Abstract
Objective
Surgical resection is associated with higher overall survival (OS) than definitive radiotherapy (RT) or chemoradiotherapy (CRT) in cT4b sinonasal squamous cell carcinoma (SCC). Our study investigates the survival benefit of surgical resection in cT4b sinonasal non‐SCC.
Methods
The 2004 to 2019 National Cancer Database was queried for patients with cT4b sinonasal non‐SCC undergoing definitive treatment with (1) surgical resection + additional therapy (RT, chemotherapy, or both), (2) RT alone, or (3) CRT. Surgical resection + additional therapy and definitive RT/CRT were compared with Kaplan–Meier and multivariable Cox regression models.
Results
Of 629 patients satisfying inclusion criteria, 513 (81.6%) underwent surgical resection + additional therapy and 116 (18.4%) underwent definitive RT/CRT. The most frequent histologic types were undifferentiated carcinoma (23.7%), adenoid cystic carcinoma (22.6%), and adenocarcinoma (20.7%). Few patients presented with clinical nodal metastasis (15.7%). There were 4 (0.8%) mortalities within 90 days of surgical resection. Patients undergoing surgical resection with positive surgical margins had higher 5‐year OS than those undergoing definitive RT/CRT (56.3% vs. 39.4%, p = .039) and similar 5‐year OS as those with negative margins (56.3% vs. 63.9%, p = .059). Patients undergoing neoadjuvant chemotherapy had similar 5‐year OS as those undergoing definitive RT/CRT (60.9% vs. 39.5%, p = .053). Age at diagnosis, tumor diameter, and surgical resection + additional therapy (aHR 0.64, 95% CI 0.45–0.91) were associated with OS (p < .05).
Conclusion
Surgical resection + additional therapy was associated with higher OS than definitive RT/CRT in cT4b sinonasal non‐SCC. Surgical resection may benefit select patient with cT4b sinonasal non‐SCC.
Level of Evidence
4.
Keywords: cT4b, National Cancer Database, sinonasal, surgical resection, survival
Our study investigates the survival benefit of surgical resection in cT4b sinonasal non‐squamous cell carcinoma (SCC). Surgical resection + additional therapy was associated with higher overall survival than definitive radiotherapy/chemoradiotherapy in cT4b sinonasal non‐SCC. Surgical resection may benefit select patients with cT4b sinonasal non‐SCC.
1. INTRODUCTION
Approximately 50% of sinonasal cancers are squamous cell carcinoma (SCC); sinonasal non‐SCC includes heterogenous histologic types such as adenocarcinoma, adenoid cystic carcinoma (AdCC), neuroendocrine carcinoma (NEC), mucoepidermoid carcinoma (MEC), melanoma, esthesioneuroblastoma, and sinonasal undifferentiated carcinoma (SNUC), and each has distinct treatment and survival. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
Surgical resection achieving negative margins with adjuvant radiotherapy (RT) or chemoradiotherapy (CRT) is the preferred definitive treatment of sinonasal non‐SCC presenting without evidence of distant metastasis. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 Invasion of the orbital apex, dura, brain, middle cranial fossa, cranial nerves, or nasopharynx, a defining feature of very locally advanced (cT4b) disease, can be considered a relative contraindication to surgical resection because of the difficulty in safely achieving negative margins, exposure to unjustifiable morbidity, and higher likelihood of poor prognosis. 26 , 27 Although the rarity and histologic heterogeneity of sinonasal non‐SCC complicate treatment choice and outcomes, the National Comprehensive Cancer Network (NCCN) recommends definitive RT/CRT, experimental therapies, and palliative care for cT4b sinonasal non‐SCC. 26 , 28
New medical therapies and advances in surgical technologies and techniques have challenged previously described contraindications in cT4b sinonasal cancer. Recent studies suggest that surgical resection improves locoregional control and survival in cT4b sinonasal SCC, even when achieving negative margins is not feasible. 17 , 26 , 27 , 29 , 30 , 31 , 32 , 33 , 34 Considering that sinonasal non‐SCC is less sensitive to RT and chemotherapy than sinonasal SCC, exploring the utility of surgical resection in cT4b sinonasal non‐SCC may benefit certain patients. 35 , 36 Our study utilizes the National Cancer Database (NCDB) to investigate the survival benefit of surgical resection in cT4b sinonasal non‐SCC. To our knowledge, our study is also the first to present a cohort of exclusively cT4b sinonasal non‐SCC.
2. METHODS
2.1. Data source
The NCDB is jointly sponsored by the American Cancer Society (ACS) and American College of Surgeons Commission on Cancer (CoC). 37 The NCDB collects data from >1500 CoC‐accredited hospitals within the United States, capturing >70% of newly diagnosed head and neck cancer. 37 The Rutgers New Jersey Medical School and University of Pennsylvania Institutional Review Boards exempted our study because of the de‐identified nature of patient data. The ACS and CoC are not responsible for the validity of conclusions derived herein.
2.2. Inclusion criteria
The NCDB was queried for adults with primary cT4b sinonasal non‐SCC diagnosed between January 2004 and December 2019, confirmed with histology (Figure 1). Sinonasal non‐SCC was identified using International Classification of Diseases for Oncology, Third Edition (ICD‐O‐3) histology, behavior, and topography codes (Table S1). Extension into and beyond the eye, skull base, dura, brain, cranial nerves, masseter, and pterygoid muscles, or nasopharynx was used to indicate cT4b disease in the frontal and sphenoid sinuses, for which no standard tumor classification system exists. 26 Although the study period includes the transition from the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition to the AJCC Cancer Staging Manual, 8th edition, classification criteria for cT4b disease did not change. Patients were excluded from survival analyses if they had history of prior malignancy; evidence of distant metastasis; treatment with palliative intent; treatment with chemotherapy without radiotherapy; or unknown vital status or follow‐up. 26 , 38 , 39 Patients were also excluded from survival analyses if they had last follow‐up within 6 months of diagnosis to account for immortal time bias. 26 , 38 , 39 , 40
FIGURE 1.
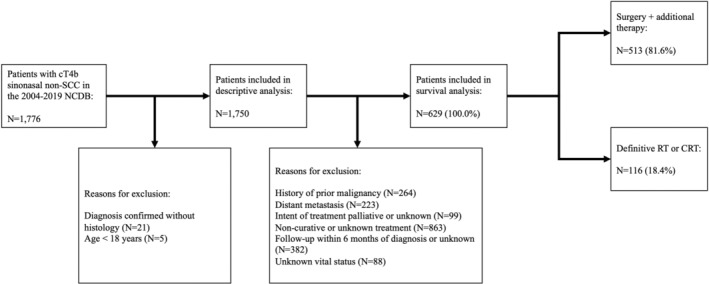
Inclusion criteria for 1750 cT4b tumors included in descriptive analyses and 629 cT4b tumors included in survival analyses. CRT, chemoradiotherapy; cT, clinical tumor; NCDB, National Cancer Database; RT, radiotherapy; SCC, squamous cell carcinoma.
Extension into bone or the pterygoid fossa was used to indicate cT4a disease in the frontal and sphenoid sinuses. Survival analyses included cT4a tumors undergoing surgical resection + RT/CRT subject to identical inclusion criteria. 38 , 39
2.3. Variables
Patient data included age at diagnosis, sex, race, Charlson–Deyo comorbidity score (CDCS), history of prior malignancy, histology, primary site, tumor diameter, clinical (cT) and pathologic tumor (pT) classification, clinical (cN), and pathologic nodal (pN) metastasis, distant metastasis, surgical approach, surgical margin status, treatment, length of stay (LOS) and 30‐day readmission following surgical resection, mortality, and survival time. Cases with a CDCS of 0 had no recorded comorbid conditions. Macroscopic, microscopic, or unspecified residual tumor were considered positive surgical margins (PSM). Local tumor destruction, local tumor excision, partial removal, total removal, debulking, radical removal, and unspecified surgery were classified as surgical resection. Neck dissection was defined as the removal and examination of ≥18 lymph nodes, a validated threshold in head and neck SCC. 41 , 42 RT was defined as external beam radiation with volume in the head and neck. Definitive treatment was defined as (1) surgical resection with ≥1 additional treatment (RT, chemotherapy, or both) or (2) RT alone or CRT with cumulative radiation dose between 66 and 80 Gy, as previously defined. 26 , 27 Patients undergoing neoadjuvant therapy were included in the surgical resection treatment arm, consistent with previous studies of cT4b sinonasal SCC. 26 The primary outcome of our study was 5‐year overall survival (OS). Survival time was calculated as the time from 6‐months of follow‐up to death or 5 years.
2.4. Statistical analysis
Patients undergoing surgical resection + additional therapy and definitive RT/CRT were compared with the chi‐square and Mann–Whitney U tests, as appropriate. Multivariable binary logistic regression models handling missing data with listwise elimination and adjusting for all significant variables on univariable regression were implemented to identify patient demographics and clinicopathologic features independently associated with undergoing definitive treatment. Assumptions of multivariable regression including (1) normality of residuals as determined by predicted probability (P–P) plots, (2) homoscedasticity of residual distributions, (3) linearity between predictor and outcome variables, and (4) absence of multicollinearity among predictor variables as assessed by correlation matrices with coefficients <0.8 and variable inflation factors <10, were tested and not violated in any models. Kaplan–Meier analysis was performed with the log‐rank test to estimate 5‐year OS. Multivariable Cox proportional hazards regression models handling missing data with listwise elimination and adjusting for all significant variables on univariable regression were implemented to identify patient demographics, clinicopathologic features, and treatment independently associated with OS. The proportionality of hazards was evaluated using time‐dependent covariates and was not violated in any regression models. The two‐sided threshold for statistical significance was set at p <.05. SPSS version 25 (IBM) was used for statistical analysis.
3. RESULTS
3.1. Patient demographics, clinicopathologic features, and treatment
A total of 1750 patients with cT4b sinonasal non‐SCC satisfied inclusion criteria (Table 1). Median age at diagnosis (interquartile range [IQR]) was 61 (49–71) years. A high proportion of patients were male (58.0%), White (82.1%), and had disease of the nasal cavity (36.6%). The most frequent histologic types were SNUC (23.2%), AdCC (20.4%), adenocarcinoma (19.4%), NEC (15.1%), and melanoma (13.6%). A minority of patients presented with nodal (21.6%) or distant (12.7%) metastases. A total of 979 (56.1%) patients underwent surgical resection, 1213 (69.3%) underwent RT, and 895 (52.5%) underwent chemotherapy. Very few patients underwent first‐course immunotherapy (N = 71, 4.1%). Age at diagnosis, melanoma histology, and cN metastasis were associated with undergoing non‐definitive treatment on multivariable binary logistic regression (p <.025) (Table 2).
TABLE 1.
Patient demographics and clinicopathologic features of 1750 cT4b tumors, n (%).
| Total | |
|---|---|
| No. | 1750 (100.0) |
| Age at diagnosis, median years (IQR) | 61 (49–71) |
| Sex | |
| Male | 1015 (58.0) |
| Female | 735 (42.0) |
| Race | |
| White | 1413 (82.1) |
| Black | 207 (12.0) |
| Other | 102 (5.9) |
| CDCS | |
| 0 | 1409 (80.5) |
| ≥1 | 341 (19.5) |
| History of prior malignancy | |
| No | 1486 (84.9) |
| Yes | 264 (15.1) |
| Histology | |
| Adenocarcinoma | 339 (19.4) |
| Adenoid cystic carcinoma | 357 (20.4) |
| Neuroendocrine carcinoma | 265 (15.1) |
| Mucoepidermoid carcinoma | 29 (1.7) |
| Melanoma | 238 (13.6) |
| Esthesioneuroblastoma | 116 (6.6) |
| Undifferentiated carcinoma | 406 (23.2) |
| Primary site | |
| Nasal cavity | 641 (36.6) |
| Maxillary sinus | 338 (19.3) |
| Ethmoid sinus | 426 (24.3) |
| Frontal sinus | 17 (1.0) |
| Sphenoid sinus | 73 (4.2) |
| Sinus: overlapping, unspecified | 169 (9.7) |
| Nasopharynx | 86 (4.9) |
| Tumor diameter, median cm (IQR) | 5.1 (3.9–6.6) |
| Clinical nodal metastasis | |
| No | 1086 (78.4) |
| Yes | 299 (21.6) |
| Distant metastasis | |
| No | 1527 (87.3) |
| Yes | 223 (12.7) |
| Treatment | |
| None | 126 (7.2) |
| Surgical resection + additional therapy | 721 (41.2) |
| Definitive RT/CRT | 166 (9.5) |
| Other | 737 (42.1) |
| Surgical approach | |
| Open | 224 (44.1) |
| Endoscopic | 284 (55.9) |
| Surgical margin status | |
| Negative | 301 (44.3) |
| Positive | 379 (55.7) |
Abbreviations: CDCS, Charlson–Deyo comorbidity score; CRT, chemoradiotherapy; IQR, interquartile range; RT, radiotherapy.
TABLE 2.
Univariable and multivariable binary logistic regression models for undergoing definitive treatment among 1750 cT4b tumors.
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| N | OR (95% CI) | p‐value | aOR a (95% CI) | p‐value | |
| Age at diagnosis (years) | 1385 | 0.97 (0.97–0.98) | <.001 | 0.97 (0.96–0.98) | <.001 |
| Sex | |||||
| Male | Ref | ||||
| Female | 0.86 (0.7–1.04) | .126 | |||
| Race | |||||
| White | Ref | ||||
| Black | 0.85 (0.62–1.16) | .305 | |||
| Other | 1.03 (0.68–1.56) | .881 | |||
| CDCS | |||||
| 0 | Ref | ||||
| ≥1 | 0.83 (0.65–1.07) | .146 | |||
| Histology | |||||
| Adenocarcinoma | 272 | Ref | Ref | ||
| Adenoid cystic carcinoma | 286 | 1.06 (0.78–1.44) | .700 | 0.90 (0.63–1.28) | .547 |
| Neuroendocrine carcinoma | 218 | 0.81 (0.58–1.14) | .227 | 0.81 (0.55–1.20) | .293 |
| Mucoepidermoid carcinoma | 17 | 0.85 (0.38–1.88) | .681 | 1.67 (0.61–4.60) | .320 |
| Melanoma | 178 | 0.45 (0.31–0.66) | <.001 | 0.57 (0.37–0.90) | .016 |
| Esthesioneuroblastoma | 79 | 1.55 (1.02–2.38) | .042 | 1.53 (0.91–2.58) | .113 |
| Undifferentiated carcinoma | 335 | 0.93 (0.69–1.26) | .643 | 0.91 (0.64–1.28) | .580 |
| Primary site | |||||
| Nasal cavity | Ref | ||||
| Accessory sinus | 0.96 (0.78–1.17) | .667 | |||
| Nasopharynx | 0.79 (0.49–1.27) | .327 | |||
| Tumor diameter (cm) | 0.99 (0.97–1.02) | .511 | |||
| Clinical nodal metastasis | |||||
| No | 1086 | Ref | Ref | ||
| Yes | 299 | 0.56 (0.42–0.75) | <.001 | 0.49 (0.36–0.66) | <.001 |
| Distant metastasis | |||||
| No | Ref | ||||
| Yes | 0.00 (0.00–0.00) | .995 |
Abbreviations: aOR, adjusted odds ratio; CDCS, Charlson–Deyo comorbidity score; CI, confidence interval; CRT, chemoradiotherapy; cT, clinical tumor; OR, odds ratio; OS, overall survival; RT, radiotherapy. Bolded values indicate statistically significant results (p<0.05).
N = 1385; number of events: 491.
Of 629 patients undergoing definitive treatment, 513 (81.6%) underwent surgical resection + additional (neoadjuvant or adjuvant) therapy and 116 (18.4%) underwent RT/CRT (Table 3). Compared with those undergoing surgical resection + additional therapy, a higher proportion of patients undergoing definitive RT/CRT had SNUC (36.2% vs. 20.9%) and cN metastasis (26.5% vs. 13.0%) (p <.001). A lower proportion of patients undergoing definitive RT/CRT had nasal cavity disease (30.2% vs. 39.2%, p <.001). Patients with PSM had similar RT (48.5% vs. 40.8%) and CRT (48.0% vs. 55.7%) utilization as those with negative surgical margins (p = .314).
TABLE 3.
Patient demographics and clinicopathologic features among 629 cT4b tumors undergoing definitive treatment, n (%).
| Surgical resection + additional therapy | RT/CRT | p‐value | Total | |
|---|---|---|---|---|
| No. | 513 (100.0) | 116 (100.0) | ‐ | 629 (100.0) |
| Age at diagnosis, median years (IQR) | 55 (45–65) | 60 (46–70) | .030 | 55 (46–66) |
| Sex | ||||
| Male | 312 (60.8) | 68 (58.6) | .662 | 380 (60.4) |
| Female | 201 (39.2) | 48 (41.4) | 249 (39.6) | |
| Race | ||||
| White | 430 (84.6) | 86 (75.4) | .054 | 516 (83.0) |
| Black | 51 (10.0) | 17 (14.9) | 68 (10.9) | |
| Other | 27 (5.3) | 11 (9.6) | 38 (6.1) | |
| CDCS | ||||
| 0 | 423 (82.5) | 95 (81.9) | .886 | 518 (82.4) |
| ≥1 | 90 (17.5) | 21 (18.1) | 111 (17.6) | |
| Histology | ||||
| Adenocarcinoma | 114 (22.2) | 16 (13.8) | <.001 | 130 (20.7) |
| Adenoid cystic carcinoma | 111 (21.6) | 31 (26.7) | 142 (22.6) | |
| Neuroendocrine carcinoma | 71 (13.8) | 18 (15.5) | 89 (14.1) | |
| Mucoepidermoid carcinoma | 8 (1.6) | 2 (1.7) | 10 (1.6) | |
| Melanoma | 47 (9.2) | 5 (4.3) | 52 (8.3) | |
| Esthesioneuroblastoma | 55 (10.7) | 2 (1.7) | 57 (9.1) | |
| Undifferentiated carcinoma | 107 (20.9) | 42 (36.2) | 149 (23.7) | |
| Primary site | ||||
| Nasal cavity | 201 (39.2) | 35 (30.2) | <.001 | 236 (37.5) |
| Maxillary sinus | 72 (14.0) | 22 (19.0) | 94 (14.9) | |
| Ethmoid sinus | 140 (27.3) | 28 (24.1) | 168 (26.7) | |
| Frontal sinus | 11 (2.1) | 0 (0.0) | 11 (1.7) | |
| Sphenoid sinus | 16 (3.1) | 11 (9.5) | 27 (4.3) | |
| Sinus: overlapping, unspecified | 63 (12.3) | 3 (2.6) | 66 (10.5) | |
| Nasopharynx | 10 (1.9) | 17 (14.7) | 27 (4.3) | |
| Tumor diameter, median cm (IQR) | 5.0 (3.7–6.2) | 6.0 (4.8–7.0) | <.001 | 5.0 (3.9–6.4) |
| Clinical nodal metastasis | ||||
| No | 342 (87.0) | 72 (73.5) | .001 | 414 (84.3) |
| Yes | 51 (13.0) | 26 (26.5) | 77 (15.7) |
Abbreviations: CDCS, Charlson–Deyo comorbidity score; CRT, chemoradiotherapy; IQR, interquartile range; RT, radiotherapy. Bolded values indicate statistically significant results (p<0.05).
Among 513 patients undergoing surgical resection + additional therapy, 439 (85.6%) had known treatment sequence; 41 (9.3%) underwent neoadjuvant therapy alone, 386 (87.9%) underwent adjuvant therapy alone, and 12 (2.7%) underwent both. A total of 51 (11.6%) patients underwent neoadjuvant chemotherapy. A total of 249 (48.5%) patients underwent surgery with known approach. An open approach was utilized more frequently than an endoscopic approach in nasal cavity (51.1% vs. 48.9%), maxillary sinus (73.0% vs. 27.0%), ethmoid sinus (58.2% vs. 41.8%), and overlapping or unspecified disease (59.4% vs. 40.6%). A total of 26 (5.2%) patients undergoing surgical resection + additional therapy also underwent neck dissection; 10 (47.6%) patients had cN metastasis and 13 (50.0%) had pN metastasis. Median (IQR) LOS following surgical resection was 4 (1–8) days. Within 30 days of surgical resection, there were 31 (6.3%) readmissions and 1 (0.2%) mortality; within 90 days, there were 4 (0.8%) mortalities.
Among 376 patients undergoing surgical resection + additional therapy with known margin status, 202 (57.3%) had PSM. A total of 33 (16.3%) patients were macroscopically positive, 69 (34.2%) were microscopically positive, and 100 (49.5%) were unspecified positive. Among 169 patients undergoing surgical resection + RT alone and 194 patients undergoing surgical resection + CRT, the rate of PSM was similar (58.0% vs. 50.0%, p = .128). Among 33 patients undergoing neoadjuvant therapy alone and 278 patients undergoing adjuvant therapy alone, the rate of PSM was higher in those undergoing adjuvant therapy alone (56.5% vs. 30.3%, p = .004).
Among 585 cT4b tumors with known pT classification, 421 (72.0%) remained pT4b; 113 (19.3%) were classified as pT4a and 51 (8.7%) as pT3 or lower. Among 141 cT4b tumors with known pN classification, 16 (11.3%) had pN metastasis.
3.2. 5‐year OS by definitive treatment
Patients undergoing definitive treatment had higher 5‐year OS than those undergoing non‐definitive treatment (53.7% vs. 39.2%, p <.001). Among patients undergoing definitive treatment, those with PSM had higher 5‐year OS than those undergoing definitive RT/CRT (56.3% vs. 39.4%, p = .039) and similar 5‐year OS as those with negative margins (56.3% vs. 63.9%, p =.059) (Figure 2, Tables 3 and 4). 5‐year OS was similar between microscopically positive, macroscopically positive, and unspecified PSM (60.3% vs. 47.5% vs. 56.5%, p = .309). Compared with definitive RT/CRT, surgical resection + additional therapy remained associated with higher OS in adenocarcinoma (56.8% vs. 16.1%), AdCC (70.1% vs. 29.8%), and sphenoid sinus disease (71.4% vs. 9.1%) (p <.005). Compared with PSM, negative margins were associated with higher OS in SNUC (64.2% vs. 25.0%), nasal cavity disease (78.4% vs. 55.2%), and cN0 disease (70.3% vs. 58.3%) (p <.015) (Table 5). Among patients with PSM, those undergoing surgical resection + RT alone and surgical resection + CRT had similar 5‐year OS (57.6% vs. 56.1%, p = .870). Among patients undergoing surgical resection + additional therapy, those also undergoing neck dissection had higher 5‐year OS than those not undergoing neck dissection (58.6% vs. 34.7%, p =.002). Patients undergoing neoadjuvant chemotherapy and definitive RT/CRT had similar 5‐year OS (60.9% vs. 39.5%, p = .053). Among patients undergoing neoadjuvant therapy, those with positive and negative margins had similar 5‐year OS (51.7% vs. 63.6%, p = .136).
FIGURE 2.
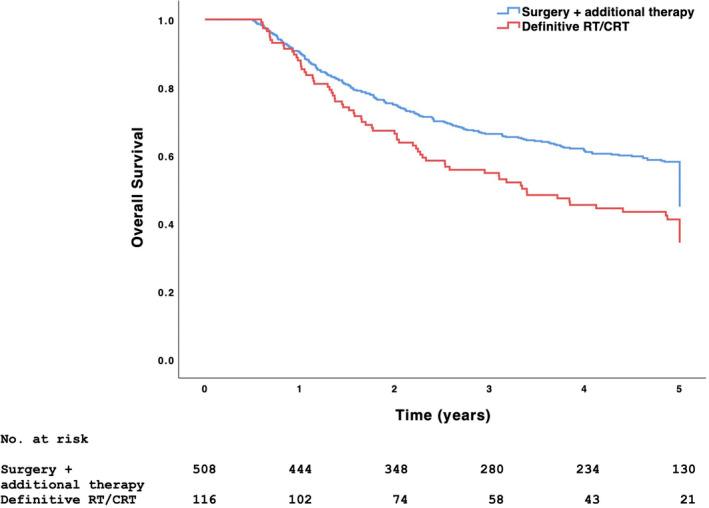
5‐year OS among 513 cT4b tumors undergoing surgical resection + additional therapy and 116 cT4b tumors undergoing definitive RT/CRT. Significance derived from the log‐rank test (p = .006). CRT, chemoradiotherapy; cT, clinical tumor; OS, overall survival; RT, radiotherapy.
TABLE 4.
Kaplan–Meier analysis of 5‐year OS (%) among 629 cT4b tumors undergoing definitive treatment.
| Surgical resection + additional therapy | RT/CRT | p‐value | |
|---|---|---|---|
| Overall | 57.1 | 39.4 | .006 |
| Histology | |||
| Adenocarcinoma | 56.8 | 16.1 | .002 |
| Adenoid cystic carcinoma | 70.1 | 29.8 | <.001 |
| Neuroendocrine carcinoma | 58.1 | 68.8 | .949 |
| Mucoepidermoid carcinoma | 25.0 | 50.0 | .960 |
| Melanoma | 34.9 | 50.0 | .231 |
| Esthesioneuroblastoma | 80.8 | 69.2 | .479 |
| Undifferentiated carcinoma | 43.3 | 55.6 | .414 |
| Primary site | |||
| Nasal cavity | 61.5 | 40.6 | .079 |
| Maxillary sinus | 57.6 | 35.0 | .148 |
| Ethmoid sinus | 51.6 | 42.3 | .337 |
| Frontal sinus | 54.5 | ‐ | ‐ |
| Sphenoid sinus | 71.4 | 9.1 | .004 |
| Sinus: overlapping, unspecified | 48.0 | 33.3 | .849 |
| Nasopharynx | 88.9 | 58.8 | .417 |
| Clinical nodal metastasis | |||
| No | 40.7 | 88.9 | .015 |
| Yes | 57.4 | 40.6 | .130 |
Abbreviations: CRT, chemoradiotherapy; OS, overall survival; RT, radiotherapy. Bolded values indicate statistically significant results (p<0.05)
TABLE 5.
Kaplan–Meier analysis of 5‐year OS (%) among 513 cT4b tumors undergoing surgical resection + additional therapy.
| NSM | PSM | p‐value | |
|---|---|---|---|
| Overall | 63.9 | 56.3 | .059 |
| Histology | |||
| Adenocarcinoma | 54.5 | 66.2 | .820 |
| Adenoid cystic carcinoma | 70.6 | 69.0 | .842 |
| Neuroendocrine carcinoma | 68.8 | 54.2 | .332 |
| Mucoepidermoid carcinoma | 66.7 | ‐ | .069 |
| Melanoma | 42.9 | 36.0 | .511 |
| Esthesioneuroblastoma | 87.5 | 81.3 | .290 |
| Undifferentiated carcinoma | 64.2 | 25.0 | .001 |
| Primary site | |||
| Nasal cavity | 78.4 | 55.2 | .009 |
| Maxillary sinus | 60.0 | 61.4 | .589 |
| Ethmoid sinus | 54.3 | 51.4 | .635 |
| Frontal sinus | 60.0 | 50.0 | .743 |
| Sphenoid sinus | 66.7 | 69.2 | .882 |
| Sinus: overlapping, unspecified | 50.0 | 50.0 | .901 |
| Nasopharynx | 71.4 | 100.0 | .728 |
| Clinical nodal metastasis | |||
| No | 70.3 | 58.3 | .011 |
| Yes | 40.0 | 44.8 | .717 |
| Additional therapy | |||
| Chemotherapy alone | 61.2 | 57.6 | .644 |
| RT alone | 40.0 | 42.9 | .666 |
| CRT | 67.3 | 56.1 | .043 |
| Therapy sequence | |||
| Adjuvant | 64.7 | 57.1 | .123 |
| Neoadjuvant | 63.6 | 51.7 | .136 |
Abbreviations: CRT, chemoradiotherapy; NSM, negative surgical margins; OS, overall survival; PSM, positive surgical margins; RT, radiotherapy. Bolded values indicate statistically significant results (p<0.05).
Among patients undergoing definitive treatment, 5‐year OS was 51.3% for adenocarcinoma, 61.0% for AdCC, 60.2% for NEC, 20.0% for MEC, 31.3% for melanoma, 79.6% for esthesioneuroblastoma, and 45.3% for SNUC (p < .001) (Figure S1). Age at diagnosis, tumor diameter, and surgical resection + additional therapy (adjusted hazard ratio [aHR] 0.64, 95% confidence interval [CI] 0.45–0.91) were associated with OS on multivariable Cox regression (p <.05) (Table 6).
TABLE 6.
Univariable and multivariable Cox proportional hazard regression models of 5‐year OS among 629 cT4b tumors undergoing definitive treatment.
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| N | HR (95% CI) | p‐value | aHR a (95% CI) | p‐value | |
| Age at diagnosis (years) | 387 | 1.02 (1.01–1.03) | <.001 | 1.02 (1.01–1.03) | .004 |
| Sex | |||||
| Male | Ref | ||||
| Female | 0.97 (0.78–1.22) | .815 | |||
| Race | |||||
| White | Ref | ||||
| Black | 0.86 (0.59–1.26) | .438 | |||
| Other | 0.64 (0.36–1.11) | .110 | |||
| CDCS | |||||
| 0 | Ref | ||||
| ≥1 | 0.97 (0.73–1.30) | .852 | |||
| Histology | |||||
| Adenocarcinoma | 95 | Ref | Ref | ||
| Adenoid cystic carcinoma | 73 | 0.92 (0.66–1.30) | .641 | 0.85 (0.54–1.33) | .479 |
| Neuroendocrine carcinoma | 57 | 0.82 (0.55–1.23) | .337 | 0.77 (0.46–1.28) | .308 |
| Mucoepidermoid carcinoma | 6 | 3.19 (1.64–6.22) | .001 | 2.06 (0.86–4.94) | .105 |
| Melanoma | 30 | 2.15 (1.44–3.21) | <.001 | 1.58 (0.92–2.72) | .095 |
| Esthesioneuroblastoma | 26 | 0.50 (0.30–0.83) | .008 | 0.60 (0.28–1.30) | .194 |
| Undifferentiated carcinoma | 101 | 1.15 (0.83–1.60) | .410 | 1.20 (0.81–1.79) | .364 |
| Primary site | |||||
| Nasal cavity | 136 | Ref | |||
| Accessory sinus | 237 | 1.27 (1.00–1.61) | .049 | 1.38 (1.00–1.90) | .051 |
| Nasopharynx | 15 | 0.72 (0.39–1.35) | .307 | 0.39 (0.15–1.00) | .050 |
| Tumor diameter (cm) | 387 | 1.02 (1.00–1.04) | .021 | 1.02 (1.00–1.04) | .036 |
| Clinical nodal metastasis | |||||
| No | Ref | ||||
| Yes | 1.23 (0.87–1.72) | 0.240 | |||
| Treatment | |||||
| Surgical resection + additional therapy | 315 | 0.70 (0.54–0.91) | .008 | 0.64 (0.45–0.91) | .014 |
| Definitive RT/CRT | 73 | Ref | Ref |
Abbreviations: aHR, adjusted hazard ratio; CDCS, Charlson–Deyo comorbidity score; CI, confidence interval; CRT, chemoradiotherapy; HR, hazard ratio; OS, overall survival; RT, radiotherapy. Bolded values indicate statistically significant results (p<0.05).
N = 387; number of uncensored deaths: 192.
Among patients undergoing surgical resection + additional therapy, open approach (HR 0.87, 95% CI 0.59–1.28, p = .476) and PSM (HR 1.35, 95% CI 0.99–1.83, p = .059) were not associated with OS on univariable Cox regression (Table S2).
3.3. 5‐year OS by cT classification
Identical inclusion criteria identified 503 patients with cT4a sinonasal non‐SCC undergoing surgical resection + RT/CRT. A higher proportion of cT4a tumors had AdCC histology (28.8% vs. 21.7%), melanoma histology (22.3% vs. 9.1%), and maxillary sinus disease (37.8% vs. 13.6%) (p <.001) (Table S3). cN metastasis (8.4% vs. 12.6%, p = .057) and PSM (49.5% vs. 53.7%, p = .246) were similar between cT4a and cT4b tumors.
Among 503 cT4a tumors and 492 cT4b tumors undergoing surgical resection + RT/CRT, no difference in 5‐year OS was observed between cT4a and cT4b tumors (62.6% vs. 57.7%, p = .097) (Figure S2). 5‐year OS was similar across most histologies for cT4a and cT4b tumors: adenocarcinoma (70.9% vs. 56.0%, p = .104), AdCC (74.8% vs. 71.0%, p = .278), NEC (73.0% vs. 60.7%, p = .147), MEC (78.9% vs. 28.6%, p <.001), melanoma (32.0% vs. 34.9%, p = .600), esthesioneuroblastoma (73.1% vs. 82.4%, p = .248), and SNUC (64.0% vs. 43.3%, p = .016). cT4a and cT4b (HR 1.17, 95% CI 0.97–1.40, p = .106) tumors had similar OS on univariable Cox regression (Table S4).
3.4. Neoadjuvant therapy sensitivity analysis
Excluding the 53 patients undergoing neoadjuvant therapy, 487 underwent definitive treatment: 386 (79.3%) underwent surgical resection + adjuvant therapy and 101 (20.7%) underwent definitive RT/CRT. Patients with PSM had higher 5‐year OS than those undergoing definitive RT/CRT (57.3% vs. 40.4%, p = .043) and similar 5‐year OS as those with negative margins (57.3% vs. 63.7%, p = .417). Surgical resection + adjuvant therapy (aHR 0.68, 95% CI 0.50–0.93, p = .017) remained associated with higher OS on multivariable Cox regression.
Excluding the 195 patients undergoing neoadjuvant therapy, 426 cT4a tumors and 374 cT4b tumors undergoing surgical resection + adjuvant RT/CRT were identified. No difference in 5‐year OS was observed between cT4a and cT4b tumors (63.0% vs. 57.2%, p = .052). cT4a and cT4b (HR 0.82, 95% CI 0.66–1.01, p = .058) tumors had similar OS on univariable Cox regression.
4. DISCUSSION
Surgical resection achieving negative margins is the preferred definitive treatment for sinonasal non‐SCC and additional RT/CRT has documented survival benefit. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Although the AJCC defines cT4b disease as unresectable, surgical resection has increasingly been considered in cT4b sinonasal cancer. 17 , 31 , 32 , 33 , 43 , 44 Surgical resection + additional therapy, for example, has been associated with higher OS than definitive RT/CRT in cT4b sinonasal SCC. 26 , 27 , 32 , 45 , 46 , 47 The heterogeneity of the cT4b classification and sinonasal non‐SCC having less sensitivity to RT and chemotherapy than sinonasal SCC raises the possibility that certain patients may benefit from surgical resection. 36 , 38 , 39 Our study investigating sinonasal non‐SCC suggests that surgical resection + additional therapy is associated with higher OS than definitive RT/CRT, despite high rate of PSM.
Our study identified 1750 patients with cT4b sinonasal non‐SCC. A minority of patients (35.9%) underwent definitive treatment with either (1) surgical resection + additional therapy or (2) RT/CRT. More patients (81.6%) underwent surgical resection + additional therapy than RT/CRT (18.4%), despite NCCN recommendations not including surgery in the multimodal management of cT4b sinonasal non‐SCC. Varying interpretations of unresectable disease among surgeons and institutions may account for this finding. 27 Patients with adenocarcinoma, AdCC, and sphenoid sinus disease may especially benefit from surgical resection. The high rate of PSM (53.7%) following surgical resection may reflect a desire to balance patient quality of life, including nasal obstruction and persistent epistaxis, with disease prognosis. Studies of cT4b sinonasal cancer report rates of PSM approaching 45%, underscoring the technical challenge of surgical resection in cT4b disease. 26 , 27 , 32 Patients undergoing surgical resection with PSM had higher 5‐year OS than those undergoing definitive RT/CRT and similar 5‐year OS as those with negative margins; the significance of these differences varied by histology. Univariable analysis did not associate PSM with worse OS suggesting that negative margins may not be necessary in certain patients and that the role of surgical resection may be to reduce overall tumor burden before administering adjuvant therapy. Patients with SNUC, nasal cavity disease, and cN0 disease, however, may especially benefit from negative margins. Among patients undergoing surgical resection + additional therapy, neck dissection frequently detected metastatic lymph nodes and was associated with higher OS. The mortality rate within 90 days of surgical resection was 0.8%, supporting the clinical practice of resecting cT4b sinonasal non‐SCC.
Estimates of survival in cT4b sinonasal non‐SCC are limited because SCC accounts for most published cases of sinonasal cancer. 28 5‐year OS of cT4b sinonasal SCC ranges from 20% to 40% depending on treatment intent and modality. 48 , 49 , 50 Surgical resection, even if limited to debulking, and appropriately selected, high‐dose adjuvant therapy are suggested to improve 5‐year OS by ≥20% compared with definitive RT/CRT. 26 , 27 , 30 , 32 , 45 Surgical resection may even be considered for tumors invading the orbital apex and cavernous sinus because definitive RT/CRT is associated with increased risk of disease progression and locoregional recurrence. 27 , 33 , 51 , 52 , 53 5‐year OS in our cohort was 53.7% and worse for patients with melanoma and SNUC, consistent with previous studies of cT4b disease. 2 , 26 , 27 , 32 , 38 , 39 , 54 , 55 , 56 , 57 Of note, SNUC is increasingly understood to represent a diverse group of poorly differentiated carcinomas, each with unique phenotypes and associated clinical outcomes.
Endoscopic approaches are suggested to reduce LOS, postoperative complications, readmission, and medical costs without compromising survival in several sinonasal cancers. 13 , 36 , 58 , 59 , 60 , 61 , 62 , 63 , 64 Most surgical resections in our study (58.5%), however, utilized an open approach. An open approach was utilized more frequently than an endoscopic approach in nasal cavity, maxillary sinus, and ethmoid sinus disease. Surgical approach not influencing OS on univariable analysis may be related to additional therapy mitigating the survival detriment associated with PSM. 27
Sinonasal cancer may be down‐staged with induction chemotherapy (IC) which can be used to select patients for (1) definitive CRT in those with robust response to IC, or (2) surgical resection + adjuvant CRT in those with poor response to IC. 65 , 66 , 67 , 68 Definitive CRT is suggested to improve locoregional control and OS more than surgical resection in those achieving favorable response to IC. 32 , 69 Although our study did not associate definitive RT/CRT with higher OS than neoadjuvant chemotherapy, the rate of PSM was lower in patients undergoing neoadjuvant therapy than those undergoing adjuvant therapy, consistent with previous studies. 70 , 71 The NCDB does not adequately capture whether patients undergoing neoadjuvant chemotherapy demonstrated poor response to IC but active clinical trials comparing IC with primary surgical resection are expected to inform multimodal management of sinonasal non‐SCC. 26
Surgical resection has historically been contraindicated in cT4b disease because sacrificing involved structures such as the eyes, brain, internal carotid artery, or nerves confers unjustifiable morbidity. Studies of cT4b head and neck cancer, however, demonstrate the safety and survival benefit of surgical resection in select patients. 27 , 32 , 38 , 39 , 55 For example, combined open and endoscopic approaches with advanced reconstructive techniques allow for complete resection of tumors infiltrating the pterygoid plates and dura. 55 Studies of cT4b oral cavity SCC, sinonasal SCC, major salivary gland cancer (MSGC), and head and neck AdCC document <2% mortality within 90 days of surgical resection. 38 , 39 , 72 Taken together, surgical resection of cT4b sinonasal non‐SCC may be more feasible and beneficial than previously assumed.
The NCCN recommends surgical resection for sinonasal non‐SCC classified as cT4a or lower. cT4b disease is typically considered unresectable because of tumor extension into critical neurovascular structures. Retrospective studies of head and neck cancer, however, suggest that some tumors classified as cT4b may be closer to cT4a in extent and outcome. 38 , 39 , 73 , 74 , 75 To explore this hypothesis, our study (1) investigated cT4b pathologic classification and (2) compared OS between cT4a and cT4b tumors undergoing surgical resection + RT/CRT. A moderate proportion of cT4b tumors (28%) were classified as pT4a or lower on pathologic examination. Although most cT4b tumors remained pT4b, cT4a and cT4b tumors undergoing surgical resection + RT/CRT had similar OS for most histologies studied. cT4a and cT4b tumors undergoing surgical resection + RT/CRT are noted to have similar OS in oral cavity SCC, sinonasal SCC, MSGC, and head and neck AdCC. 17 , 38 , 39 , 72 Local factors such as inflammation and bone erosion may prevent accurate evaluation of tumor size and extent, leading to up‐staging of cT4a tumors. If future research confirms these findings, a critical revision of cT4b classification criteria may be necessary to optimize treatment selection and survival.
Limitations inherent in retrospective study of the NCDB include inaccurate histologic diagnosis and variable miscoding. The NCDB does not report medical comorbidities, tobacco use, depth of invasion, imaging studies, multidisciplinary tumor board recommendations, quality of life, locoregional recurrence, and disease‐free survival. The NCDB also does not report participation in clinical trials and experimental therapies which may have influenced treatment decisions for some patients. Evaluating surgical margins in large tumors is challenging and the possibility of occult disease persists following microscopically negative resection. The NCDB also does not clarify whether these margins were obtained from the specimen or tumor bed, or whether the reported margins were obtained from the initial or final resection; negative surgical margins may therefore not be considered a low‐risk feature in our cohort. Although defining neck dissection as the removal and examination of ≥18 lymph nodes may have underestimated the delivery of neck dissection, some patients undergoing more selective nodal resections had known pN classification. The advent of free flap reconstruction in more recent years of the study period (2004–2019) may have allowed for wider resections with negative surgical margins. Despite these limitations, our findings may provide valuable insight into the multimodal management of cT4b sinonasal non‐SCC.
5. CONCLUSION
Patients with cT4b sinonasal non‐SCC undergoing definitive treatment more frequently underwent surgical resection + additional therapy than RT/CRT, representing a deviation from NCCN recommendations. Surgical resection + additional therapy was associated with higher OS than definitive RT/CRT, despite high rate of PSM; the significance of these differences varied by histology. Although a moderate proportion of cT4b tumors were classified as pT4a or lower, some cT4b tumors of certain histologies may have similar extent and outcome as cT4a tumors. A clinical trial investigating surgical resection in cT4b sinonasal non‐SCC may assist in identifying optimal treatment strategies and improving survival.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Figure S1. 5‐year OS among 629 cT4b tumors undergoing definitive treatment with stratification by histology (p <.001).
Figure S2. 5‐year OS among 503 cT4a and 492 cT4b tumors undergoing surgical resection + RT/CRT (p = 0.097). Significance derived from the log‐rank test. CRT, chemoradiotherapy; cT, clinical tumor; OS, overall survival; radiotherapy, RT.
Table S1. Histology, behavior, and topography codes included, n (%).
Table S2. Univariable and multivariable Cox proportional hazard regression models of 5‐year OS among 513 cT4b tumors undergoing surgical resection + additional therapy.
Table S3. Patient demographics and clinicopathologic features among 503 cT4a tumors and 492 cT4b tumors undergoing surgical resection + RT/CRT, n (%).
Table S4. Univariable and multivariable Cox proportional hazard regression models of 5‐year OS among 503 cT4a tumors and 492 cT4b tumors undergoing surgical resection + RT/CRT.
Patel AM, Haleem A, Revercomb L, et al. Surgical resection and overall survival in cT4b sinonasal non‐squamous cell carcinoma. Laryngoscope Investigative Otolaryngology. 2024;9(5):e70025. doi: 10.1002/lio2.70025
REFERENCES
- 1. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population‐based data. Head Neck. 2012;34(6):877‐885. doi: 10.1002/hed.21830 [DOI] [PubMed] [Google Scholar]
- 2. Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: a population‐based analysis of site‐specific incidence and survival. Laryngoscope. 2015;125(11):2491‐2497. doi: 10.1002/lary.25465 [DOI] [PubMed] [Google Scholar]
- 3. Spiro JD, Soo KC, Spiro RH. Nonsquamous cell malignant neoplasms of the nasal cavities and paranasal sinuses. Head Neck. 1995;17(2):114‐118. doi: 10.1002/hed.2880170207 [DOI] [PubMed] [Google Scholar]
- 4. Triantafillou V, Maina IW, Kuan EC, et al. Sinonasal mucoepidermoid carcinoma: a review of the national cancer database. Int Forum Allergy Rhinol. 2019;9(9):1046‐1053. doi: 10.1002/alr.22379 [DOI] [PubMed] [Google Scholar]
- 5. Klebaner D, Saddawi‐Konefka R, Finegersh A, et al. Immunotherapy in sinonasal melanoma: treatment patterns and outcomes compared to cutaneous melanoma. Int Forum Allergy Rhinol. 2020;10(9):1087‐1095. doi: 10.1002/alr.22628 [DOI] [PubMed] [Google Scholar]
- 6. Issa K, Ackall F, Jung SH, et al. Survival outcomes in sinonasal carcinoma with neuroendocrine differentiation: a NCDB analysis. Am J Otolaryngol. 2021;42(2):102851. doi: 10.1016/j.amjoto.2020.102851 [DOI] [PubMed] [Google Scholar]
- 7. Carey RM, Godovchik J, Workman AD, et al. Patient, disease, and treatment factors associated with overall survival in esthesioneuroblastoma. Int Forum Allergy Rhinol. 2017;7(12):1186‐1194. doi: 10.1002/alr.22027 [DOI] [PubMed] [Google Scholar]
- 8. Ganti A, Raman A, Shay A, et al. Treatment modalities in sinonasal mucosal melanoma: a national cancer database analysis. Laryngoscope. 2020;130(2):275‐282. doi: 10.1002/lary.27995 [DOI] [PubMed] [Google Scholar]
- 9. Elsamna ST, Ahsanuddin S, Mir GS, et al. Surgical margin status and survival following resection of Sinonasal mucosal melanoma. Laryngoscope. 2021;131(11):2429‐2435. doi: 10.1002/lary.29574 [DOI] [PubMed] [Google Scholar]
- 10. Tsutsumi K, Ahmed KH, Goshtasbi K, et al. Impact of esthesioneuroblastoma treatment delays on overall patient survival. Laryngoscope. 2023;133(4):764‐772. doi: 10.1002/lary.30136 [DOI] [PubMed] [Google Scholar]
- 11. Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys. 2007;67(3):691‐702. doi: 10.1016/j.ijrobp.2006.09.023 [DOI] [PubMed] [Google Scholar]
- 12. Kaki PC, Patel AM, Maxwell R, et al. Choice of Adjuvant Radiotherapy Facility in Sinonasal Squamous Cell Carcinoma. Laryngoscope. 2024. doi: 10.1002/lary.31794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rawal RB, Farzal Z, Federspiel JJ, Sreenath SB, Thorp BD, Zanation AM. Endoscopic resection of Sinonasal malignancy: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2016;155(3):376‐386. doi: 10.1177/0194599816646968 [DOI] [PubMed] [Google Scholar]
- 14. de Almeida JR, Witterick IJ, Gullane PJ, et al. Physical morbidity by surgical approach and tumor location in skull base surgery. Head Neck. 2013;35(4):493‐499. doi: 10.1002/hed.23006 [DOI] [PubMed] [Google Scholar]
- 15. Robin TP, Jones BL, Gordon OM, et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017;123(16):3040‐3049. doi: 10.1002/cncr.30686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014;11(8):460‐472. doi: 10.1038/nrclinonc.2014.97 [DOI] [PubMed] [Google Scholar]
- 17. Sakata K, Maeda A, Rikimaru H, et al. Advantage of extended craniofacial resection for advanced malignant tumors of the nasal cavity and paranasal sinuses: long‐term outcome and surgical management. World Neurosurg. 2016;89:240‐254. doi: 10.1016/j.wneu.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 18. Snyderman CH, Carrau RL, Kassam AB, et al. Endoscopic skull base surgery: principles of endonasal oncological surgery. J Surg Oncol. 2008;97(8):658‐664. doi: 10.1002/jso.21020 [DOI] [PubMed] [Google Scholar]
- 19. Goel AN, Yang JY, Wang MB, Lee JT, St John MA, Long JL. Predictors, costs, and causes of readmission after surgery for sinonasal cancer: a national perspective. Int Forum Allergy Rhinol. 2018;8(9):1056‐1064. doi: 10.1002/alr.22134 [DOI] [PubMed] [Google Scholar]
- 20. Vedula S, Kheir L, Hu P, Patel AM, Roden DF, Park RC. Adjuvant radiation and survival following surgical resection of Sinonasal adenocarcinoma. Laryngoscope. 2023;133(10):2603‐2612. doi: 10.1002/lary.30567 [DOI] [PubMed] [Google Scholar]
- 21. Shay A, Ganti A, Raman A, et al. Survival in low‐grade and high‐grade sinonasal adenocarcinoma: a national cancer database analysis. Laryngoscope. 2020;130(1):E1‐E10. doi: 10.1002/lary.28052 [DOI] [PubMed] [Google Scholar]
- 22. Abiri A, Yasaka TM, Lehrich BM, et al. Adjuvant therapy and prognosticators of survival in head and neck mucosal melanoma. Laryngoscope. 2022;132(3):584‐592. doi: 10.1002/lary.29807 [DOI] [PubMed] [Google Scholar]
- 23. Goel AN, Lee JT, Wang MB, Suh JD. Treatment delays in surgically managed sinonasal cancer and association with survival. Laryngoscope. 2020;130(1):2‐11. doi: 10.1002/lary.27892 [DOI] [PubMed] [Google Scholar]
- 24. Robbins KT, Ronen O, Saba NF, et al. Progress and emerging strategies to preserve function in the treatment of sinonasal cancer. Head Neck. 2023;45(11):2955‐2966. doi: 10.1002/hed.27510 [DOI] [PubMed] [Google Scholar]
- 25. Thawani R, Kim MS, Arastu A, et al. The contemporary management of cancers of the sinonasal tract in adults. CA Cancer J Clin. 2023;73(1):72‐112. doi: 10.3322/caac.21752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karp JM, Hu KS, Persky M, et al. Including surgical resection in the multimodal management of very locally advanced sinonasal cancer. Otolaryngol Head Neck Surg. 2022;167(3):494‐500. doi: 10.1177/01945998211067503 [DOI] [PubMed] [Google Scholar]
- 27. Li R, Tian S, Lin L, Liu Q, Wang S. Comparative outcome of surgical and nonsurgical therapy for T4bN0M0 sinonasal squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2019;276(11):3113‐3122. doi: 10.1007/s00405-019-05601-7 [DOI] [PubMed] [Google Scholar]
- 28. Povolotskiy R, Farber NI, Bavier RD, Cerasiello SY, Eloy JA, Hsueh WD. Endoscopic versus open resection of non‐squamous cell carcinoma sinonasal malignancies. Laryngoscope. 2020;130(8):1872‐1876. doi: 10.1002/lary.28270 [DOI] [PubMed] [Google Scholar]
- 29. Lisan Q, Kolb F, Temam S, Tao Y, Janot F, Moya‐Plana A. Management of orbital invasion in sinonasal malignancies. Head Neck. 2016;38(11):1650‐1656. doi: 10.1002/hed.24490 [DOI] [PubMed] [Google Scholar]
- 30. Orlandi E, Cavalieri S, Granata R, et al. Locally advanced epithelial sinonasal tumors: the impact of multimodal approach. Laryngoscope. 2020;130(4):857‐865. doi: 10.1002/lary.28202 [DOI] [PubMed] [Google Scholar]
- 31. Yeung JT, Caminer DM, Young IM, Sughrue ME, Teo C. Radical exenteration of the Skull Base for end‐stage, locally advanced Sinonasal malignancies: challenging the dictum of Unresectability. World Neurosurg. 2021;150:e102‐e107. doi: 10.1016/j.wneu.2021.02.092 [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Zhang J, Yang B, et al. T4b sinonasal squamous cell carcinoma: surgery plus radiotherapy may contribute to prolonged survival. Laryngoscope. 2023;133(9):2222‐2231. doi: 10.1002/lary.30545 [DOI] [PubMed] [Google Scholar]
- 33. Sugawara T, Aoyagi M, Ogishima T, et al. Extended orbital exenteration for sinonasal malignancy with orbital apex extension: surgical technique and clinical analysis. J Neurosurg. 2015;123(1):52‐58. doi: 10.3171/2014.9.JNS141256 [DOI] [PubMed] [Google Scholar]
- 34. Siddiqui F, Smith RV, Yom SS, et al. ACR appropriateness criteria® nasal cavity and paranasal sinus cancers. Head Neck. 2017;39(3):407‐418. doi: 10.1002/hed.24639 [DOI] [PubMed] [Google Scholar]
- 35. Konuthula N, Khan MN, Parasher A, et al. The presentation and outcomes of mucosal melanoma in 695 patients. Int Forum Allergy Rhinol. 2017;7(1):99‐105. doi: 10.1002/alr.21831 [DOI] [PubMed] [Google Scholar]
- 36. Farber NI, Bavier RD, Crippen MM, Vatsa N, Hsueh WD, Eloy JA. Comparing endoscopic resection and open resection for management of sinonasal mucosal melanoma. Int Forum Allergy Rhinol. 2019;9(12):1492‐1498. doi: 10.1002/alr.22422 [DOI] [PubMed] [Google Scholar]
- 37. Janz TA, Graboyes EM, Nguyen SA, et al. A comparison of the NCDB and SEER database for research involving head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(2):284‐294. doi: 10.1177/0194599818792205 [DOI] [PubMed] [Google Scholar]
- 38. Patel EJ, Oliver JR, Vaezi A, et al. Primary surgical treatment in very advanced (T4b) Oral cavity squamous cell carcinomas. Otolaryngol Head Neck Surg. 2021;165(3):431‐437. doi: 10.1177/0194599820984358 [DOI] [PubMed] [Google Scholar]
- 39. Papazian MR, Chow M, Oliver J, et al. Surgical treatment in very advanced (T4b) adenoid cystic carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2023;168(6):1411‐1419. doi: 10.1002/ohn.203 [DOI] [PubMed] [Google Scholar]
- 40. Newman NB, Brett CL, Kluwe CA, et al. Immortal time bias in National Cancer Database Studies. Int J Radiat Oncol Biol Phys. 2020;106(1):5‐12. doi: 10.1016/j.ijrobp.2019.07.056 [DOI] [PubMed] [Google Scholar]
- 41. Divi V, Chen MM, Nussenbaum B, et al. Lymph node count from neck dissection predicts mortality in head and neck cancer. J Clin Oncol. 2016;34(32):3892‐3897. doi: 10.1200/JCO.2016.67.3863 [DOI] [PubMed] [Google Scholar]
- 42. Haimowitz S, Cohen DA, Dhanda A, Barron K, Povolotskiy R, Roden D. Mucosal melanoma of the Oral cavity: what is the role of elective neck dissection? Laryngoscope. 2023;133(2):317‐326. doi: 10.1002/lary.30152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Battaglia P, Turri‐Zanoni M, Dallan I, et al. Endoscopic endonasal transpterygoid transmaxillary approach to the infratemporal and upper parapharyngeal tumors. Otolaryngol Head Neck Surg. 2014;150(4):696‐702. doi: 10.1177/0194599813520290 [DOI] [PubMed] [Google Scholar]
- 44. Mattavelli D, Ferrari M, Bolzoni Villaret A, et al. Transnasal endoscopic surgery in selected nasal‐ethmoidal cancer with suspected brain invasion: indications, technique, and outcomes. Head Neck. 2019;41(6):1854‐1862. doi: 10.1002/hed.25621 [DOI] [PubMed] [Google Scholar]
- 45. Jansen EP, Keus RB, Hilgers FJ, Haas RL, Tan IB, Bartelink H. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys. 2000;48(1):27‐35. doi: 10.1016/s0360-3016(00)00594-0 [DOI] [PubMed] [Google Scholar]
- 46. Resto VA, Chan AW, Deschler DG, Lin DT. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2008;30(2):222‐229. doi: 10.1002/hed.20681 [DOI] [PubMed] [Google Scholar]
- 47. Kawashima M, Ogino T, Hayashi R, et al. Influence of postsurgical residual tumor volume on local control in radiotherapy for maxillary sinus cancer. Jpn J Clin Oncol. 2001;31(5):195‐202. doi: 10.1093/jjco/hye038 [DOI] [PubMed] [Google Scholar]
- 48. Farrell NF, Mace JC, Detwiller KY, et al. Predictors of survival outcomes in sinonasal squamous cell carcinoma: an analysis of the National Cancer Database. Int Forum Allergy Rhinol. 2021;11(6):1001‐1011. doi: 10.1002/alr.22737 [DOI] [PubMed] [Google Scholar]
- 49. Al‐Qurayshi Z, Smith R, Walsh JE. Sinonasal squamous cell carcinoma presentation and outcome: a national perspective. Ann Otol Rhinol Laryngol. 2020;129(11):1049‐1055. doi: 10.1177/0003489420929048 [DOI] [PubMed] [Google Scholar]
- 50. Toyomasu Y, Demizu Y, Matsuo Y, et al. Outcomes of patients with Sinonasal squamous cell carcinoma treated with particle therapy using protons or carbon ions. Int J Radiat Oncol Biol Phys. 2018;101(5):1096‐1103. doi: 10.1016/j.ijrobp.2018.04.041 [DOI] [PubMed] [Google Scholar]
- 51. Chopra S, Kamdar DP, Cohen DS, et al. Outcomes of nonsurgical management of locally advanced carcinomas of the sinonasal cavity. Laryngoscope. 2017;127(4):855‐861. doi: 10.1002/lary.26228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoppe BS, Nelson CJ, Gomez DR, et al. Unresectable carcinoma of the paranasal sinuses: outcomes and toxicities. Int J Radiat Oncol Biol Phys. 2008;72(3):763‐769. doi: 10.1016/j.ijrobp.2008.01.038 [DOI] [PubMed] [Google Scholar]
- 53. Chen NX, Chen L, Wang JL, et al. A clinical study of multimodal treatment for orbital organ preservation in locally advanced squamous cell carcinoma of the nasal cavity and paranasal sinus. Jpn J Clin Oncol. 2016;46(8):727‐734. doi: 10.1093/jjco/hyw064 [DOI] [PubMed] [Google Scholar]
- 54. Chen MM, Chang CM, Dermody S, et al. A consideration for surgical management in select T4b Oral cavity squamous cell carcinoma. Ann Otol Rhinol Laryngol. 2022;131(6):609‐616. doi: 10.1177/00034894211038213 [DOI] [PubMed] [Google Scholar]
- 55. Gangopadhyay A, Bhatt S, Nandy K, Rai S, Rathod P, Puj KS. Survival impact of surgical resection in locally advanced T4b Oral squamous cell carcinoma. Laryngoscope. 2021;131(7):E2266‐E2274. doi: 10.1002/lary.29394 [DOI] [PubMed] [Google Scholar]
- 56. Patel ZM, Li J, Chen AY, Ward KC. Determinants of racial differences in survival for sinonasal cancer. Laryngoscope. 2016;126(9):2022‐2028. doi: 10.1002/lary.25897 [DOI] [PubMed] [Google Scholar]
- 57. Nichols AC, Bhattacharyya N. Racial differences in stage and survival in head and neck squamous cell carcinoma. Laryngoscope. 2007;117(5):770‐775. doi: 10.1097/MLG.0b013e318033c800 [DOI] [PubMed] [Google Scholar]
- 58. Swegal W, Koyfman S, Scharpf J, et al. Endoscopic and open surgical approaches to locally advanced sinonasal melanoma: comparing the therapeutic benefits. JAMA Otolaryngol Head Neck Surg. 2014;140(9):840‐845. doi: 10.1001/jamaoto.2014.1321 [DOI] [PubMed] [Google Scholar]
- 59. Higgins TS, Thorp B, Rawlings BA, Han JK. Outcome results of endoscopic vs craniofacial resection of sinonasal malignancies: a systematic review and pooled‐data analysis. Int Forum Allergy Rhinol. 2011;1(4):255‐261. doi: 10.1002/alr.20051 [DOI] [PubMed] [Google Scholar]
- 60. Shipchandler TZ, Batra PS, Citardi MJ, Bolger WE, Lanza DC. Outcomes for endoscopic resection of sinonasal squamous cell carcinoma. Laryngoscope. 2005;115(11):1983‐1987. doi: 10.1097/01.mlg.0000178330.09881.6b [DOI] [PubMed] [Google Scholar]
- 61. Kılıç S, Kılıç SS, Baredes S, et al. Comparison of endoscopic and open resection of sinonasal squamous cell carcinoma: a propensity score‐matched analysis of 652 patients. Int Forum Allergy Rhinol. 2018;8(3):421‐434. doi: 10.1002/alr.22040 [DOI] [PubMed] [Google Scholar]
- 62. Eloy JA, Vivero RJ, Hoang K, et al. Comparison of transnasal endoscopic and open craniofacial resection for malignant tumors of the anterior skull base. Laryngoscope. 2009;119(5):834‐840. doi: 10.1002/lary.20186 [DOI] [PubMed] [Google Scholar]
- 63. Barinsky GL, Azmy MC, Kilic S, et al. Comparison of open and endoscopic approaches in the resection of Esthesioneuroblastoma. Ann Otol Rhinol Laryngol. 2021;130(2):136‐141. doi: 10.1177/0003489420939582 [DOI] [PubMed] [Google Scholar]
- 64. de Almeida JR, Su SY, Koutourousiou M, et al. Endonasal endoscopic surgery for squamous cell carcinoma of the sinonasal cavities and skull base: oncologic outcomes based on treatment strategy and tumor etiology. Head Neck. 2015;37(8):1163‐1169. doi: 10.1002/hed.23731 [DOI] [PubMed] [Google Scholar]
- 65. Khan MN, Konuthula N, Parasher A, et al. Treatment modalities in sinonasal undifferentiated carcinoma: an analysis from the national cancer database. Int Forum Allergy Rhinol. 2017;7(2):205‐210. doi: 10.1002/alr.21861 [DOI] [PubMed] [Google Scholar]
- 66. Nyirjesy SC, Fenberg R, Heller MA, et al. Response to induction chemotherapy in sinonasal malignancies: a single‐institutional experience. Head Neck. 2023;45(6):1445‐1454. doi: 10.1002/hed.27357 [DOI] [PubMed] [Google Scholar]
- 67. Kuo P, Torabi SJ, Kraus D, Judson BL. Survival outcomes for induction vs adjuvant chemotherapy in squamous cell carcinoma of the maxillary sinus. Otolaryngol Head Neck Surg. 2019;160(4):658‐663. doi: 10.1177/0194599818804777 [DOI] [PubMed] [Google Scholar]
- 68. Murr AT, Lenze NR, Weiss JM, et al. Sinonasal squamous cell carcinoma survival outcomes following induction chemotherapy vs standard of care therapy. Otolaryngol Head Neck Surg. 2022;167(5):846‐851. doi: 10.1177/01945998221083097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Amit M, Abdelmeguid AS, Watcherporn T, et al. Induction chemotherapy response as a guide for treatment optimization in Sinonasal undifferentiated carcinoma. J Clin Oncol. 2019;37(6):504‐512. doi: 10.1200/JCO.18.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Z, Qu Y, Wang K, et al. The value of preoperative radiotherapy in the treatment of locally advanced nasal cavity and paranasal sinus squamous cell carcinoma: a single institutional experience. Oral Oncol. 2020;101:104512. doi: 10.1016/j.oraloncology.2019.104512 [DOI] [PubMed] [Google Scholar]
- 71. Hirakawa H, Hanai N, Ozawa T, et al. Prognostic impact of pathological response to neoadjuvant chemotherapy followed by definitive surgery in sinonasal squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1305‐E1311. doi: 10.1002/hed.24217 [DOI] [PubMed] [Google Scholar]
- 72. Patel AM, Haleem A, Choudhry HS, Brant JA, Brody RM, Carey RM. Surgical resection improves overall survival in cT4b major salivary gland cancer. Otolaryngol Head Neck Surg. 2024;170(5):1349‐1363. doi: 10.1002/ohn.686 [DOI] [PubMed] [Google Scholar]
- 73. Liao CT, Chang JTC, Wang HM, et al. Surgical outcome of T4a and resected T4b oral cavity cancer. Cancer. 2006;107(2):337‐344. doi: 10.1002/cncr.21984 [DOI] [PubMed] [Google Scholar]
- 74. Liao CT, Lee LY, Hsueh C, et al. Comparative outcomes in oral cavity cancer with resected pT4a and pT4b. Oral Oncol. 2013;49(3):230‐236. doi: 10.1016/j.oraloncology.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 75. Mair MD, Sawarkar N, Nikam S, et al. Impact of radical treatments on survival in locally advanced T4a and T4b buccal mucosa cancers: selected surgically treated T4b cancers have similar control rates as T4a. Oral Oncol. 2018;82:17‐22. doi: 10.1016/j.oraloncology.2018.04.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 5‐year OS among 629 cT4b tumors undergoing definitive treatment with stratification by histology (p <.001).
Figure S2. 5‐year OS among 503 cT4a and 492 cT4b tumors undergoing surgical resection + RT/CRT (p = 0.097). Significance derived from the log‐rank test. CRT, chemoradiotherapy; cT, clinical tumor; OS, overall survival; radiotherapy, RT.
Table S1. Histology, behavior, and topography codes included, n (%).
Table S2. Univariable and multivariable Cox proportional hazard regression models of 5‐year OS among 513 cT4b tumors undergoing surgical resection + additional therapy.
Table S3. Patient demographics and clinicopathologic features among 503 cT4a tumors and 492 cT4b tumors undergoing surgical resection + RT/CRT, n (%).
Table S4. Univariable and multivariable Cox proportional hazard regression models of 5‐year OS among 503 cT4a tumors and 492 cT4b tumors undergoing surgical resection + RT/CRT.


