Abstract
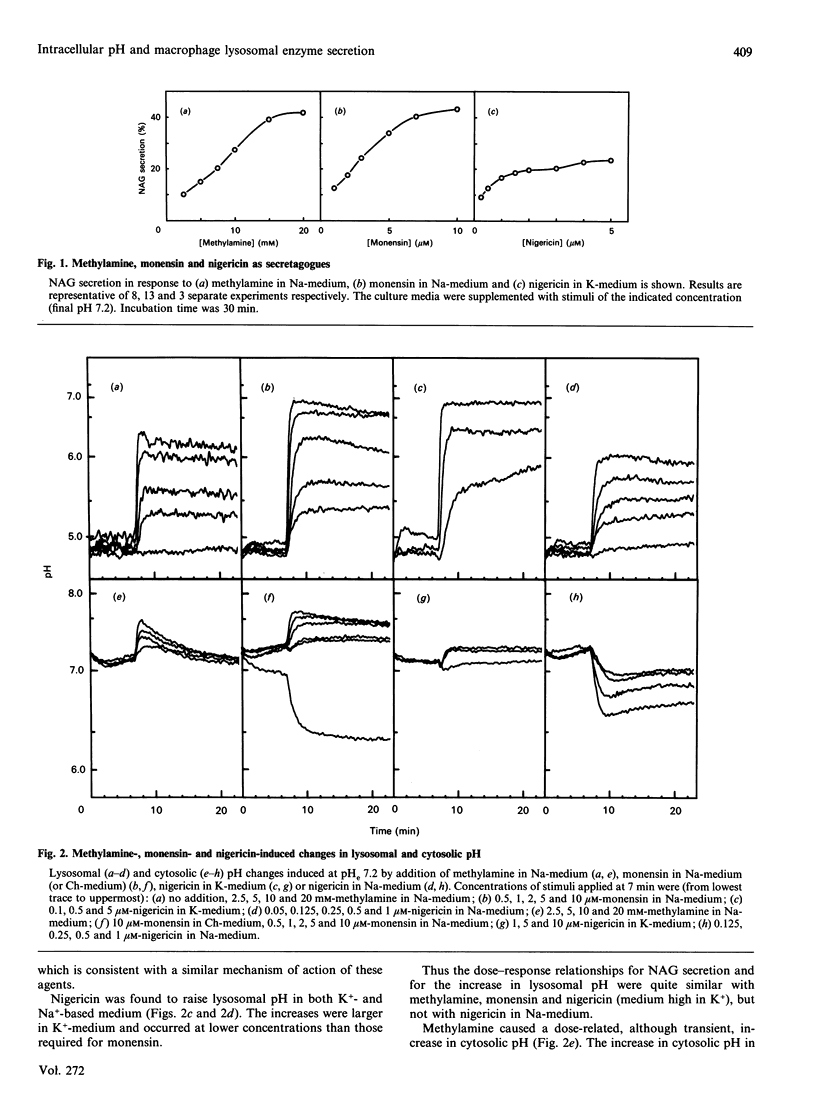
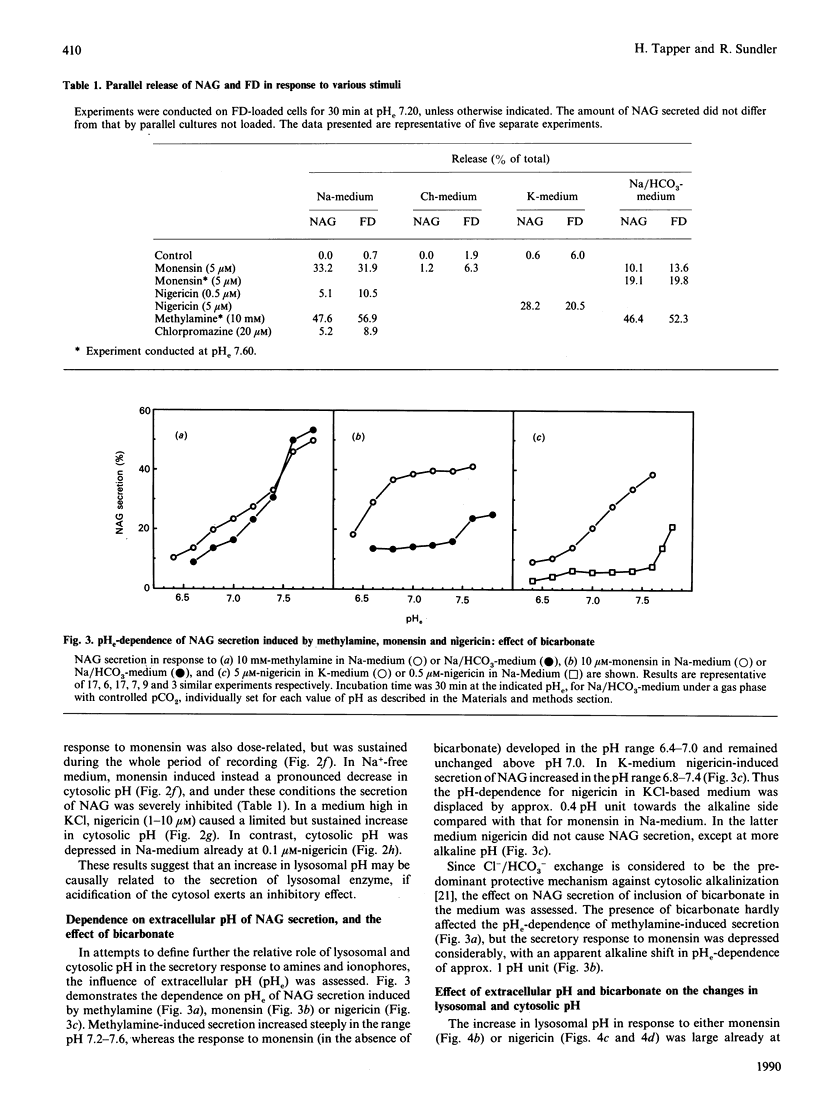
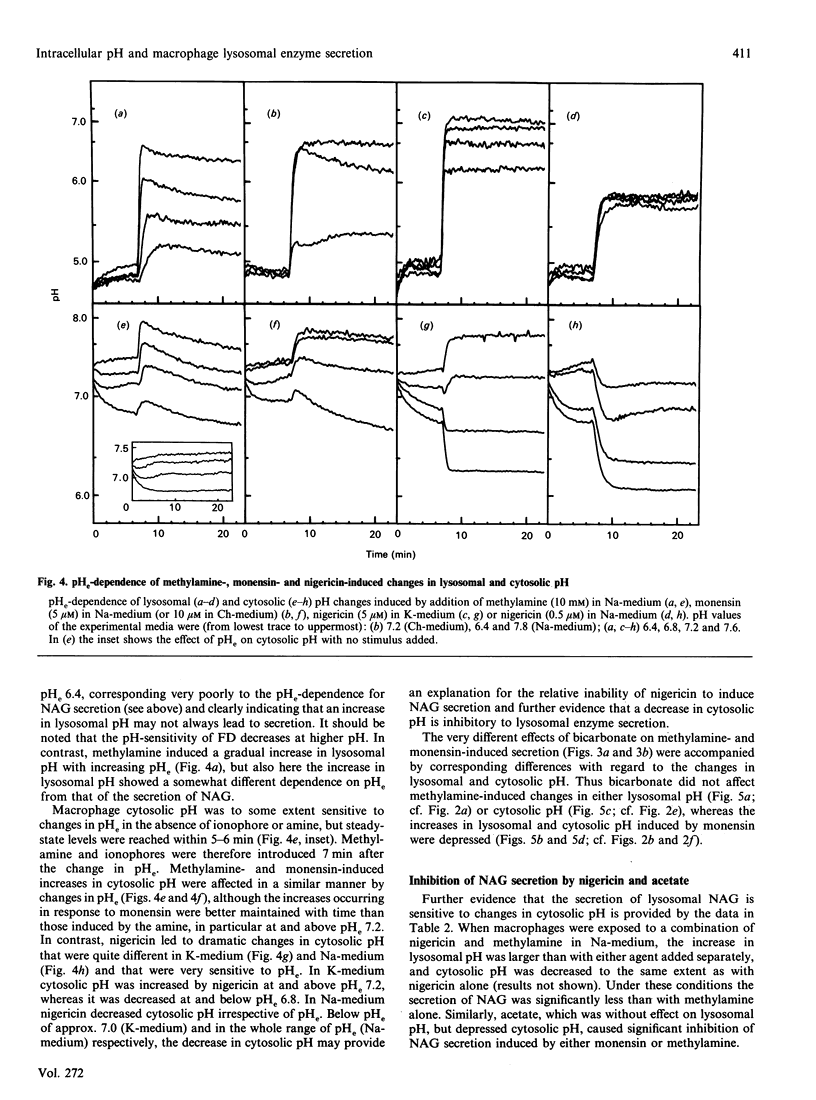
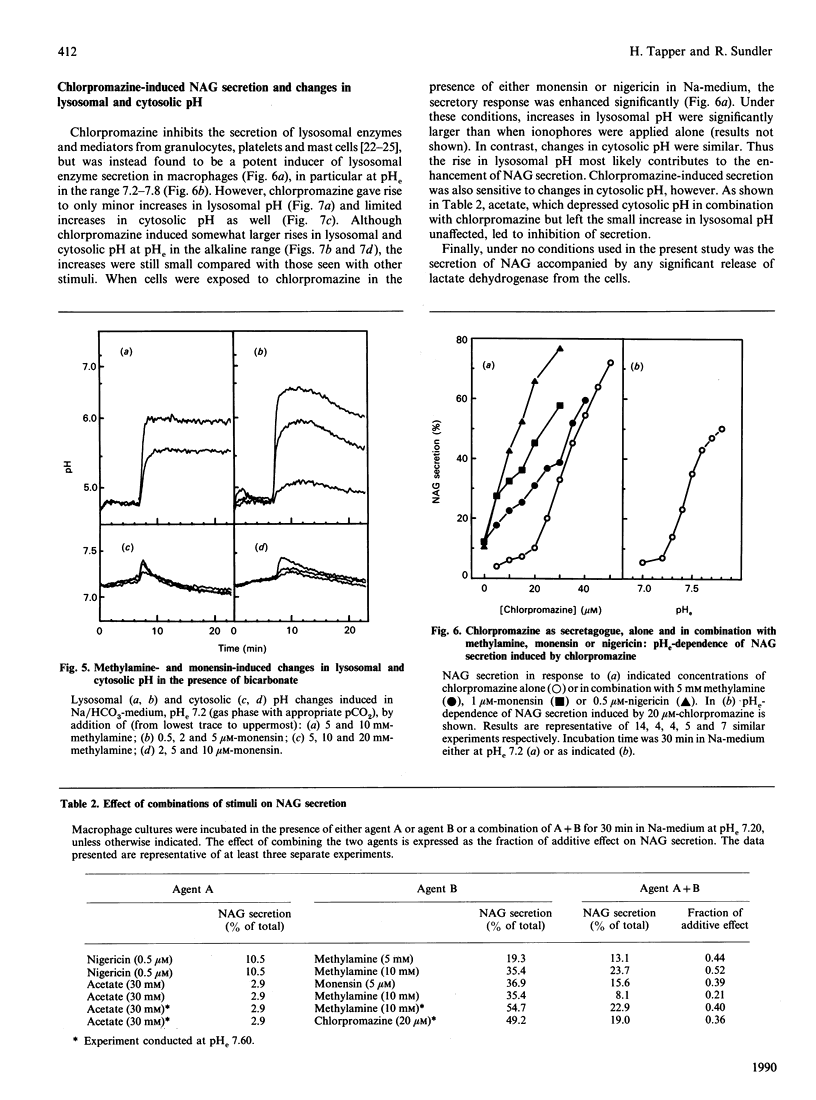
Rapid and parallel secretion of lysosomal beta-N-acetylglucosaminidase and preloaded fluorescein-labelled dextran was initiated in macrophages by agents affecting intracellular pH (methylamine, chlorpromazine, and the ionophores monensin and nigericin). In order to evaluate the relative role of changes in lysosomal and cytosolic pH, these parameters were monitored by using pH-sensitive fluorescent probes [fluorescein-labelled dextran or 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein]. All agents except chlorpromazine caused large increases in lysosomal pH under conditions where they induced secretion. By varying extracellular pH and ion composition, the changes in lysosomal and cytosolic pH could be dissociated. Secretion was then found to be significantly modulated by changes in cytosolic pH, being enhanced by alkalinization and severely inhibited by cytosolic acidification. However, changes in cytosolic pH in the absence of stimulus were unable to initiate secretion. Dissociation of the effects on lysosomal and cytosolic pH was also achieved by combining stimuli with either nigericin or acetate. Further support for a role of intracellular pH in the control of lysosomal enzyme secretion was provided by experiments where bicarbonate was included in the medium. The present study demonstrates that an increase in lysosomal pH is sufficient to initiate lysosomal enzyme secretion in macrophages and provides evidence for a significant regulatory role of cytosolic pH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Kahn A. J., Crouch E. C., Jeffrey J. J., Teitelbaum S. L. Isolated osteoclasts resorb the organic and inorganic components of bone. J Cell Biol. 1986 Apr;102(4):1164–1172. doi: 10.1083/jcb.102.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Novak E. K., Swank R. T. Effects of ammonia on processing and secretion of precursor and mature lysosomal enzyme from macrophages of normal and pale ear mice: evidence for two distinct pathways. J Cell Biol. 1985 Jun;100(6):1894–1904. doi: 10.1083/jcb.100.6.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. A., Mitchell J. B. Viability measurements in mammalian cell systems. Anal Biochem. 1989 May 15;179(1):1–7. doi: 10.1016/0003-2697(89)90191-7. [DOI] [PubMed] [Google Scholar]
- Dean R. T., Jessup W., Roberts C. R. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biochem J. 1984 Jan 1;217(1):27–40. doi: 10.1042/bj2170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Douglas W. W., Nemeth E. F. On the calcium receptor activating exocytosis: inhibitory effects of calmodulin-interacting drugs on rat mast cells. J Physiol. 1982 Feb;323:229–244. doi: 10.1113/jphysiol.1982.sp014070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink J. G. Chlorpromazine inhibits phagocytosis and exocytosis in rabbit polymorphonuclear leukocytes. Biochem Pharmacol. 1979 Apr 1;28(7):965–968. doi: 10.1016/0006-2952(79)90287-9. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Sundler R. Evidence for a catalytic role of phospholipase A in phorbol diester- and zymosan-induced mobilization of arachidonic acid in mouse peritoneal macrophages. Biochim Biophys Acta. 1986 May 21;876(3):533–542. doi: 10.1016/0005-2760(86)90041-x. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Henderson E. G., Sha'afi R. I. The effects of alterations of transmembrane Na+ and K+ gradients by ionophores (nigericin, monensin) on serotonin transport in human blood platelets. Biochim Biophys Acta. 1977 Jul 14;468(2):284–295. doi: 10.1016/0005-2736(77)90121-3. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., D'Arcy Hart P., Young M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J Cell Biol. 1981 Jun;89(3):645–652. doi: 10.1083/jcb.89.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol. 1989 Mar;108(3):855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup W., Shirazi M. F., Dean R. T. Inhibition of some spontaneous secretory processes in macrophages and fibroblasts by ammonium chloride. Biochem Pharmacol. 1983 Sep 15;32(18):2703–2710. doi: 10.1016/0006-2952(83)90079-5. [DOI] [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxnat M., Müller H. J., Galla H. J. Membrane solubility of chlorpromazine. Hygroscopic desorption and centrifugation methods yield comparable results. Biochem J. 1984 Dec 15;224(3):1023–1026. doi: 10.1042/bj2241023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H. Regulation of intracellular pH in eukaryotic cells. Biochem J. 1988 Feb 15;250(1):1–8. doi: 10.1042/bj2500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoni R., Kreis T. E. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987 Sep;105(3):1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Takai Y., Minakuchi R., Yu B., Nishizuka Y. Inhibitory action of chlorpromazine, dibucaine, and other phospholipid-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1980 Sep 25;255(18):8378–8380. [PubMed] [Google Scholar]
- Moriyama Y., Takano T., Ohkuma S. Similarity of lysosomal H+-ATPase to mitochondrial F0F1-ATPase in sensitivity to anions and drugs as revealed by solubilization and reconstitution. Biochim Biophys Acta. 1986 Jan 16;854(1):102–108. doi: 10.1016/0005-2736(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol. 1981 Sep;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opstvedt A., Rongved S., Aarsaether N., Lillehaug J. R., Holmsen H. Differential effects of chlorpromazine on secretion, protein phosphorylation and phosphoinositide metabolism in stimulated platelets. Biochem J. 1986 Aug 15;238(1):159–166. doi: 10.1042/bj2380159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachell P. T., Pearce F. L. Effect of calmodulin inhibitors on histamine secretion from mast cells. Agents Actions. 1985 Mar;16(1-2):43–44. doi: 10.1007/BF01999642. [DOI] [PubMed] [Google Scholar]
- Pohlmann R., Krüger S., Hasilik A., von Figura K. Effect of monensin on intracellular transport and receptor-mediated endocytosis of lysosomal enzymes. Biochem J. 1984 Feb 1;217(3):649–658. doi: 10.1042/bj2170649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B., Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981 Sep;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES W. J., Jr, FIMOGNARI G. M. AN IMPROVED PROCEDURE FOR THE PREPARATION OF CRYSTALLINE LACTIC DEHYDROGENASE FROM HOG HEART. J Biol Chem. 1963 Dec;238:3853–3858. [PubMed] [Google Scholar]
- Riches D. W., Channon J. Y., Leslie C. C., Henson P. M. Receptor-mediated signal transduction in mononuclear phagocytes. Prog Allergy. 1988;42:65–122. [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Evidence for a mechanism for the initiation of acid hydrolase secretion by macrophages that is functionally independent of alternative pathway complement activation. Biochem J. 1982 Mar 15;202(3):639–645. doi: 10.1042/bj2020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Primary amines induce selective release of lysosomal enzymes from mouse macrophages. Biochem J. 1980 Jun 15;188(3):933–936. doi: 10.1042/bj1880933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988 Oct;107(4):1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Takano Y., Imai K., Tanaka A., Fujimori K., Yamada M., Yamamoto K. Stimulation of the release of lysosomal and nonlysosomal granular enzymes from macrophages treated with monensin. Cell Struct Funct. 1984 Sep;9(3):265–277. doi: 10.1247/csf.9.265. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Van Oost B. A., Smith J. B., Holmsen H., Vladutiu G. D. Lysosomotropic agents selectively potentiate thrombin-induced acid hydrolase secretion from platelets. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2374–2378. doi: 10.1073/pnas.82.8.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Wileman T., Boshans R. L., Schlesinger P., Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984 Jun 15;220(3):665–675. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Duve C. Lysosomes revisited. Eur J Biochem. 1983 Dec 15;137(3):391–397. doi: 10.1111/j.1432-1033.1983.tb07841.x. [DOI] [PubMed] [Google Scholar]


