014. [DOI] [PubMed] [Google Scholar]
- 36.Taylor T.D., Klotz M.W., Lawton R.A. Titanium tattooing associated with zirconia implant abutments: a clinical report of two cases. Int J Oral Maxillofac Implants. 2014;29:958–960. doi: 10.11607/jomi.3700. [DOI] [PubMed] [Google Scholar]
- 37.Chen W.Q., Zhang S.M., Qiu J. Surface analysis and corrosion behavior of pure titanium under fluoride exposure. J Prosthet Dent. 2020;124:239.e1-.e8. doi: 10.1016/j.prosdent.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Kheder W., Al Kawas S., Khalaf K., Samsudin A.R. Impact of tribocorrosion and titanium particles release on dental implant complications - a narrative review. Jpn Dent Sci Rev. 2021;57:182–189. doi: 10.1016/j.jdsr.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sicilia A., Cuesta S., Coma G., Arregui I., Guisasola C., Ruiz E., et al. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implants Res. 2008;19:823–835. doi: 10.1111/j.1600-0501.2008.01544.x. [DOI] [PubMed] [Google Scholar]
- 40.Kitagawa M., Murakami S., Akashi Y., Oka H., Shintani T., Ogawa I., et al. Current status of dental metal allergy in Japan. J Prosthodont Res. 2019;63:309–312. doi: 10.1016/j.jpor.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Bilgic A., Bozca B.C., Subası G.Y., Dicle Ö., Uzun S., Yılmaz E., et al. Standard patch test results and clinical relevance: a cross-sectional study of 10-year retrospective experience. Indian J Dermatol. 2022;67:258–264. doi: 10.4103/ijd.ijd_965_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comino-Garayoa R., Cortes-Breton Brinkmann J., Pelaez J., Lopez-Suarez C., Martinez-Gonzalez J.M., Suarez M.J. Allergies to titanium dental implants: what do we really know about them? A scoping review. Biol (Basel) 2020;9 doi: 10.3390/biology9110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sendyk D.I., Chrcanovic B.R., Albrektsson T., Wennerberg A., Zindel Deboni M.C. Does surgical experience influence implant survival rate? A systematic review and meta-analysis. Int J Prosthodont. 2017;30:341–347. doi: 10.11607/ijp.5211. [DOI] [PubMed] [Google Scholar]
- 44.Greenstein G., Cavallaro J., Romanos G., Tarnow D. Clinical recommendations for avoiding and managing surgical complications associated with implant dentistry: a review. J Periodo. 2008;79:1317–1329. doi: 10.1902/jop.2008.070067. [DOI] [PubMed] [Google Scholar]
- 45.Manor Y., Oubaid S., Mardinger O., Chaushu G., Nissan J. Characteristics of early versus late implant failure: a retrospective study. J Oral Maxillofac Surg. 2009;67:2649–2652. doi: 10.1016/j.joms.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 46.Do T.A., Le H.S., Shen Y.W., Huang H.L., Fuh L.J. Risk factors related to late failure of dental implant-a systematic review of recent studies. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17113931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zitzmann N.U., Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodo. 2008;35:286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y., Pu R., Qian Y., Shi J., Si M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: a systematic review and meta-analysis. Photo Photo Ther. 2021;34 doi: 10.1016/j.pdpdt.2021.102231. [DOI] [PubMed] [Google Scholar]
- 49.Alasqah M.N., Alrabiah M., Al-Aali K.A., Mokeem S.A., Binmahfooz A.M., ArRejaie A.S., et al. Peri-implant soft tissue status and crestal bone levels around adjacent implants placed in patients with and without type-2 diabetes mellitus: 6 years follow-up results. Clin Implant Dent Relat Res. 2018;20:562–568. doi: 10.1111/cid.12617. [DOI] [PubMed] [Google Scholar]
- 50.Reis I., do Amaral G., Hassan M.A., Villar C.C., Romito G.A., Spin-Neto R., et al. The influence of smoking on the incidence of peri-implantitis: a systematic review and meta-analysis. Clin Oral Implants Res. 2023;34:543–554. doi: 10.1111/clr.14066. [DOI] [PubMed] [Google Scholar]
- 51.Yu H., Qiu L. Analysis of fractured dental implant body from five different implant systems: a long-term retrospective study. Int J Oral Maxillofac Surg. 2022;51:1355–1361. doi: 10.1016/j.ijom.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Balshi T.J. An analysis and management of fractured implants: a clinical report. Int J Oral Maxillofac Implants. 1996;11:660–666. [PubMed] [Google Scholar]
- 53.Lee D.W., Kim N.H., Lee Y., Oh Y.A., Lee J.H., You H.K. Implant fracture failure rate and potential associated risk indicators: an up to 12-year retrospective study of implants in 5,124 patients. Clin Oral Implants Res. 2019;30:206–217. doi: 10.1111/clr.13407. [DOI] [PubMed] [Google Scholar]
- 54.Yi Y., Heo S.J., Koak J.Y., Kim S.K. Mechanical complications of implant-supported restorations with internal conical connection implants: a 14-year retrospective study. J Prosthet Dent. 2023;129:732–740. doi: 10.1016/j.prosdent.2021.06.053. [DOI] [PubMed] [Google Scholar]
- 55.Shemtov-Yona K., Rittel D., Machtei E.E., Levin L. Effect of dental implant diameter on fatigue performance. Part II: failure analysis. Clin Implant Dent Relat Res. 2014;16:178–184. doi: 10.1111/j.1708-8208.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 56.Chrcanovic B.R., Kisch J., Albrektsson T., Wennerberg A. Factors influencing the fracture of dental implants. Clin Implant Dent Relat Res. 2018;20:58–67. doi: 10.1111/cid.12572. [DOI] [PubMed] [Google Scholar]
- 57.Stoichkov B., Kirov D. Analysis of the causes of dental implant fracture: a retrospective clinical study. Quintessence Int. 2018;49:279–286. doi: 10.3290/j.qi.a39846. [DOI] [PubMed] [Google Scholar]
- 58.Chen S.T., Buser D., Sculean A., Belser U.C. Complications and treatment errors in implant positioning in the aesthetic zone: diagnosis and possible solutions. Periodontol 2000. 2023 doi: 10.1111/prd.12474. [DOI] [PubMed] [Google Scholar]
- 59.Romandini M., Pedrinaci I., Lima C., Soldini M.C., Araoz A., Sanz M. Prevalence and risk/protective indicators of buccal soft tissue dehiscence around dental implants. J Clin Periodo. 2021;48:455–463. doi: 10.1111/jcpe.13417. [DOI] [PubMed] [Google Scholar]
- 60.Putra R.H., Yoda N., Astuti E.R., Sasaki K. The accuracy of implant placement with computer-guided surgery in partially edentulous patients and possible influencing factors: a systematic review and meta-analysis. J Prosthodont Res. 2022;66:29–39. doi: 10.2186/jpr.JPR_D_20_00184. [DOI] [PubMed] [Google Scholar]
- 61.Wei S.M., Zhu Y., Wei J.X., Zhang C.N., Shi J.Y., Lai H.C. Accuracy of dynamic navigation in implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2021;32:383–393. doi: 10.1111/clr.13719. [DOI] [PubMed] [Google Scholar]
- 62.Tahmaseb A., Wu V., Wismeijer D., Coucke W., Evans C. The accuracy of static computer-aided implant surgery: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):416–435. doi: 10.1111/clr.13346. [DOI] [PubMed] [Google Scholar]
- 63.Mendes V., Dos Santos G.O., Calasans-Maia M.D., Granjeiro J.M., Moraschini V. Impact of bisphosphonate therapy on dental implant outcomes: an overview of systematic review evidence. Int J Oral Maxillofac Surg. 2019;48:373–381. doi: 10.1016/j.ijom.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Ata-Ali J., Ata-Ali F., Penarrocha-Oltra D., Galindo-Moreno P. What is the impact of bisphosphonate therapy upon dental implant survival? A systematic review and meta-analysis. Clin Oral Implants Res. 2016;27:e38–e46. doi: 10.1111/clr.12526. [DOI] [PubMed] [Google Scholar]
- 65.Chappuis V., Avila-Ortiz G., Araujo M.G., Monje A. Medication-related dental implant failure: systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(Suppl 16):55–68. doi: 10.1111/clr.13137. [DOI] [PubMed] [Google Scholar]
- 66.Gelazius R., Poskevicius L., Sakavicius D., Grimuta V., Juodzbalys G. Dental implant placement in patients on bisphosphonate therapy: a systematic review. J Oral Maxillofac Res. 2018;9 doi: 10.5037/jomr.2018.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guazzo R., Sbricoli L., Ricci S., Bressan E., Piattelli A., Iaculli F. Medication-related osteonecrosis of the jaw and dental implants failures: a systematic review. J Oral Implant. 2017;43:51–57. doi: 10.1563/aaid-joi-16-00057. [DOI] [PubMed] [Google Scholar]
- 68.Papadakis I., Spanou A., Kalyvas D. Success rate and safety of dental implantology in patients treated with antiresorptive medication: a systematic review. J Oral Implant. 2021;47:169–180. doi: 10.1563/aaid-joi-D-19-00088. [DOI] [PubMed] [Google Scholar]
- 69.Kwon T.G., Lee C.O., Park J.W., Choi S.Y., Rijal G., Shin H.I. Osteonecrosis associated with dental implants in patients undergoing bisphosphonate treatment. Clin Oral Implants Res. 2014;25:632–640. doi: 10.1111/clr.12088. [DOI] [PubMed] [Google Scholar]
- 70.López-Cedrún J.L., Sanromán J.F., García A., Peñarrocha M., Feijoo J.F., Limeres J., et al. Oral bisphosphonate-related osteonecrosis of the jaws in dental implant patients: a case series. Br J Oral Maxillofac Surg. 2013:874–879. doi: 10.1016/j.bjoms.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Lazarovici T.S., Yahalom R., Taicher S., Schwartz-Arad D., Peleg O., Yarom N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J Oral Maxillofac Surg. 2010;68:790–796. doi: 10.1016/j.joms.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 72.Pichardo S.E.C., van der Hee J.G., Fiocco M., Appelman-Dijkstra N.M., van Merkesteyn J.P.R. Dental implants as risk factors for patients with medication-related osteonecrosis of the jaws (MRONJ) Br J Oral Maxillofac Surg. 2020;58:771–776. doi: 10.1016/j.bjoms.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 73.Ruggiero S.L., Dodson T.B., Aghaloo T., Carlson E.R., Ward B.B., Kademani D. American association of oral and maxillofacial surgeons' position paper on medication-related osteonecrosis of the jaws-2022 update. J Oral Maxillofac Surg. 2022;80:920–943. doi: 10.1016/j.joms.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Haney E.M., Warden S.J. Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: evidence from clinical studies. J Musculoskelet Neuron Inter. 2008;8:133–145. [PubMed] [Google Scholar]
- 75.Lespessailles E., Toumi H. Proton pump inhibitors and bone health: an update narrative review. Int J Mol Sci. 2022;23 doi: 10.3390/ijms231810733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hofbauer L.C., Henneicke H. β-blockers and bone health. J Clin Invest. 2018;128:4745–4747. doi: 10.1172/JCI122992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Waeli H., Reboucas A.P., Mansour A., Morris M., Tamimi F., Nicolau B. Non-steroidal anti-inflammatory drugs and bone healing in animal models-a systematic review and meta-analysis. Syst Rev. 2021;10:201. doi: 10.1186/s13643-021-01690-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heitz-Mayfield L.J., Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):325–345. doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- 79.Ting M., Alluri L.S.C., Sulewski J.G., Suzuki J.B., Paes Batista da Silva A. Laser treatment of peri-implantitis: a systematic review of radiographic outcomes. Dent J (Basel) 2022;10 doi: 10.3390/dj10020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Misch C.E., Perel M.L., Wang H.L., Sammartino G., Galindo-Moreno P., Trisi P., et al. Implant success, survival, and failure: the international congress of oral implantologists (ICOI) pisa consensus conference. Implant Dent. 2008;17:5–15. doi: 10.1097/ID.0b013e3181676059. [DOI] [PubMed] [Google Scholar]
- 81.Greenstein G., Cavallaro J. Failed dental implants: diagnosis, removal and survival of reimplantations. J Am Dent Assoc. 2014;145:835–842. doi: 10.14219/jada.2014.28. [DOI] [PubMed] [Google Scholar]
- 82.Lagervall M., Jansson L.E. Treatment outcome in patients with peri-implantitis in a periodontal clinic: a retrospective study. J Periodo. 2013;84:1365–1373. doi: 10.1902/jop.2012.120555. [DOI] [PubMed] [Google Scholar]
- 83.de Waal Y.C., Raghoebar G.M., Meijer H.J., Winkel E.G., van Winkelhoff A.J. Prognostic indicators for surgical peri-implantitis treatment. Clin Oral Implants Res. 2016;27:1485–1491. doi: 10.1111/clr.12584. [DOI] [PubMed] [Google Scholar]
- 84.Ravidà A., Siqueira R., Saleh I., Saleh M.H.A., Giannobile A., Wang H.L. Lack of clinical benefit of implantoplasty to improve implant survival rate. J Dent Res. 2020;99:1348–1355. doi: 10.1177/0022034520944158. [DOI] [PubMed] [Google Scholar]
- 85.Bowkett A., Laverty D., Patel A., Addy L. Removal techniques for failed implants. Br Dent J. 2016;220:109–114. doi: 10.1038/sj.bdj.2016.88. [DOI] [PubMed] [Google Scholar]
- 86.Lee J.B. Selectable implant removal methods due to mechanical and biological failures. Case Rep Dent. 2017;2017 doi: 10.1155/2017/9640517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy M., Loutan L., Garavaglia G., Hashim D. Removal of osseointegrated dental implants: a systematic review of explantation techniques. Clin Oral Invest. 2020;24:47–60. doi: 10.1007/s00784-019-03127-0. [DOI] [PubMed] [Google Scholar]
- 88.Monje A., Nart J. Management and sequelae of dental implant removal. Periodontol 2000. 2022;88:182–200. doi: 10.1111/prd.12418. [DOI] [PubMed] [Google Scholar]
- 89.Anitua E., Orive G. A new approach for atraumatic implant explantation and immediate implant installation. Oral Surg Oral Med Oral Pathol Oral Radio. 2012;113:e19–e25. doi: 10.1016/j.tripleo.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 90.Anitua E., Murias-Freijo A., Alkhraisat M.H. Conservative implant removal for the analysis of the cause, removal torque, and surface treatment of failed nonmobile dental implants. J Oral Implant. 2016;42:69–77. doi: 10.1563/aaid-joi-D-14-00207. [DOI] [PubMed] [Google Scholar]
- 91.Solderer A., Al-Jazrawi A., Sahrmann P., Jung R., Attin T., Schmidlin P.R. Removal of failed dental implants revisited: questions and answers. Clin Exp Dent Res. 2019;5:712–724. doi: 10.1002/cre2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marini E., Cisterna V., Messina A.M. The removal of a malpositioned implant in the anterior mandible using piezosurgery. Oral Surg Oral Med Oral Pathol Oral Radio. 2013;115:e1-5. doi: 10.1016/j.oooo.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 93.Messina A.M., Marini L., Marini E. A step-by-step technique for the piezosurgical removal of fractured implants. J Craniofac Surg. 2018;29:2116–2118. doi: 10.1097/SCS.0000000000004553. [DOI] [PubMed] [Google Scholar]
- 94.Muroff F.I. Removal and replacement of a fractured dental implant: case report. Implant Dent. 2003;12:206–210. doi: 10.1097/01.id.0000084168.57434.f1. [DOI] [PubMed] [Google Scholar]
- 95.Oguz Y., Cinar D., Bayram B. Removal of fractured implants and replacement with new ones. J Oral Implant. 2015;41:85–87. doi: 10.1563/AAID-JOI-D-12-00249. [DOI] [PubMed] [Google Scholar]
- 96.Jin S.Y., Kim S.G., Oh J.S., You J.S., Jeong M.A. Incidence and management of fractured dental implants: case reports. Implant Dent. 2017;26:802–806. doi: 10.1097/ID.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 97.Gargallo-Albiol J., Tavelli L., Barootchi S., Monje A., Wang H.L. Clinical sequelae and patients' perception of dental implant removal: a cross-sectional study. J Periodo. 2021;92:823–832. doi: 10.1002/JPER.20-0259. [DOI] [PubMed] [Google Scholar]
- 98.Sukegawa S., Saika M., Tamamura R., Nakano K., Takabatake K., Kawai H., et al. Clinical retrospective study of dental implant removal: do patients who require implant removal desire implant prosthesis again? Med Oral Patol Oral Cir Bucal. 2020;25:e784–e790. doi: 10.4317/medoral.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pons R., Carreño M., Amerio E., Gargallo-Albiol J., Nart J., Monje A. Hard tissue dimensional changes following implant removal due to peri-implantitis: a retrospective study. Clin Implant Dent Relat Res. 2021;23:432–443. doi: 10.1111/cid.13004. [DOI] [PubMed] [Google Scholar]
- 100.Anitua E., Murias-Freijo A., Piñas L., Tejero R., Prado R., Orive G. Nontraumatic implant explantation: a biomechanical and biological analysis in sheep tibia. J Oral Implant. 2016;42:3–11. doi: 10.1563/aaid-joi-D-14-00193. [DOI] [PubMed] [Google Scholar]
- 101.Covani U., Barone A., Cornelini R., Crespi R. Clinical outcome of implants placed immediately after implant removal. J Periodo. 2006;77:722–727. doi: 10.1902/jop.2006.040414. [DOI] [PubMed] [Google Scholar]
- 102.Monje A., Suárez-López Del Amo F. Application of biologics for ridge preservation/reconstruction after implant removal. Clin Adv Periodontics. 2022;12:270–276. doi: 10.1002/cap.10218. [DOI] [PubMed] [Google Scholar]
- 103.Machtei E.E., Mahler D., Oettinger-Barak O., Zuabi O., Horwitz J. Dental implants placed in previously failed sites: survival rate and factors affecting the outcome. Clin Oral Implants Res. 2008;19:259–264. doi: 10.1111/j.1600-0501.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 104.Oh S.L., Shiau H.J., Reynolds M.A. Survival of dental implants at sites after implant failure: a systematic review. J Prosthet Dent. 2020;123:54–60. doi: 10.1016/j.prosdent.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 105.Kim Y.K., Park J.Y., Kim S.G., Lee H.J. Prognosis of the implants replaced after removal of failed dental implants. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2010;110:281–286. doi: 10.1016/j.tripleo.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 106.Gomes G.H., Misawa M.Y.O., Fernandes C., Pannuti C.M., Saraiva L., Huynh-Ba G., et al. A systematic review and meta-analysis of the survival rate of implants placed in previously failed sites. Braz Oral Res. 2018;32 doi: 10.1590/1807-3107bor-2018.vol32.0027. [DOI] [PubMed] [Google Scholar]
- 107.Zhou W., Wang F., Monje A., Elnayef B., Huang W., Wu Y. Feasibility of dental implant replacement in failed sites: a systematic review. Int J Oral Maxillofac Implants. 2016;31:535–545. doi: 10.11607/jomi.4312. [DOI] [PubMed] [Google Scholar]
- 108.Jemt T., Häger P. Early complete failures of fixed implant-supported prostheses in the edentulous maxilla: a 3-year analysis of 17 consecutive cluster failure patients. Clin Implant Dent Relat Res. 2006;8:77–86. doi: 10.1111/j.1708-8208.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 109.Horwitz J., Zuabi O., Peled M., Machtei E.E. Immediate and delayed restoration of dental implants in periodontally susceptible patients: 1-year results. Int J Oral Maxillofac Implants. 2007;22:423–429. [PubMed] [Google Scholar]
- 110.Schwartz-Arad D., Laviv A., Levin L. Failure causes, timing, and cluster behavior: an 8-year study of dental implants. Implant Dent. 2008;17:200–207. doi: 10.1097/ID.0b013e3181777906. [DOI] [PubMed] [Google Scholar]
- 111.Park Y.S., Lee B.A., Choi S.H., Kim Y.T. Evaluation of failed implants and reimplantation at sites of previous dental implant failure: survival rates and risk factors. J Periodontal Implant Sci. 2022;52:230–241. doi: 10.5051/jpis.2105020251. [DOI] [PMC free article] [PubMed] [Google Scholar]