Abstract
Mitochondria play a key role in aging. Here, we measured integrated mitochondrial functions in experimentally evolved lines of the seed beetle Acanthoscelides obtectus that were selected for early (E) or late (L) reproduction for nearly 4 decades. The 2 lines have markedly different lifespans (8 days and 13 days in the E and L lines, respectively). The contribution of the NADH pathway to maximal flux was lower in the L compared to the E beetles at young stages, associated with increased control by complex I. In contrast, the contribution of the Succinate pathway was higher in the L than in the E line, whereas the Proline pathway showed no differences between the lines. Our data suggest that selection of age at reproduction leads to a modulation of complex I activity in mitochondria and that mitochondria are a functional link between evolutionary and mechanistic theories of aging.
Keywords: Complex I, Electron transport system pathways, Life expectancy, Oxidative phosphorylation
Graphical Abstract
Graphical Abstract.
Animal’s aging is characterized by the stochastic impairments of global and specific cellular functions, and an increased susceptibility to disease (1). Fourteen key biological pathways alterations have been identified as the hallmarks of aging (reviewed by (2)). Changes at the mitochondrial level, which is one of them, can be linked with all the other hallmarks. Mitochondria mediate multiple functions, such as oxidative phosphorylation (OXPHOS), production and management of reactive oxygen species (ROS), regulation of cellular metabolism, anabolism, biosynthesis of subcellular components, calcium and iron homeostasis, apoptosis, intracellular signaling, regulation of innate immunity, and modulation of stem cell activity (reviewed by (3,4)). Moreover, mitochondrial dysfunction can also be classified as an antagonistic hallmark of aging, that is, minor dysfunction can have beneficial effects, whereas major dysfunction can have deleterious effects (5). Thus, mitochondria are poised to act as a signaling hub in the intersecting mechanisms of aging and changes in mitochondrial function can lead to lifespan extension.
In multiple species, targeted inhibition of components of the mitochondrial complexes, is associated with lifespan extension (6–19), except for complex II (CII) (7,11,20). These lifespan extension effects of mitochondrial inhibition exhibit a threshold effect; for example, excess of complex I (CI) suppression decreased lifespan (14,16). The lifespan impact of impeding mitochondrial function may depend on the life stage at which alteration occurs. In Caenorhabditis elegans, reductive mitochondrial changes within larval stages extend lifespan (6,9,17), whereas inducing such changes in adulthood has no effect (6,9). In contrast, in killifish, long-term pharmacological inhibition of CI during the adult stage resulted in increased lifespan (16). The available literature shows that changes in mitochondrial function aimed at increasing lifespan need to be of relatively low magnitude, linked to specific mitochondrial components and pathways, and occur within a specific life phase or species. Despite the insights gleaned from these studies, the capacity of specific steps involved in OXPHOS was not measured in most. Still unknown are the amplitude of change in mitochondrial function required to elicit lifespan extension and the specific life-stage where mitochondrial alteration produces the largest effect. Most importantly, when a selection for life-history traits is performed, it is not known if specific changes in mitochondrial function are associated with the lifespan extension.
To evaluate if the microevolution of longevity is associated with modulation of mitochondrial function, we undertook an experimental evolutionary approach using seed beetles (Acanthoscelides obtectus). Selection studies that isolated early (E) and late (L) reproducing beetles for over 37 years gave rise to 2 distinct lines with markedly different lifespans (21,22). Studies show that beetles from the L line age slower and thus live longer (14.1 days in females, 13.1 days in males) (23) than those from the E line (8.4 days in females, 8.2 days in males) (23). These differences are observed regardless of sex and reproductive status (ie, virgin and mated; (22–25)). Developmental time from eggs to adult is also slightly longer in L beetles (32.2 days in females, 31.3 days in males) compared to E beetles (29.0 days in females, 28.2 days in males) (23). Although differences in mitochondrial electron transport system (ETS) enzyme activities between E and L lines have been reported (24), these outcomes have only been measured in early adult life (ie, day 1) and for enzyme activities rather than functional OXPHOS process. This invertebrate model offers multiple advantages. Long-term experimental evolution enables testing key mitochondrial characteristics associated with aging in selection lines with distinct life-history phenotypes, but of common ancestry. The short lifespan of the species allows cross-sectional and longitudinal comparisons to determine which parameters at which life-stage are associated with longevity. The role of sex as a determinant of longevity can furthermore be studied easily in contrast to other species such as C elegans (males and hermaphrodites). Finally, we can better control the rearing conditions compared to other models since the beetles develop within the bean seeds, adults are aphageous and laboratory conditions are close to natural conditions for the species.
The objective here is to determine if changes in life-history traits resulting from selection are associated with adjustments in mitochondrial function throughout adult life in E and L seed beetles. Moreover, because the effects of CI inhibition on lifespan differed between males and females in these beetles (14), we also investigated the role of sex on these outcomes. Several OXPHOS pathways and steps were included: the NADH pathway, the Succinate pathway, the Proline dehydrogenase (ProDH) pathway (Proline being an amino acid recognized as an energy source in insect flight muscle; (26)), and the cytochrome c oxidase (CIV) activity. We estimated the control of OXPHOS by various complexes with specific inhibitor titrations (27,28). This provides the first observation on how a long-term selection for late reproduction/long life and early reproduction/short life has shaped mitochondrial function.
Method
Experimental Populations
The lines were developed in Belgrade (Serbia) from an ancestral base population by allowing the seed beetles to reproduce either in the first 2 days (E lines) or at age of 10 days (L lines) for over 235 generations since 1986 ((21); Supplementary Figure 1). Еach selection regime (E and L) consists of 4 replicate populations. Acanthoscelides obtectus (Coleoptera: Bruchidae) were kept on organic, dry, common white beans (Phaseolus vulgaris) in dark incubators (Fisherbrand Isotemp, Fisher Scientific, Ottawa, ON, Canada) set at 30°C. Prior to use, beans were frozen and then brought to room temperature.
In our analysis, we used 1–5 individuals per sex per replicate population per line. Adult beetles aged 1, 5, and 8 days old were collected. These timepoints reflect the beginning (day 1), middle (day 5) and end (day 8) of the short-lived E line beetles’ lifespan. The sex of each beetle was determined by the pattern of dorsal side of their abdomen (white and brown checkerboard pattern in females, solid brown pattern in males). Each beetle was placed at 4°C for 5–10 minutes to facilitate their weight measurement using an analytical balance (Mettler Toledo XS205). Individual whole beetles were immediately put in 3 mL of ice-cold Mitochondrial Respiration Medium 05, (MiR05; 0.5 mM EGTA, 3 mM MgCl2-6H2O, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, and 1 g/L BSA essentially fatty-acid free, pH 7.1; (27)). Tissue homogenates were prepared on ice using a Potter-Elvehjem device connected to an overhead stirrer (Wheaton Instruments, Millville, NJ, USA) at a speed of 0.8 for 5 passes for days 1 and 5, and 5 to 7 passes for day 8, as needed. Preliminary assays determined the appropriate homogenization technique that displayed the optimal rate of respiration, coupling preservation, as well as mitochondrial outer membrane integrity.
Measurements of Specific OXPHOS Pathway
Immediately after homogenate preparation, mitochondrial respiration was measured at 30°C using an OROBOROS Oxygraph-2k (OROBOROS Instruments Inc., Innsbruck, Austria). Chambers were calibrated with 2 mL of MiR05 prior to experimentation. The protocol used included 2 respiratory states: LEAK (non-phosphorylation, no ADP) and OXPHOS (coupled OXPHOS, with saturating ADP). Tests confirmed no limitation of OXPHOS capacity by the phosphorylation system since the oxygen consumption following addition of dinitrophenol (uncoupler) did not increase. As a result, respiration in the OXPHOS state and electron transfer (ET) capacity were considered equivalent in these beetles.
For respiration measurements, pyruvate (5 mM), malate (5 mM), glutamate (10 mM) and 0.5 mL of each homogenate were added for a final chamber volume of 2 mL (LEAK state). Then, the following substrates and inhibitors were added: 2.5 mM ADP (OXPHOS state through the NADH pathway via CI), 10 μM cytochrome c (outer mitochondrial membrane integrity test), 10 mM Succinate (OXPHOS state for the combined NADH and Succinate pathways, the NS pathway), 10 mM l-Proline (OXPHOS state for the combined NS and ProDH pathways, the NSPro pathway), 1 μM rotenone (OXPHOS state through the Succinate pathway via CII and part of the ProDH pathway, SPro pathway), 5 μM antimycin A (non-mitochondrial residual oxygen consumption, ROX), 2 mM ascorbate and 0.5 mM N,N,Nʹ,Nʹ-tetramethyl-p-phenylenediamine (TMPD; CIV activity), and 100 mM sodium azide (chemical background). Mitochondrial respiration was measured in pmol O2 per second per mg of tissue and corrected for ROX, except for CIV, which was corrected for the chemical background. The cytochrome c control efficiency to indicate the integrity of the outer mitochondrial membrane was calculated using the following equation: . A significant increase in the cytochrome c control efficiency was observed with age in the E line (median of 0.15, 0.24, and 0.35 at days 1, 5, and 8, respectively) and in the L line (median of 0.19, 0.30, and 0.27 at days 1, 5, and 8, respectively), and in the L line compared to the E line at days 1 (p = .003) and 5 (p = .043). To avoid any bias induced by changes in outer membrane integrity, OXPHOS capacity values used for comparison between ages and lines were all taken after addition of exogenous cytochrome c.
A portion of each homogenate remaining in the chambers was stored at −80°C for citrate synthase (CS) activity assays performed at 30°C, in replicate, as previously described (27). Enzymatic activity was expressed in international units (IU) per milligram of sample, where IU is 1 μmol of substrate transformed per minute.
Control of Mitochondrial Respiratory Capacity
Additional sets of experiments were conducted to quantify the control exerted on mitochondrial respiration by specific complexes, that is, CI, CII, and CIV over maximal combined pathway flux, as previously described (27,28). For these experiments, both chambers of each oxygraph were loaded with the same homogenate. In one chamber, the maximal capacity was measured, with the NSPro pathway active. In the other chamber, the specific pathway or complex was measured: the specific NADH pathway through CI (with pyruvate, glutamate, malate, ADP, and cytochrome c), the Succinate pathway through CII (with Succinate, rotenone, ADP, and cytochrome c), or CIV (pyruvate, malate, glutamate, ADP, cytochrome c, Succinate, Proline, rotenone, antimycin A, ascorbate, and TMPD). Once the O2 flux stabilized, the specific inhibitor of CI (rotenone), CII (malonic acid), or CIV (sodium azide) was titrated in both chambers. The titration included 13–17 additions with rotenone (final concentrations between 0 and 3.00 µM), 12–21 additions with malonic acid (final concentrations between 0 and 25.5 mM) and 14 additions of sodium azide (final concentrations between 0 and 108 mM). Each protocol was repeated 5–10 times, on independent homogenate preparations, for each timepoint (1 and 5 days old) and random line selection of male beetles (2–3 L lines, 2 E lines). Titration protocols were tested in beetles beforehand to ensure that a proper titration curve was obtained.
Data Analysis
High-resolution respirometry data analysis was completed using Datlab 7.4 software (OROBOROS Instruments Inc.). Data were adjusted for the instrumental and chemical backgrounds. Mitochondrial respiration was expressed in Flux per mass (pmol O2 per second per milligram tissue mass, corrected for ROX), or as the flux control ratio (FCR; the pathway or complex in question normalized to maximal OXPHOS capacity, the NSPro pathway).
To determine pathway control, mitochondrial respiration was expressed in Flux per mass (pmol O2 per second per milligram tissue mass) corrected for ROX. Raw data points from each animal homogenate (ie, both chambers) were plotted as threshold plots with relative NSPro flux as a function of relative complex inhibition. The best-fitting polynomial trendline for each threshold plot was applied based on the accuracy of the curve to the data and the R2. The order of polynomials varied between 3 and 6. Afterwards, using the specific polynomial curve equation for each animal, the amount of inhibition of the overall flux after 25% and 50% inhibition of the specific complex was determined and then compared between the beetle ages and selection lines. We also determined the slope and y-intercept of each animal’s CIV threshold plot, using only the data points that indicated CIV inhibition (ie, after the initial plateau where relative NSPro flux remained unaffected by CIV inhibition).
Statistical Analysis
SigmaPlot 13 (Aspire Software International, Ashburn, VA, USA) was used to complete all statistical analyses. Specific details are in the Figure legends. In most cases, a 3-way ANOVA was used to determine the overall effects of beetle line, age, and sex. Then a 2-way ANOVA was used to determine the overall effects of line and age with sexes pooled (Figures 1 and 2), and within each sex (Supplementary Figures 2–4), followed by Tukey tests. If needed, data were transformed to meet the normality and equal variance requirements. The criteria for normality and homogeneity of variance were tested using Shapiro–Wilk and Brown–Forsythe tests, respectively. With the sex-pooled data (Figures 1 and 2), if the 2 requirements were not met, the effect of line and day were evaluated separately with a 1-way ANOVA or ANOVA on ranks. For the data with the sex separated (Supplementary Figures 2–4), if the 2 requirements were not met, the effect of line and sex were evaluated for each day with a 2-way ANOVA and then effects of age and line were considered separately with a 2-way ANOVA. For the effect of age, we used a 1-way ANOVA (followed by a Tukey test) or a Kruskal–Wallis ANOVA on ranks (followed by Dunn’s test). For the effect of the selection line (ie, longevity), t tests were completed if both tests passed, otherwise Mann–Whitney rank sum tests or Kruskal–Wallis ANOVA on ranks were used. For the inhibition curve data (CI, CII, CIV; Figures 3–5), the relative NSPro flux at 25% and 50% inhibition, at days 1 and 5, were compared using t test statistical analyses. For CIV inhibition, 2-way ANOVA was used to compare y-intercepts. Significance was considered at p ≤ .05. Figures were prepared using GraphPad Prism 9, Boston, MA, USA.
Figure 1.
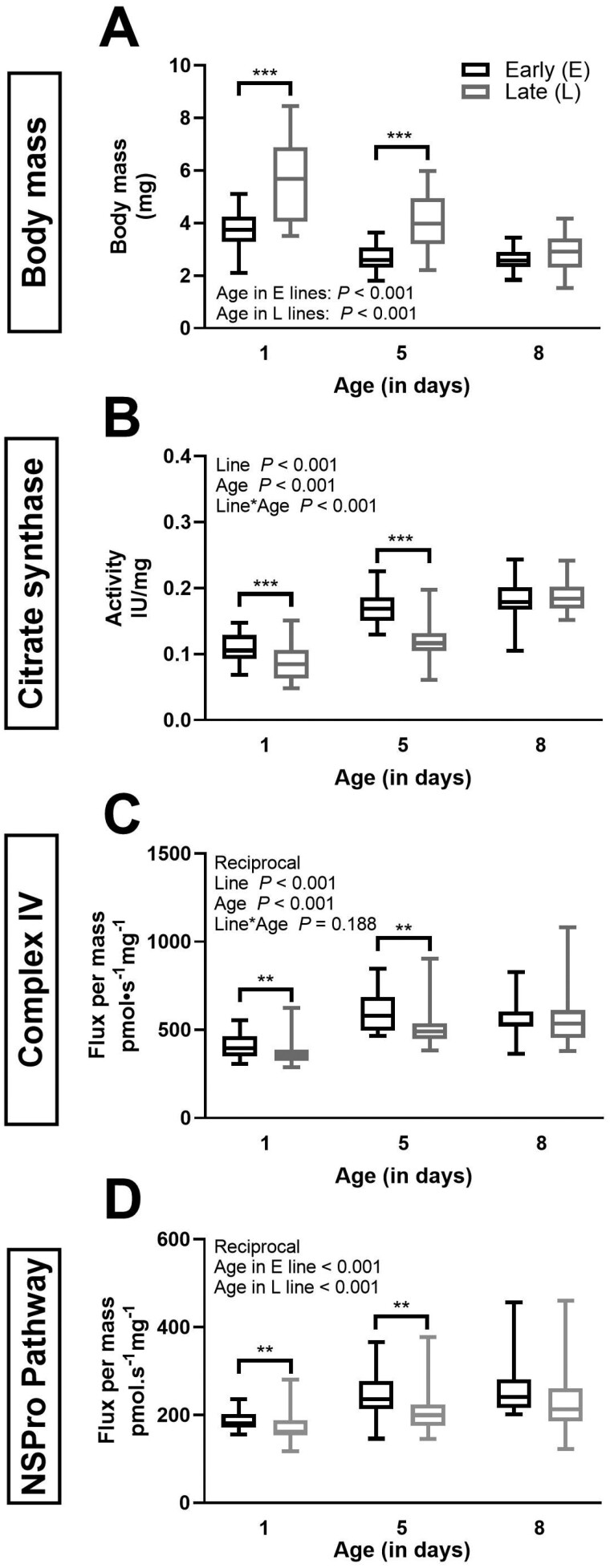
Body mass and biomarkers of mitochondrial content in E and L lines beetles of various ages. Body mass in mg (panel A), citrate synthase (CS) in specific activity per milligram of seed beetle tissue (panel B), as well as complex IV (CIV; panel C) and NADH + Succinate + Proline (NSPro) combined pathway flux expressed as flux per mass of seed beetle tissue (panel D). Box plots indicate the minimum, 25th percentile, median, 75th percentile, and maximum. The data are a pool of females and males (in the E line, n = 32 at day 1, n = 28 at day 5, and n = 22 at day 8, in the L line, n = 28 at days 1 and 5, and n = 30 at day 8). Two-way ANOVA p values for the effects of line and age as well as the interaction between line and age are indicated in each panel. Body mass data (panel A) did not meet the assumptions and the following tests were performed: 1-way ANOVA (line effect at day 5) and Kruskal–Wallis ANOVA on ranks (age effect within L and E lines, and effect of selection line at days 1 and 8). CS (panel B) data did not undergo any transformations. Data underwent reciprocal transformation to meet the assumptions of the 2-way ANOVA for CIV (panel C) and the 1-way ANOVA for NSPro (panel D). Significant differences between the beetle lines for specific ages are denoted *p ≤ .05), **p ≤ .01, and ***p ≤ .001.
Figure 2.
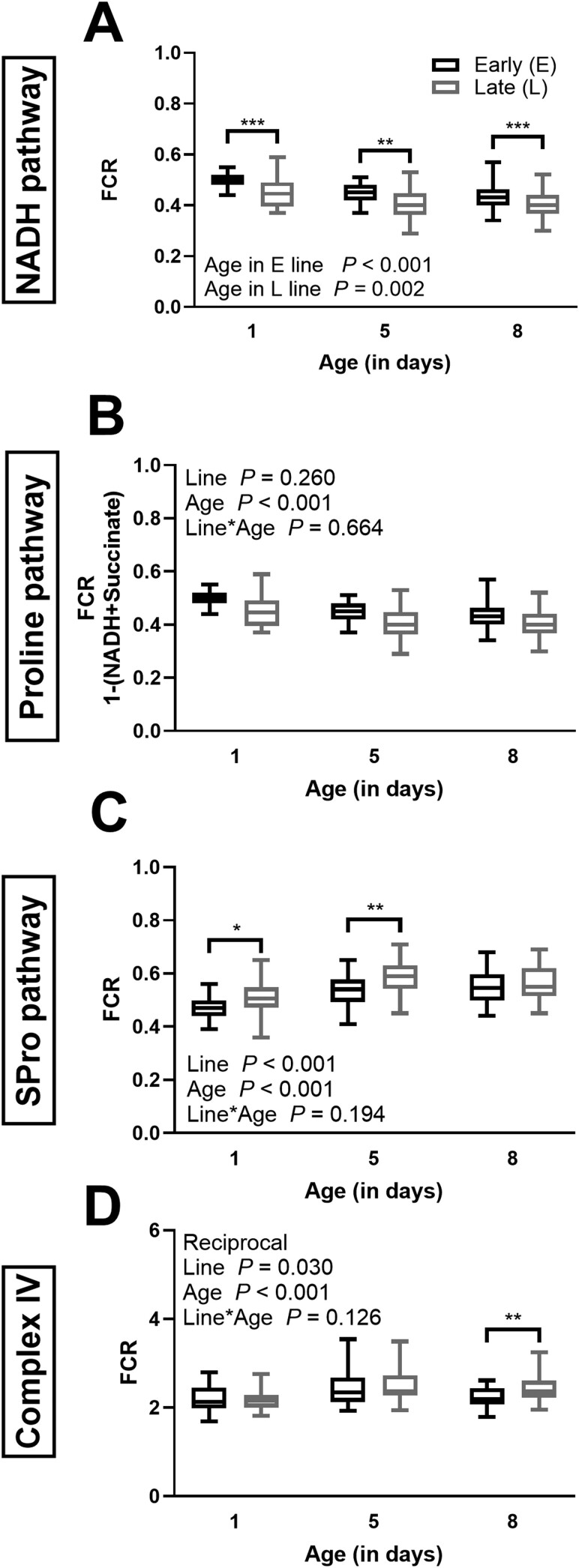
Mitochondrial respiratory capacity of specific respiratory pathways and steps in E and L lines of various ages. Respiration was measured for the NADH pathway through complex I (A), the Proline pathway through Proline dehydrogenase (b), the Succinate and Proline pathways (SPro pathway) through complex II and Proline dehydrogenase (C), and complex IV activity (D). The respiration is expressed as the flux control ratio (FCR; normalized to maximal OXPHOS capacity of the NADH, Succinate, and Proline pathways combined flux) in seed beetle tissue at days 1, 5, and 8 of their lifespan. Box plots indicate the minimum, 25th percentile, median, 75th percentile, and maximum. The data are a pool of females (in the E line, n = 32 at day 1, n = 28 at day 5, and n = 22 at day 8, in the L line, n = 28 at days 1 and 5, and n = 30 at day 8). Two-way ANOVA p values for the effects of line and age as well as the interaction between line and age are indicated in panels whose data met the assumptions of the ANOVA. Data in panel D underwent reciprocal transformations to meet the assumptions of the ANOVA. Data in panel A did not meet the assumptions and the following tests were performed: 1-way ANOVA (age effect within each line), Kruskal–Wallis (line effect at days 1 and 5), and 1-way ANOVA (day 1). Significant differences between the beetle lines are denoted *p ≤ .05, **p ≤ .01, and ***p ≤ .001.
Figure 3.
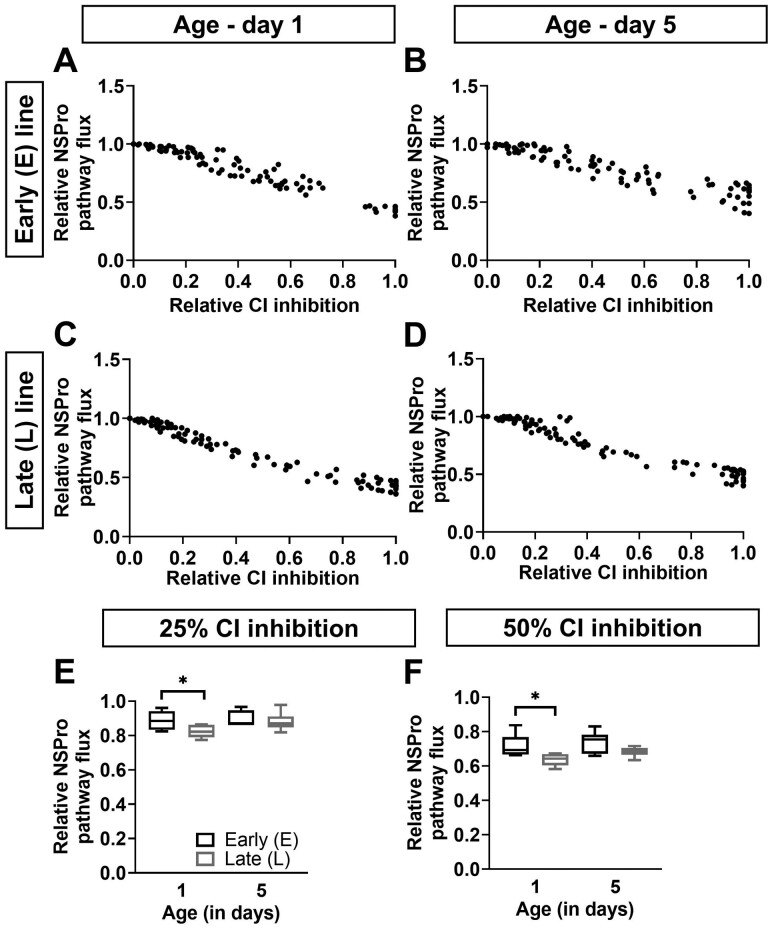
Effect of complex I inhibition on maximal pathway flux. (A–D) The relative maximal pathway flux in the presence of NADH, Succinate, and Proline (NSPro) is presented as a function of relative complex I (CI) inhibition using a rotenone titration at days 1 (panels A and C) and 5 (panels B and D) in males of the early (E; panels A and B) and late (L; panels C and D) seed beetle selection lines. Dots represent the raw data plots. (E and F) The relative NSPro flux at 25% (panel E) and 50% (panel F) CI inhibition, at days 1 and 5 in males of the E and L lines. n = 6 at both ages and for both lines. Significant differences between the lines based on t test statistical analyses are denoted *p ≤ .05.
Figure 4.
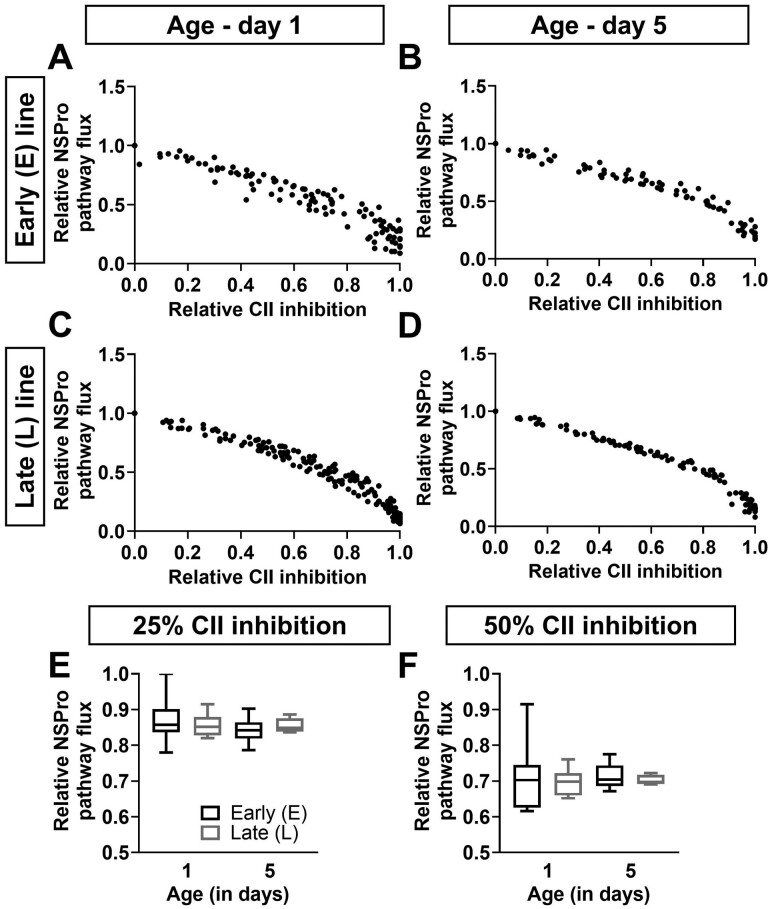
Effect of complex II inhibition on maximal pathway flux. (A–D) The relative maximal pathway flux in the presence of NADH, Succinate, and Proline (NSPro) is presented as a function of relative complex II (CII) inhibition using a malonate titration at days 1 (panels A and C) and 5 (panels B and D) in males of the early (E; panels A and B) and late (L; panels C and D) seed beetle selection lines. Dots represent the raw data plots. (E and F) The relative NSPro flux at 25% (panel E) and 50% (panel F) CII inhibition, at days 1 and 5 in males of the E and L lines. n = 6–7 for the E line and n = 7–10 for the L line at both ages. T tests showed no significant differences between the beetle lines.
Figure 5.
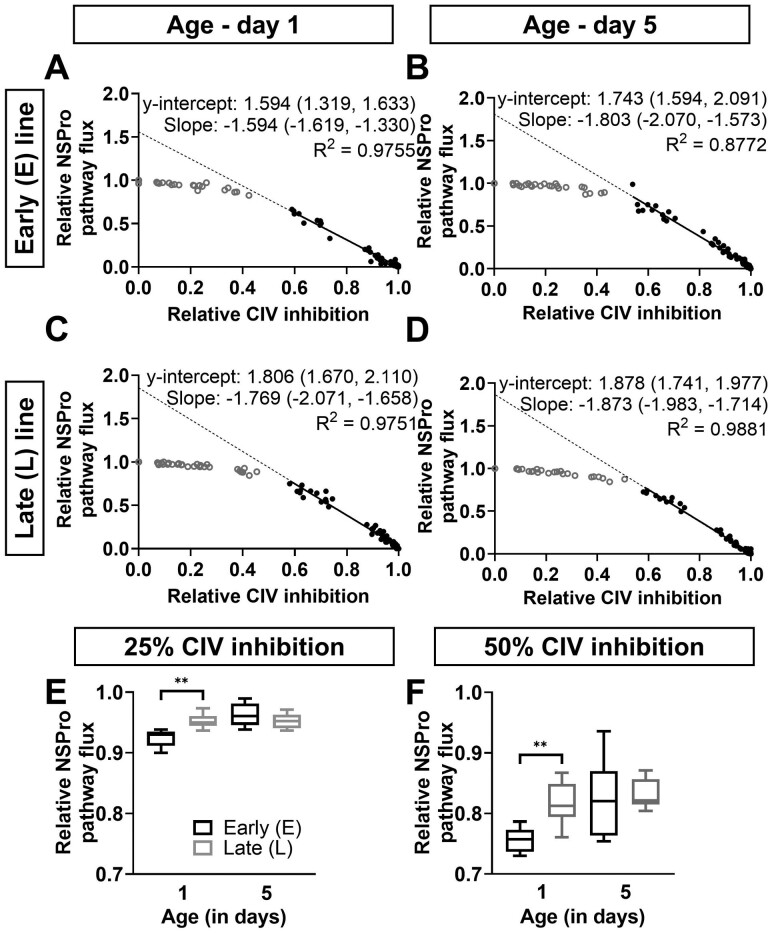
Effect of complex IV inhibition on maximal pathway flux. (A–D) The relative maximal pathway flux in the presence of NADH, Succinate, and Proline (NSPro) is presented as a function of relative complex IV (CIV) inhibition using a sodium azide titration at days 1 (panels A and C) and 5 (panels B and D) in males of the early (E; panels A and B) and late (L; panels C and D) seed beetle selection lines. Dots represent the raw data plots. Data up to the threshold of inhibition are represented by open, gray circles, whereas data after the inflection point are represented by closed, black circles. The y-intercept represents the intercept of the linear regression of the data after the inflection point with zero CIV inhibition. Y-intercepts are listed on the graphs as median (min, max) and represent the apparent excess CIV capacity. Graphs also display the slopes as median (min, max) as well as the goodness of fit (R2). Data were analyzed by 2-way ANOVA and met the assumptions without undergoing any transformations. Two-way ANOVA results comparing y-intercepts are: p = .007 (the effect of selection line), p = .066 (the effect of age), and p = .095 (interaction). Significant differences between the y-intercepts of selection lines are denoted * (p = .003). (E and F) The relative NSPro flux at 25% (panel E) and 50% (panel F) CIV inhibition, at days 1 and 5 in males of the E and L lines. n = 5–7 at both ages and in both lines. Significant differences between the lines as the result of t test statistical analyses are denoted **p ≤ .01.
Results
Body Mass and Mitochondrial Content
The effect of the selection line on whole-body weight of beetles was evident at days 1 and 5 in both sexes (Supplementary Figure 2) and with sexes pooled together, where L beetles were consistently heavier than E beetles (Figure 1A; p < .001 in each case). At day 8, the significance between the lines was lost in both females (Supplementary Figure 2A; p = .051) and males (Supplementary Figure 2B; p = .128), and with sexes pooled (Figure 1A; p = .087). Body weights decreased with age in both sexes and selection lines, especially between days 1 and 8 (Supplementary Figure 2 and Figure 1A; p < .001; see Supplementary Table 1 for details on the age effect).
Citrate synthase, CIV activities, and respiration rate with combined pathways, expressed per mg of tissue are correlated with mitochondrial fractional area or mitochondrial content (29–31). For these 3 biomarkers, the sexes were pooled because the effect of age and line showed similar trend in both sexes (Supplementary Figure 3), even if the 3-way ANOVA showed a significant effect of sex on mitochondrial content for CIV (p = .004) and for the combined NADH + Succinate + Proline (NSPro) pathways (p = .005), but not for CS activity (p = .834). Once the sexes pooled, the 3 markers of mitochondrial content were affected similarly by selection line. All the biomarkers of mitochondrial content were lower in the L line compared to the E line at days 1 and 5, that is, CS activity (Figure 1B; p < .001 both for days), CIV activity (Figure 1C; p = .005 and .004 for days 1 and 5, respectively) and NSPro flux (Figure 1D; p = .006 and .003 for days 1 and 5, respectively). The results for the 3 biomarkers also showed an increase with age (Figure 1B–D, Supplementary Table 1). The CS results showed an interaction between the effect of age and the effect of selection line (p < .001). Since the effect of Line here is significant at days 1 and 5, but not at day 8, the same as the differences in body mass, we wanted to verify if it could be explained by body mass. We effectively noticed that the 3 markers of mitochondrial content decreased as the body mass increased, both with the lines pooled (R2 = 0.496 for CS, R2 = 0.401 for CIV, R2 = 0.262 for NSPro Flux) or not pooled (for the CS: E line: y = −0.037x + 0.263, R² = 0.507; L line: y = −0.023x + 0.227, R² = 0.520). This relationship between the mitochondrial content and body mass, as previously shown (32), could then partly explain the line divergences here.
Effect of Age and Selection Line on the Qualitative Properties of Mitochondria
Flux control ratios reflect oxygen flux normalized to maximal respiratory capacity (NSPro pathway), and allow to study the contribution of specific pathways to respiration, independently of mitochondrial content. For the NADH pathway, because the data did not meet the conditions to perform a 3-way ANOVA, a 2-way ANOVA was used to evaluate the effect of sex for each stage separately, using line and sex as the factors. This analysis showed an effect of sex only at day 5 and in the L line (p = .012). The sexes were pooled on Figure 2A as the effect of age and line showed similar trend in both sexes (Supplementary Figure 4A and B). The FCR for the NADH pathway was lower in the L lines compared to the E lines at the 3 time points (p < .001 at days 1 and 8, p = .006 at day 5), and declined with age (Figure 2A; Supplementary Table 1). The differences in NADH pathway between the E and the L lines are hardly dictated by body mass since they were significant at days 1, 5, and 8, whereas no differences in mass were observed at day 8. Furthermore, when lines were pooled, no mass effect was detected for the FCR of the NADH pathway (R2 = 0.056). When lines were analyzed separately, FCR increased with mass (E line: y = 0.034x + 0.358, R² = 0.310; L line: y = 0.016x + 0.350, R² = 0.191). This is the opposite of what would be expected if the lower FCR for NADH in the L line was explained by mass divergences.
To calculate the contribution of the ProDH pathway flux to maximal respiration, the combined NS pathway flux was subtracted from the maximum OXPHOS capacity, and data were expressed as FCR. The 3-way ANOVA showed a significant effect of sex (p = .003), a decline with age, but no effect of selection line (Figure 2B, Supplementary Figure 4C and D, Supplementary Table 1).
The Succinate pathway through CII expressed in FCR (Figure 2C) generally showed a reverse relation with selection line or age when compared to the NADH pathway through CI. The 3-way ANOVA showed no effect of sex (p = .117; Supplementary Figure 4E and F). With the sexes pooled, the L line showed a stronger contribution of the SPro pathway to OXPHOS compared to the E line (Figure 2C; p < .001). In contrast to the NADH pathway, the SPro pathway FCR increased with age in both sexes (Figure 2C; p < .001; Supplementary Table 1). Here again, the differences in SPro pathway between the E and the L lines cannot be dictated by body mass differences. There is no correlation between body mass and FCR for SPro when lines are pooled (R2 = 0.074) and when lines are analyzed separately, we observe a decrease in FCR with mass (y = −0.037x + 0.629, R² = 0.218 in the E line and y = −0.019x + 0.632, R² = 0.193 in the L line). Again, it is at the opposite of what would be expected if higher FCR for SPro pathway in the L line was explained by weight divergences.
When CIV activity is expressed as a FCR, there is no sex effect detected by a 3-way ANOVA analysis (p = .828; Supplementary Figure 4G and H). When the sexes are pooled (Figure 2D), the FCR of CIV increased with age (p < .001), whereas the selection line diverged only at day 8 (p = .009), with the L line exhibiting a higher FCR compared to the E line.
LEAK state respiration expressed as FCR, known as the coupling-control ratio and used as a marker of the quality of the mitochondrial preparation, showed most values below 0.05 (median and range: 0.011 [0.000–0.087]), which is indicative of good coupling of mitochondrial OXPHOS. Furthermore, there was no effect of age (p = .465) or selection line (p = .894), indicating no change in quality of mitochondria.
Control of Mitochondrial Respiratory Capacity by CI, CII, and CIV
The inhibitor titration protocols were performed only in males since data were consistent between sexes. For each animal, the inhibitor titration was performed simultaneously on the maximal pathway flux with NSPro activated and on a specific complex. This approach allowed us to quantify excess (ie, unused) capacity of each complex as well as determine the control a given complex exerts over total mitochondrial flux.
Rotenone titration of the NADH pathway (x-axis) and of the maximal pathway flux (y-axis) is displayed as scatter plots (Figure 3A–D). At 25% and 50% CI inhibition, the curves showed a stronger inhibition of the NSPro pathway in the L line compared to the E line at day 1 only (Figure 3E and F; p = .038 and p = .028 at 25% and 50% inhibition, respectively). Furthermore, the control by CI tended to decrease (ie, the overall NSPro flux was higher, less affected) in the L line when aging (Figure 3E and F; p = .057 and p = .030 at 25% and 50% inhibition, respectively). At day 5, the impact of CI inhibition was equivalent in both lines, at both 25% (Figure 3E; p = .627, 87% and 87% for E and L lines, respectively) and 50% inhibition (Figure 4F; p = .073, 75% and 68% in E and L lines, respectively).
The malonate titration (Succinate pathway, Figure 4A–D) showed a similar control on the NSPro pathway in the L and E lines at days 1 and 5. When 25% or 50% of CII was inhibited (Figure 4E and F), the decrease in NSPro pathway flux was similar in both lines (at 25% inhibition, p = .494 at day 1 and p = .452 at day 5; at 50% inhibition, p = .597 at day 1 and p = .522 at day 5). Furthermore, the control by CII did not change with age in both lines (p = .362 and p = .953 at 25% and 50% inhibition, respectively, for E line; p = .878 and p = .676 at 25% and 50% inhibition, respectively, for L line). Even if the Succinate pathway compensated for the NADH pathway in the L line compared to the E line (Figure 4C and D), CII did not show any adjustment in control over the maximal pathway flux between the lines.
We examined the apparent excess capacity of CIV at maximal convergent pathway flux through the ETS (Figure 5A–D) and when complex III (CIII) and CIV exert maximal control (27). We observed 2 distinct phases: (1) the elimination of excess capacity above the threshold (initial slope; gray dots) and (2) the slope below the threshold (black dots and solid line), where further inhibition of CIV caused a linear inhibition of pathway flux. We extrapolated the solid line to give a visual indication of the y-intercept (dotted line). A higher y-intercept indicates a greater excess capacity of CIV, and the y-intercept minus 1 represents the level of inhibition required to begin affecting the overall combined pathways flux (ie, the excess capacity of the complex).
Here, the differences in y-intercepts show a higher excess capacity of CIV in the L line compared to the E line at day 1 (Figure 5A and C; median y-intercept of 1.594 and 1.806 for the E line and L line, respectively; p = .003), and no difference between the lines at day 5 (Figure 5B and D; 1.743 and 1.878 for the E line and the L line, respectively; p = .380). There was no effect of age on the y-intercept in either selection line (p = .066).
When 25% of CIV was inhibited (Figure 5E), the capacity of the combined pathways was more preserved in the L line compared to the E line at day 1 (95% vs 93% of overall NSPro flux in the L line vs E line; p = .005), but these differences faded at day 5 (96% vs 95%; p = .320). The same pattern was observed at 50% inhibition (Figure 5F; 81% vs 76% in the L vs E lines at day 1, p = .007; 82% vs 82% at day 5, p = .807). The excess capacity was lower at day 1 compared to day 5 in the E line only. At 25% CIV inhibition, the residual respiration was 96% at day 5 and 93% at day 1 (p = .007, Figure 5E), and a similar trend was seen at 50% CIV inhibition (Figure 5F; 82% vs 76%, p = .052). In the L line, there was no effect of age (95% vs 95% at days 5 vs 1, p = .979 at 25% CIV inhibition; and 82% vs 81%, p = .442 at 50% CIV inhibition).
Discussion
In this study, we provide evidence that selection for early or late reproduction led to modification of mitochondrial function in A obtectus. We observed that adult L beetles, had a lower flux and contribution of the NADH pathway through CI, compared to E, which was observed at each time point. Furthermore, at day 1, there was a higher control of overall flux by CI in the long live line. The decreased capacity of the NADH pathway through CI in L line coincides with expanded capacity of the Succinate pathway through CII. These differences were observed in both sexes. Additionally, the ProDH pathway did not vary between the selection lines, indicating that this pathway does not play any significant role in modulating the lifespan of A obtectus.
Our results show that a selection for late reproduction leads to reduced OXPHOS capacity through the NADH pathway (CI), which is associated with increased lifespan in the beetles. Our data align with studies done in various species showing an inverse relationship between longevity and CI activity (33,34), contents (35,36), or gene expression (6–11,13,15,17,37) as well as studies showing lifespan extension using pharmacological or molecular approaches to inhibit CI activity (16–18). But our study used an experimental evolution approach, by first using long-term selection for age at reproduction (and longevity) and then looking at association with mitochondrial functions. Previous results in seed beetles, using mitonuclear introgression lines, demonstrated that selection for and the evolution of a longer lifespan is associated with alterations in bioenergetic properties of mitochondria (24). Specifically, ETS activity was depressed when mitonuclear interactions were disrupted, and these epistatic effects were proportionally largest for CI. This supports the importance of CI in the evolution of aging in E and L lines, as well as the strong age-specific role of CI in the control OXPHOS. Additionally, genes involved in CI activity are differently expressed in E and L, especially in reproductive tissues (38). However, a study by Đorđević et al. (24) found no differences in CI activity between the E and L lines but reported an increase in CIII activity in the L compared to the E. The difference between their results and ours could be explained by differences in the methods of measurement. Their enzyme activities were expressed per mg of protein and their data were obtained for only day 1. Furthermore, because the assays were performed on frozen mitochondria, the functionality of the global OXPHOS process was not considered. In contrast, our FCR data pinpointed an alteration at the NADH pathway through CI relative to overall flux in the L compared to the E line. This expresses properties of the entire OXPHOS process on intact mitochondria, which is closer to the conditions of intact cells. Furthermore, using the titration curves of the CI inhibitor, our data support that the alteration noticed in the NADH pathway was, at least in part, specifically due to a decrease in CI, and not in other steps involved in the NADH pathway (such as pyruvate dehydrogenase, transaminase, and substrate transporters). Our titration curves showed that L, compared to E beetles, display lower excess of CI since inhibition by rotenone exerted a significant effect on the global pathway flux with NSPro pathway active at a low concentration of rotenone. At 50% inhibition of CI, E beetles maintained 69% of maximal NSPro flux, whereas flux in L beetles was reduced to 64% of maximal NSPro flux. That means there is little to no excess of CI capacity in L beetles, so CI has a stronger control on overall pathway flux. This decrease in CI capacity in the L compared to the E line is consistent with a previous study showing that exposure to low doses of the CI inhibitor, tebufenpyrad, in 1-day-old short-lived beetles extended lifespan by nearly 15% (14). The results presented herein and by Šešlija Jovanović et al. (14) together advocate for an adaptative role of CI activity and mitochondrial function in modulation of age at reproduction and consequently adult lifespan extension. Our study also addresses the change in the ProDH pathway associated with longevity. Whereas an increase in Proline metabolism is suspected to be involved in controlling adult lifespan in Saccharomyces cerevisiae (39,40) and C elegans (41,42), our results did not show any change in oxidative capacity of Proline pathway associated with longevity in the lines of A obtectus. Nevertheless, it is possible that manipulation of Proline metabolism in this species could still affect lifespan as the 2 selection regimes maintain an important capacity to carry out the OXPHOS process using Proline as a substrate.
Our results in A obtectus showed a higher capacity of the Succinate pathway in long-lived beetles. It must be noted that the use of rotenone in the presence of Proline inhibits CI but only partially inhibits the ProDH pathway (26), and therefore these measurements cannot be attributed to the Succinate pathway alone. Since the SPro pathway’s FCR increased with time despite the reduction in ProDH pathway FCR, it can be inferred that the observed age-related increase in the SPro pathway FCR could be even greater than that observed here. Furthermore, the difference due to line selection can be attributed to changes in Succinate pathway flux, as there was no difference in the Proline pathway between the lines. These conclusions are congruent with previous experiments using E and L mitonuclear lines that also demonstrated increased CII activity (24) in the L line. Other animal models with a defect in CI have also shown an adjustment favoring higher capacity of CII (43,44). In humans, isolated CI disorders represent a high proportion of respiratory chain diseases, whereas CII deficiencies are very rare and account for only about 1% (45), suggesting that any dysfunction of CII is selected against considering its function in the citric acid cycle. Another modification associated with lifespan is an increase in CIV capacity compared to the overall pathway flux (FCR) in the L compared to the E line. The CIV inhibitor titrations in males also showed that excess CIV capacity in the L line is higher, compared to the E line at day 1, and both lines are equivalent at day 5. These results, in conjunction with the lower CI capacity in L beetles early in life, might suggest that an excess in CIV capacity in the L line at day 1 could be secondary to the adjustment in CI capacity. A potential impact of the larger excess of CIV in the L line and lower CI activity would result in less reductive and more oxidative mitochondria, leading to diminished reduction state and lower risks to produce ROS.
In our data, the long-lived line when compared to the short-lived line shows (1) a lower contribution of the NADH pathway through CI (2), a higher capacity of the Succinate pathway through CII (3), a lower mitochondrial content, and (4) a higher CIV excess capacity. Such changes have been previously linked to reductions in ROS production which can be associated with increase in lifespan (46). CI is considered the main site of ROS production in mitochondria (reviewed by (47)). In our experimental model, it has been shown that L beetles are more resistant to oxidative stress, but that the low inhibition of CI by tebufenpyrad has mitohormetic effects only in E beetles (14). These results urge studies of direct mitochondrial ROS production and management in the 2 lines.
Our study emphasizes the importance of understanding the specific changes in integrated mitochondrial function that are connected to evolution of life-history traits and lifespan. Through our use of E and L lines of A obtectus, which have evolved different ages of reproduction since 1986, we pinpointed mitochondrial divergences between subsets of the same animal species originating from the same ancestral line but with disparate lifespans. In the long-lived beetles, a lower contribution of the NADH pathway and a higher control on overall flux by CI, commend CI as an important regulator of lifespan in these beetles. Previous studies manipulating specifically CI activity, either through biochemical or molecular interventions, have shown a direct link between CI relative activity or capacity and lifespan. In our model system, mitochondrial CI capacity modulation is a consequence of selection for early or late reproduction and is associated with lifespan differences. Evolutionary theories of aging postulate that evolution of lifespan is a consequence of selection for life-history strategies, particularly age of reproduction (48). Our results suggest that modulation of CI could be the link between selection for age at reproduction and lifespan and could reconcile evolutionary theories with mechanistic theories of aging.
Supplementary Material
Acknowledgments
Thank you to Martin Lessard for help in some of the experiments.
Contributor Information
Heather E Mast, Faculty Saint-Jean, University of Alberta, Edmonton, Alberta, Canada; Department of Medicine, University of Alberta, Edmonton, Alberta, Canada.
Pierre U Blier, Département de Biologie, Université du Québec à Rimouski, Rimouski, Quebec, Canada.
Mirko Ɖorđević, Department of Evolutionary Biology, Institute for Biological Research “Siniša Stanković” – National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia.
Uroš Savković, Department of Evolutionary Biology, Institute for Biological Research “Siniša Stanković” – National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia.
Claudia D Holody, Faculty Saint-Jean, University of Alberta, Edmonton, Alberta, Canada; Department of Anesthesiology and Pain Medicine, University of Alberta, Edmonton, Alberta, Canada.
Stephane L Bourque, Department of Anesthesiology and Pain Medicine, University of Alberta, Edmonton, Alberta, Canada; Women and Children’s Health Research Institute, University of Alberta, Edmonton, Alberta, Canada.
Hélène Lemieux, Faculty Saint-Jean, University of Alberta, Edmonton, Alberta, Canada; Women and Children’s Health Research Institute, University of Alberta, Edmonton, Alberta, Canada.
Funding
This study was supported by the following grants to H.L.: a discovery grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN 2021-02924), a research grant and a startup grant from Faculty Saint-Jean, and an equipment grant from the Canadian Foundation for Innovation. For M.Đ. and U.S. work was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (grant number: 451-03-66/2024-03/200007).
Conflict of Interest
None.
Data Availability
Data are available on Dryad: https://doi.org/doi:10.5061/dryad.vdncjsz47
Author Contributions
H.E.M., H.L., P.U.B., M.Ɖ., U.S., and C.D.H. conceived the study; H.E.M., H.L., P.U.B., M.Ɖ., and U.S. designed experiments; H.E.M. and C.D.H. performed the experiments; H.E.M. and H.L. analyzed data; H.E.M., H.L., P.U.B., M.Ɖ., U.S., C.D.H., and S.L.B. contributed intellectually; H.E.M. and H.L. wrote the manuscript; P.U.B., M.Ɖ., U.S., C.D.H., and S.L.B. reviewed and edited the manuscript.
References
- 1. Akbari M, Kirkwood TBL, Bohr VA.. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res Rev. 2019;54:100940. 10.1016/j.arr.2019.100940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmauck-Medina T, Molière A, Lautrup S, et al. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany NY). 2022;14:6829–6839. 10.18632/aging.204248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava S. The mitochondrial basis of aging and age-related disorders. Genes. 2017;8:398. 10.3390/genes8120398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun N, Youle RJ, Finkel T.. The mitochondrial basis of aging. Mol Cell. 2016;61:654–666. 10.1016/j.molcel.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G.. The hallmarks of aging. Cell. 2013;153:1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dillin A, Hsu AL, Arantes-Oliveira N, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. 10.1126/science.1077780 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton B, Dong Y, Shindo M, et al. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. 10.1101/gad.1308205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen M, Hsu AL, Dillin A, Kenyon C.. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005;1:119–128. 10.1371/journal.pgen.0010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rea SL, Ventura N, Johnson TE.. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. 10.1371/journal.pbio.0050259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Copeland JM, Cho J, LoT, Jr, et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. 10.1016/j.cub.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 11. Yang W, Hekimi S.. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9:433–447. 10.1111/j.1474-9726.2010.00571.x [DOI] [PubMed] [Google Scholar]
- 12. Durieux J, Wolff S, Dillin A.. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owusu-Ansah E, Song W, Perrimon N.. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell. 2013;155:699–712. 10.1016/j.cell.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Šešlija Jovanović D, Dorđević M, Savković U, Lazarević J.. The effect of mitochondrial complex I inhibitor on longevity of short-lived and long-lived seed beetles and its mitonuclear hybrids. Biogerontology. 2014;15:487–501. 10.1007/s10522-014-9520-5 [DOI] [PubMed] [Google Scholar]
- 15. Rauthan M, Ranji P, Abukar R, Pilon M.. A mutation in Caenorhabditis elegans NDUF-7 activates the mitochondrial stress response and prolongs lifespan via ROS and CED-4. G3. 2015;5:1639–1648. 10.1534/g3.115.018598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baumgart M, Priebe S, Groth M, et al. Longitudinal RNA-seq analysis of vertebrate aging identifies mitochondrial complex I as a small-molecule-sensitive modifier of lifespan. Cell Systems. 2016;2:122–132. 10.1016/j.cels.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 17. Labbadia J, Brielmann RM, Neto MF, Lin YF, Haynes CM, Morimoto RI.. Mitochondrial stress restores the heat shock response and prevents proteostasis collapse during aging. Cell Rep. 2017;21:1481–1494. 10.1016/j.celrep.2017.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu C, Hwang W, Jeong DE, et al. Genetic inhibition of an ATP synthase subunit extends lifespan in C. elegans. Sci Rep. 2018;8:14836. 10.1038/s41598-018-32025-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feng J, Bussière F, Hekimi S.. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. 10.1016/s1534-5807(01)00071-5 [DOI] [PubMed] [Google Scholar]
- 20. Kuang J, Ebert PR.. The failure to extend lifespan via disruption of complex II is linked to preservation of dynamic control of energy metabolism. Mitochondrion. 2012;12:280–287. 10.1016/j.mito.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 21. Tucić N, Gliksman I, Šešlija D, Milanović D, Mikuljanac S, Stojković O.. Laboratory evolution of longevity in the bean weevil (Acanthoscelides obtectus). J Evol Biol. 1996;9:485–503. 10.1046/j.1420-9101.1996.9040485.x [DOI] [Google Scholar]
- 22. Stojković B, Savković U.. Gender differences in longevity in early and late reproduced lines of the seed beetle. Arch Biol Sci. 2011;63:129–136. 10.2298/abs1101129s [DOI] [Google Scholar]
- 23. Đorđević M, Savković U, Lazarević J, Tucić N, Stojković B.. Intergenomic interactions in hybrids between short-lived and long-lived lines of a seed beetle: analyses of life history traits. Evol Biol. 2015;42:461–472. 10.1007/s11692-015-9340-9 [DOI] [Google Scholar]
- 24. Đorđević M, Stojković B, Savković U, et al. Sex-specific mitonuclear epistasis and the evolution of mitochondrial bioenergetics, ageing, and life history in seed beetles. Evolution. 2017;71:274–288. 10.1111/evo.13109 [DOI] [PubMed] [Google Scholar]
- 25. Stojković B, Šešlija Jovanović D, Tucić B, Tucić N.. Homosexual behaviour and its longevity cost in females and males of the seed beetle Acanthoscelides obtectus. Physiol Entomol. 2010;35:308–316. 10.1111/j.1365-3032.2010.00742.x [DOI] [Google Scholar]
- 26. Teulier L, Weber JM, Crevier J, Darveau CA.. Proline as a fuel for insect flight: enhancing carbohydrate oxidation in hymenopterans. Proc Biol Sci. 2016;283:20160333–20160338. 10.1098/rspb.2016.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lemieux H, Blier PU, Gnaiger E.. Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep. 2017;7:2840. 10.1038/s41598-017-02789-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodríguez E, Hakkou M, Hagen TM, Lemieux H, Blier PU.. Divergences in the control of mitochondrial respiration are associated with life-span variation in marine bivalves. J Gerontol A Biol Sci Med Sci. 2021;76:796–804. 10.1093/gerona/glaa301 [DOI] [PubMed] [Google Scholar]
- 29. Larsen S, Nielsen J, Hansen CN, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–3360. 10.1113/jphysiol.2012.230185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Flemming D.. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. 10.1007/s00125-007-0594-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Picard M, Taivassalo T, Ritchie D, et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011;6:e18317. 10.1371/journal.pone.0018317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darveau CA, Hochachka PW, Roubik DW, Suarez RK.. Allometric scaling of flight energetics in orchid bees: evolution of flux capacities and flux rates. J Exp Biol. 2005;208:3593–3602. 10.1242/jeb.01777 [DOI] [PubMed] [Google Scholar]
- 33. Miwa S, Jow H, Baty K, et al. Low abundance of the matrix arm of complex I in mitochondria predicts longevity in mice. Nat Commun. 2014;5:3837. 10.1038/ncomms4837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munkácsy E, Rea SL.. The paradox of mitochondrial dysfunction and extended longevity. Exp Gerontol. 2014;56:221–233. 10.1016/j.exger.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gómez J, Mota-Martorell N, Jové M, Pamplona R, Barja G.. Mitochondrial ROS production, oxidative stress and aging within and between species: evidences and recent advances on this aging effector. Exp Gerontol. 2023;174:112134. 10.1016/j.exger.2023.112134 [DOI] [PubMed] [Google Scholar]
- 36. Rodríguez-Nuevo A, Torres-Sanchez A, Duran JM, De Guirior C, Martínez-Zamora MA, Böke E.. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature. 2022;607:756–761. 10.1038/s41586-022-04979-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Curran SP, Ruvkun G.. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. 10.1371/journal.pgen.0030056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Immonen E, Sayadi A, Stojković B, et al. Experimental life history evolution results in sex-specific evolution of gene expression in seed beetles. Genome Biol Evol. 2023;15:evac177. 10.1093/gbe/evac177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mukai Y, Kamei Y, Liu X, et al. Proline metabolism regulates replicative lifespan in the yeast Saccharomyces cerevisiae. Microbial Cell (Graz, Austria). 2019;6:482–490. 10.15698/mic2019.10.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishimura A, Yoshikawa Y, Ichikawa K, Takemoto T, Tanahashi R, Takagi H.. Longevity regulation by proline oxidation in yeast. Microorganisms. 2021;9:1650–1611. 10.3390/microorganisms9081650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pang S, Curran SP.. Adaptive capacity to bacterial diet modulates aging in C. elegans. Cell Metab. 2014;19:221–231. 10.1016/j.cmet.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edwards C, Canfield J, Copes N, et al. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015;16:8. 10.1186/s12863-015-0167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kayser EB, Sedensky MM, Morgan PG.. The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mech Ageing Dev. 2004;125:455–464. 10.1016/j.mad.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 44. Cormier RPJ, Champigny CM, Simard CJ, St-Coeur PD, Pichaud N.. Dynamic mitochondrial responses to a high-fat diet in Drosophila melanogaster. Sci Rep. 2019;9:4531. 10.1038/s41598-018-36060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loeffen JL, Smeitink JA, Trijbels JM, et al. Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum Mutat. 2000;15:123–134. [DOI] [PubMed] [Google Scholar]
- 46. Barja G. Towards a unified mechanistic theory of aging. Exp Gerontol. 2019;124:110627. 10.1016/j.exger.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 47. Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. 10.1016/j.exger.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lemaître JF, Moorad J, Gaillard JM, Maklakov AA, Nussey DH.. A unified framework for evolutionary genetic and physiological theories of aging. PLoS Biol. 2024;22:e3002513. 10.1371/journal.pbio.3002513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Dryad: https://doi.org/doi:10.5061/dryad.vdncjsz47