Abstract
Hippocampal volume is a marker of brain health and is reduced with aging and neurological disease. Exercise may be effective at increasing and preserving hippocampal volume, potentially serving as a treatment for conditions associated with hippocampal atrophy (e.g., dementia). This meta-analysis aimed to identify whether exercise training has a positive effect on hippocampal volume and how population characteristics and exercise parameters moderate this effect. Studies met the following criteria: 1) controlled trials; 2) interventions of physical exercise; 3) included at least one time-point of hippocampal volume data before the intervention and one after; 4) assessed hippocampal volume using either manual or automated segmentation algorithms. Animal studies, voxel-based morphometry analyses, and multi-modal interventions (e.g., cognitive training or meditation) were excluded. The primary analysis in n = 23 interventions from 22 published studies revealed a significant positive effect of exercise on total hippocampal volume. The overall effect was significant in older samples (65 years of age or older) and in interventions that lasted over 24 weeks and had less than 150 minutes per week of exercise. These findings suggest that moderate amounts of exercise for interventions greater than 6 months have a positive effect on hippocampal volume including in older populations vulnerable to hippocampal atrophy.
Keywords: Exercise, physical activity, hippocampus, aging, magnetic resonance imaging
1.1. Introduction
The hippocampus plays a critical role in memory formation (Davachi & Wagner, 2002; Eichenbaum, Otto, & Cohen, 1992; Squire, 1981; Tambini, Nee, & D’Esposito, 2018) and is reduced in volume in late adulthood (Ward et al., 2015) and in several neurologic (Wolk, Das, Mueller, Weiner, & Yushkevich, 2017) and psychiatric (Walter et al., 2016) conditions. For example, late life hippocampal atrophy predicts memory decline and conversion to dementia (den Heijer et al., 2011; Erickson, Prakash, et al., 2010). Moreover, hippocampal volume is often reduced in psychiatric patient populations, including Major Depressive Disorder (Malykhin & Coupland, 2015) and Schizophrenia (Shepherd, Laurens, Matheson, Carr, & Green, 2012). Accordingly, the hippocampus has emerged as an important brain structure targeted by clinical trials to determine if volume losses can be prevented or reversed. These hypotheses are based on the premise that the hippocampus appears to be highly modifiable and “plastic” in that it is selectively responsive to different environmental stimuli (Churchill et al., 2002; Toda & Gage, 2018). Exercise is one of the most promising treatments for influencing hippocampal volume. In rodents, exercise increases cell proliferation in both younger and older animals, particularly in the dentate gyrus (Cahill et al., 2015; Klein et al., 2017; Van Praag, Kempermann, & Gage, 1999; Yuede et al., 2009), and these effects occur independently of environmental enrichment and social interaction (Van Praag et al., 1999). Exercise may also induce macroscale changes, such as synaptogenesis, altered neuronal morphology, and angiogenesis (Vivar, Potter, & van Praag, 2013) and induce biochemical changes, including upregulation of key neurotrophins and growth factors, such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 (IGF-1), as well as reduce oxidative stress and inflammation (Bianchi, Locatelli, & Rizzi, 2017; Ross, Lithgow, Hayes, & Florida-James, 2019; Stillman, Cohen, Lehman, & Erickson, 2016). In humans, one year of aerobic exercise led to a 2% increase in hippocampal volume (Erickson et al., 2011), with other studies showing similar positive effects (Pajonk et al., 2010; Teixeira et al., 2018). Thus, exercise may be a low-cost and highly accessible approach to preserve hippocampal volume across the lifespan (Firth et al., 2018).
Nonetheless, the enhancing effects of exercise on hippocampal volume have not been consistent across published studies (Krogh et al., 2014; Maass et al., 2015; Scheewe et al., 2013). This variability raises questions about the clinical utility of exercise as an effective method for increasing hippocampal volume. There are many reasons that some studies may find positive effects of exercise and other studies fail to replicate. These discrepancies could be explained by variability in the sample characteristics (e.g., age and clinical diagnosis) or the parameters of the exercise intervention, such as the intensity, frequency, duration, or modality, such as aerobic or resistance training. Study quality characteristics may further explain variability, such as statistical power, blinding, adherence and compliance to the intervention, handling of missing data, and whether factors such as cardiorespiratory fitness were modified. A meta-analysis can overcome some of these limitations by examining the effect of exercise on hippocampal volume across studies and as a function of these potential moderating factors.
We had two main aims in this meta-analysis. First, we sought to determine whether exercise training has a positive effect on hippocampal volume relative to control interventions. Second, we assessed potential moderators by stratifying by age, patient status, intervention duration, and minutes per week of exercise to determine whether these factors account for heterogeneity in the literature. We hypothesized that the effects of exercise would be strongest in older and patient samples, and that effects would be strongest for interventions that were longer in duration and had more minutes of exercise each week. Both aerobic and resistance forms of exercise were included in this meta-analysis because aerobic and resistance training are combined in many studies. Although aerobic and resistance training may preferentially affect distinct molecular pathways in the brain (Cassilhas et al., 2012) both forms of exercise have been shown to increase neurotrophic factors that likely mediate effects on hippocampal volume (Cassilhas, Tufik, & de Mello, 2016; Liu-Ambrose, Barha, & Best, 2018; Tsai, Pai, Ukropec, & Ukropcová, 2019). The current meta-analysis extends the work of a prior meta-analysis of exercise and hippocampal volume (Firth et al., 2018) by assessing a larger number of studies, testing effects of intervention parameters, and by assessing effects of purely exercise-specific interventions.
2.1. Materials and Methods
2.1.1. Review Protocol
The aims, search terms, and approach for this meta-analysis were registered in PROSPERO before any analyses were completed (Identifier: CRD42018103690).
2.1.2. Literature Search
Literature searches were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, & Altman, 2009) in the electronic databases PubMed, EMBASE, and Cochrane (John Wiley & Sons) Central Register of Controlled Trials (CENTRAL) from their inception until January 28, 2020. The search string was developed for PubMed in close collaboration with a health sciences librarian (J.E.F.) and translated afterward for use in EMBASE and Cochrane Central. All languages were included. Reference lists from included articles were examined to identify other relevant manuscripts. All database search strings contained both controlled vocabulary and keywords representing the concepts of hippocampus and exercise (Supplementary Material).
2.1.3. Study Selection
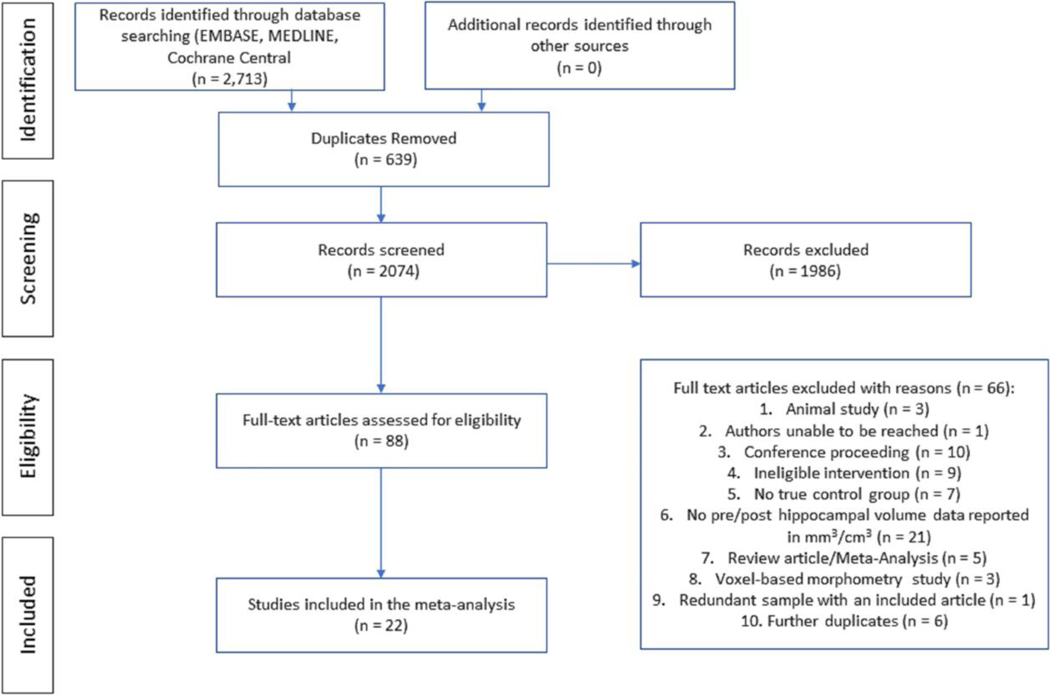
Studies included met the following criteria: 1) controlled trials; 2) use of exercise or physical activity as a method for potentially influencing brain morphology; 3) inclusion of at least two time-points of hippocampal volume data in mm3 or cm3, one pre-intervention and one post- intervention, using automated or manual segmentation methods. Studies were included if they used any exercise or physical activity intervention, such as walking, running, dancing, swimming, cycling, and strength or resistance training. Balance training treatment interventions were excluded due to balance training being commonly used as a control intervention to other exercise interventions. Animal studies and interventions with a multi-modal component (e.g., cognitive training or meditation) were excluded. The outcomes of interest were hippocampal volume at baseline and post-intervention, and whether effects differed by intervention and control groups. Figure 1 displays a flow chart of the study selection process.
Figure 1.
Study Selection flow chart. The 66 studies excluded are listed with reasons for exclusion.
No restrictions were placed on participant characteristics. Studies were excluded if they were limited to the following: 1) cross-sectional design; 2) intervention without a control condition; 3) voxel-based morphometry (VBM) studies because volume estimates in mm3 are not an outcome from VBM. Studies comparing aerobic exercise with resistance exercise with no control group were excluded. For studies with a cross-over design where one group participated in the intervention first, followed by the control group, the data from the initial group assignment was used for the analysis.
2.1.4. Quality Assessment
We followed PRISMA guidelines (Moher et al., 2009) and evaluated the methodological quality of included trials according to Cochrane’s Methods Bias Tool (Green & Higgins, 2005). Using the Cochrane criteria, two members of the research team (K.A.W., A.M.W., C.M.S., J.C.P., or K.I.E.) independently assessed risk of bias and overall study quality for each study. Any discrepancies in bias ratings were resolved by a discussion between the reviewing study team members. The Cochrane domains of bias and quality were used to rate each study as low risk of bias, high risk of bias, or unclear (insufficient detail or not reported) according to Section 8.5 of the Cochrane handbook (Green & Higgins, 2005). All studies that met eligibility requirements were included in the analysis, regardless of quality. This approach further allows the current analysis to bring attention to discrepancies in reporting and biases that may lead to unusual or inconsistent results. To assess the possibility of publication bias, a precision funnel by Hedges’ g funnel plot was examined and a trim and fill analysis was conducted to adjust for potential missing studies (Supplementary Materials). Publication bias was further tested using Egger’s regression (Egger, Smith, Schneider, & Minder, 1997) and fail-safe n analysis (Rosenthal, 1979).
2.1.5. Data Extraction
The principal measure was hippocampal volume with means and standard deviations in mm3 or cm3. The means and standard deviations for hippocampal volumes for treatment and control groups were extracted before and after the intervention for overall hippocampal volume as well as left and right hippocampal volumes. Additional variables extracted were the study authors, date, sample sizes with baseline MRI in the treatment and control groups, mean baseline age of the study participants, population type (i.e., healthy or patients), number of weeks of exercise, number of minutes per week, type of intervention control condition, segmentation method, MR magnet field strength, conflicts of interest disclosure, type of exercise (i.e., aerobic, resistance, or both), intensity of exercise (i.e., light, moderate, vigorous), compliance (% sessions attended among the treatment and control groups), adherence rate, whether the intervention was supervised, and percent men or women. Data not available from the published article were requested directly from the authors by K.I.E., K.A.W., and C.M.S. Data were double entered into an electronic (Excel) database by two members of the study team for each study (K.A.W., C.M.S., A.M.W., R.L.L., and J.C.P.). Upon compilation of the complete dataset, accuracy of data extraction was assessed by K.A.W. Discrepancies were resolved by C.M.S. and K.A.W. The dataset will be submitted to a public repository at the time of publication (https://nda.nih.gov/).
2.1.6. Calculation of Effect Sizes
Data were analyzed using Comprehensive Meta-Analysis Software, Version 3 (Borenstein, Rothstein, & Cohen, 2005). Intervention effects were represented by Hedges’ g, a bias-adjusted estimate of the standardized mean difference that applies an additional correction for small sample sizes. Effect sizes were interpreted as small (≤0.2), moderate (0.2–0.8), or large (≥ 0.8) (Cohen, 1988). Weighted mean effect size values, along with standard deviations and 95% confidence intervals (CI), were estimated using a random-effects model. Baseline sample sizes were used in analyses. Data analyzed with the intent-to-treat framework were used where available. Positive effect sizes indicate a positive Hedges’ g for hippocampal volume among the exercise groups relative to controls. We also conducted a paired t-test sensitivity meta-analysis to test whether the effects of exercise were significant when performed separately in the treatment group and in the control group to determine if effects of exercise on the hippocampus are driven by a significant increase in treatment groups or a significant decrease in control groups.
In instances where a study had two intervention groups with different exercise types, e.g., aerobic exercise and resistance exercise (Ehlers et al., 2017; Niemann, Godde, & Voelcker-Rehage, 2014; ten Brinke et al., 2015), we combined the mean and standard deviation of the two intervention groups to form one group by calculating the weighted mean and unbiased pooled standard deviation using the R package psychmeta (see Supplemental Material). This was done to avoid using the same control group data twice in the meta-analysis. In instances where the total hippocampal volume needed to be combined from the left and right hippocampal volumes, the standard deviation was calculated by taking the square root of the sum of the variances for left and right hippocampal volumes.
For our primary analysis, we performed the meta-analysis across all studies to optimize statistical power. Statistical heterogeneity was then examined using the I2 statistic. I2 of more than 75% or between 50%–75% indicates substantial and moderate heterogeneity, respectively. An I2 of less than 40% indicates limited heterogeneity.
2.1.7. Stratification Analyses
Given the wide range of age, population type, and characteristics of the interventions, we conducted analyses in which we stratified the data based on these factors. Studies were stratified by the National Institutes of Health (NIH) definition for “older adult” https://grants.nih.gov/grants/glossary.htm (< 65 or ≥ 65 years of age) which clearly delineated studies of young-middle age adults (mean ages 12–41) from studies of older adults (mean ages 65–76). Note that Tarumi (2019) (mean age 64.7) was included in the “older” group. Analyses were also stratified by patient status (healthy participants or patients), exercise intervention duration of ≤ or > 24 weeks (based on the distribution of intervention weeks across studies and because it has been proposed that interventions lasting between 24–52 weeks are required to reliably elicit changes in brain structure and cognition (Baker et al., 2010; Colcombe et al., 2006; Gregory, Parker, & Thompson, 2012), and minutes per week of exercise < 150 minutes or ≥ 150 minutes based on recommended minimum minutes of moderate-to-vigorous exercise per week (Health & Services, 2018). Exercise intensity was not distinguishable between studies, so intensity was not assessed as a potential moderator.
2.1.8. Sensitivity Analyses
We conducted post-hoc analyses to address whether a specific study or set of studies were influential in the hypothesized effects of exercise on hippocampal volume. To address this, we performed a leave-one-out analysis where the effect of exercise on hippocampal volume was re-tested leaving out each study sequentially for analyses with total, left, and right hippocampus.
3.1. Results
3.1.1. Characteristics of Studies Included
The search included 2713 potentially relevant studies, which was narrowed based on title and abstract to 88 records for which the full text was accessed. Of these, 22 studies consisting of 23 controlled trials met eligibility criteria, representing data from 1204 participants. The study selection process, including reasons for exclusion at the full-text level, are listed in the PRISMA study flow chart (Figure 1). Studies were published between 2010 and 2020. Participant characteristics of included studies are displayed in Table 1 and intervention characteristics are displayed in Table 2. The total study sample sizes across exercise and control interventions at baseline ranged from 16 to 120 participants and the weighted average age was 58.7 years (range = 11.60 – 76.07 years). Approximately 56% of participants were female. Participant pools were heterogeneous with regard to age and mental and physical health status. Population types were healthy controls and patient groups that have been shown in prior meta-analyses and multiple studies to display reduced overall hippocampal volume: healthy older adults (Fraser, Shaw, & Cherbuin, 2015), mild cognitive impairment (MCI)/early Alzheimer’s disease (AD) (Shi, Liu, Zhou, Yu, & Jiang, 2009), depression (Videbech & Ravnkilde, 2004), obesity/Type II diabetes (Araki et al., 1994; Cherbuin, Sargent-Cox, Fraser, Sachdev, & Anstey, 2015), schizophrenia/psychosis (Nelson, Saykin, Flashman, & Riordan, 1998), and radiation treated children (Nieman et al., 2015). Studies were included in the “healthy” group if they were comprised of community-dwelling adults excluded for major neurologic, psychiatric, or cognitive disorders.
Table 1.
Participant and Study Characteristics.
| Reference | Year | Control Sample Size | Exercise Sample Size | Average Age | % Female | Population | Segmentation |
|---|---|---|---|---|---|---|---|
| Broadhouse (2020) | 2020 | 24 | 19 | 69.5 | 69 | MCI | Freesurfer |
| Callisaya (2017) | 2017 | 24 | 26 | 66.2 | 48 | Obese & Type II Diabetes | FIRST |
| Ehlers (2017) | 2017 | 48 | 45 | 65.4 | 68.4 | Healthy | Freesurfer |
| Erickson (2011) | 2011 | 60 | 60 | 66.6 | 66.5 | Healthy | FIRST |
| Frederiksen (2018) | 2018 | 35 | 36 | 68.8 | 41 | AD | Freesurfer |
| Jonasson (2017) | 2017 | 29 | 29 | 68.9 | 55 | Healthy | Freesurfer |
| Kleemeyer (2016) | 2016 | 27 | 25 | 66 | 62 | Healthy | Freesurfer |
| Krogh (2014) | 2014 | 38 | 41 | 41.3 | 67 | Depressed | Manual |
| Lin (2015) | 2015 | 20 | 28 | 24.6 | 100 | Psychosis | Freesurfer |
| Maass (2015) | 2014 | 17 | 15 | 68.4 | 55 | Healthy | Manual |
| Morris (2017) | 2017 | 28 | 31 | 72.9 | 51 | Early AD | Freesurfer |
| Niemann (2015) | 2014 | 13 | 17 | 68.9 | 65 | Healthy | Manual |
| Pajonk (2010) | 2010 | 8 | 8 | 36.1 | 0 | Schizophrenia | Manual |
| Riggs (2017) | 2017 | 12 | 16 | 11.6 | 42 | Radiation Treated | Manual |
| Rosano (2017) | 2017 | 16 | 10 | 76.1 | 81 | Healthy | FIRST |
| Schewee (2013) | 2013 | 27 | 25 | 28.9 | 31 | Healthy | FIRST |
| Scheewe (2013) | 2013 | 14 | 18 | 29.6 | 19 | Schizophrenia | FIRST |
| Tarumi (2019) | 2019 | 32 | 29 | 64.7 | 21.5 | Amnesic MCI | Freesurfer |
| Teixeira (2018) | 2018 | 22 | 22 | 69.2 | 60.5 | Amnesic MCI | Freesurfer |
| ten Brinke (2015) | 2015 | 13 | 26 | 75.2 | 100 | MCI | FIRST |
| Thomas (2016) | 2016 | 24 | 30 | 33.7 | 56 | Healthy | Freesurfer |
| Venkatraman (2020) | 2019 | 48 | 50 | 72.5 | 56 | At risk for cognitive decline | Freesurfer |
| Wagner (2015) | 2015 | 17 | 17 | 24.4 | 0 | Healthy | Freesurfer |
Sample sizes reflect number of participants contributing to baseline means and standard deviations. AD = Alzheimer’s disease, MCI = mild cognitive impairment. Kleemeyer et al. (2016) also reported analyses with FIRST as a cross-validation of the primary (Freesurfer) analyses. Freesurfer results are reported here.
Table 2.
Exercise Intervention Characteristics.
| Reference | Type of Exercise | Mins of Exercise over total intervention | Weeks of Exercise | Mins per Week | Compliance (% of sessions attended) | Adherence (% completion rate) |
|---|---|---|---|---|---|---|
| Broadhouse(2020) | Resistance | 5850 | 26 | 225 | NR | NR |
| Callisaya (2017) | Aerobic with resistance† | 4320 | 26 | 180 | 79% | 79% exercise 75% control |
| Ehlers (2017) | Aerobic | 4320 | 26 | 180 | 73% | NR |
| Erickson (2011) | Aerobic | 5925 | 52 | 90 | 79% | 81% |
| Frederiksen (2018) | Aerobic | 2880 | 16 | 180 | NR | NR |
| Jonasson (2017) | Aerobic | 3240 | 24 | 135 | 83.62% | 97% |
| Kleemeyer (2016) | Aerobic* | 4290 | 24 | 165 | 92–94% | 91% |
| Krogh (2014) | Aerobic* | 1620 | 12 | 135 | 35% | 70% |
| Lin (2015) | Aerobic | 2160 | 12 | 180 | NR | 47% exercise 58 control |
| Maass (2015) | Aerobic | 1080 | 12 | 90 | NR | 100% |
| Morris (2017) | Aerobic† | 3900 | 26 | 150 | 79.9–85% | 89% |
| Niemann (2015) | Aerobic Group | 8190 | 52 | 157.5 | NR | NR |
| Pajonk (2010) | Aerobic | 1080 | 12 | 90 | 86% | 67% |
| Riggs (2017) | Aerobic | 3240 | 12 | 270 | 84% | 91% |
| Rosano (2017) | Aerobic with resistance | 14400 | 96 | 150 | 66.74–90.6% | 100% |
| Scheewe (2013) Scz | Aerobic with resistance | 3120 | 26 | 120 | NR | 81% |
| Scheewe (2013) Healthy | Aerobic with resistance | 3120 | 26 | 120 | NR | 96% |
| Tarumi (2019) | Aerobic | 5460 | 52 | 105 | 69% | NR |
| Teixeira (2018) | Aerobic and Resistance | 3120 | 26 | 120 | NR | NR |
| ten Brinke (2015) | Aerobic Group & Resistance Group | 3120 | 26 | 120 | 54–60% | 74% |
| Thomas (2016) | Aerobic | 900 | 6 | 150 | 97% | 96% |
| Venkatraman (2020) | Aerobic | 16200 | 96 | 150 | NR | 79% |
| Wagner (2015) | Aerobic* | 1080 | 6 | 180 | NR | 100% |
Denotes mixed supervised/unsupervised protocol. * Denotes high intensity, all other studies used moderate-high intensity. Scz = schizophrenia.
Twenty-one of 22 studies included aerobic exercise with stationary bicycles, walking, or dance. Six aerobic interventions also included a non-aerobic exercise component, using resistance or coordination training. Four of these studies mixed the two exercise training types and two of these studies had an aerobic-only arm. Fifteen trials consisted of aerobic only training. One study consisted of resistance only training. Control conditions included life as usual (n = 5), waitlist control (n = 1), stretching, balance, relaxation, and gentle movement (n= 11), occupational therapy (n = 1), table football (n =1), health education (n = 2), and low intensity exercise (n = 1). One study (Scheewe et al., 2013) had two separate interventions and matched control groups (schizophrenia and healthy), which were represented separately in the present meta-analysis (see Table 2 for details).
All studies used a T1-weighted structural image to collect hippocampal volume data. Two studies (Maass et al., 2015; Rosano et al., 2017) used 7T scanners. Pajonk et al. (2010) used a 1.5T scanner and Riggs et al. (2017) used both 3T and 1.5T scanners. All other studies used 3T imaging for all participants. Segmentation methods (manual or automated) are presented with study characteristics in Table 1.
Two studies (Lin et al., 2015; Venkatraman et al., 2020) disclosed potential conflicts of interest that did not appear to be related to the study or a potential source of bias in the results. Hippocampal data separated by left and right hemisphere were unavailable for Scheewe et al. (2013) (two interventions) and Pajonk et al. (2010). Thus, left and right hippocampal volumes were analyzed for 20 of the 23 studies.
3.1.2. Quality Assessment
In total, six of the 22 studies were of high methodological quality because they scored positively on all six of the quality criteria (Table 3). Given the small number of studies eligible for this meta-analysis, the results below include all studies, regardless of their rated quality.
Table 3.
Cochrane Bias Ratings. Sequence generation refers to the randomization approach. Allocation concealment refers to whether the investigators could be aware of what group potential participants would be allocated to. Blinding participants refers to whether the participants were blinded and if this poses a risk for the outcome. Blinding assessors refers to whether the investigator was blinded and if this poses a risk for the outcome. Incomplete outcome data refers to whether a large percentage of participants/scans were excluded or not completed. Difference between groups refers to whether attrition was reported as statistically different between the treatment and control groups. For both blinding variables, we considered risk low because the outcome was hippocampal volume, which is unlikely to be influenced by participant or assessor bias.
| References | Sequence Generation | Allocation Concealment | Blinding Participants | Blinding Assessors | Incomplete Outcome Data | Difference Between Groups |
|---|---|---|---|---|---|---|
| Broadhouse(2020) | L | L | L | L | L | NR |
| Callisaya (2017) | L | L | L | L | L | L |
| Ehlers (2017) | L | L | L | NR | L | L |
| Erickson (2011) | L | L | L | L | NR | L |
| Frederiksen (2018) | L | L | L | L | H | NR |
| Jonasson (2017) | L | L | NR | NR | L | L |
| Kleemeyer (2016) | L | L | L | NR | NR | L |
| Krogh (2014) | L | L | L | L | L | H |
| Lin (2015) | L | L | L | L | L | L |
| Maass (2015) | H | NR | L | NR | L | L |
| Morris (2017) | L | L | L | L | L | L |
| Niemann (2015) | L | L | L | L | H | H |
| Pajonk (2010) | L | L | L | L | L | L |
| Riggs (2017) | L | L | L | NR | L | L |
| Rosano (2017) | L | L | NR | NR | H | L |
| Scheewe (2013) | L | L | L | L | H | H |
| Tarumi (2019) | L | L | L | L | H | L |
| Teixeira (2018) | H | L | L | NR | L | L |
| ten Brinke (2015) | L | L | L | L | L | L |
| Thomas (2016) | L | L | L | NR | L | L |
| Venkatraman (2020) | L | L | L | L | L | L |
| Wagner (2015) | H | L | L | L | L | L |
H = high risk, L = low risk, NR = not reported; Reasons for high risk of bias ratings. Sequence generation: Maass: “pseudo randomization”; Teixeira: non-randomized; Wagner: Participants “assigned”; does not report “randomization”. Incomplete outcome data: Niemann: high number of participants excluded; Rosano: Participants in the exercise group attended a median of 66.74% of the max number of 187 sessions, participants in the control group attended 90.6% of the max number of 39 sessions.; Frederiksen & Tarumi: High attrition across both groups. Scheewe: High attrition, participants who did not comply at least 50% were excluded. Difference between groups: Krogh: large differences in participation between the exercise (80%) and control (58%) groups; Niemann: did not report group differences in exclusion. Scheewe: more drop out in the control group than the exercise group.
3.1.3. Effects of Exercise on Hippocampal Volume.
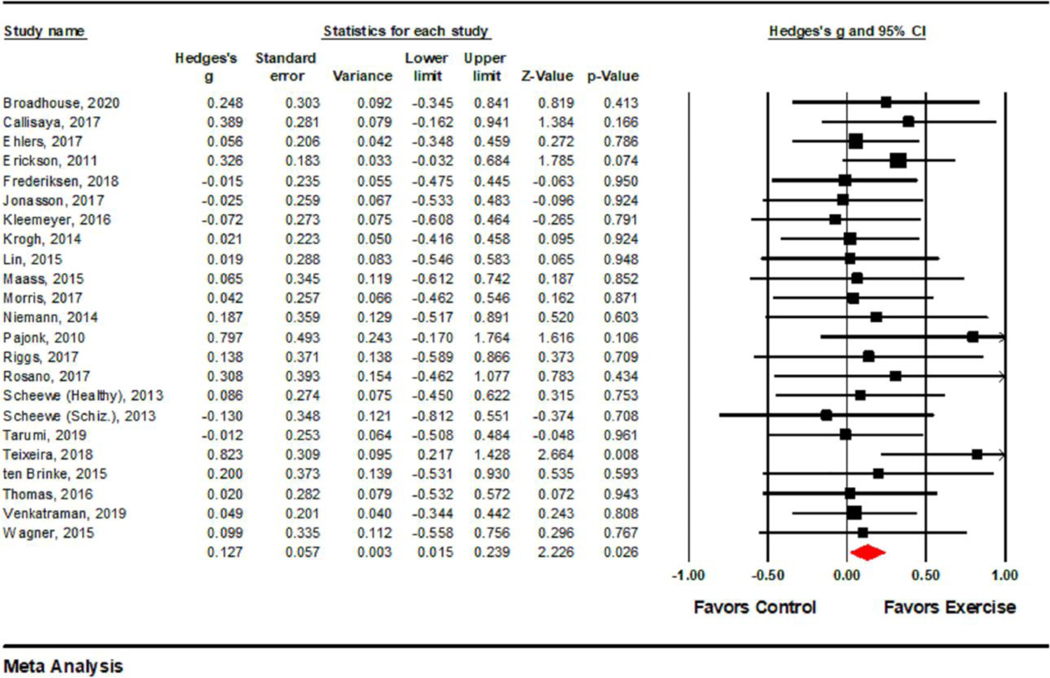
A summary of the effects of exercise on hippocampal volume are presented in Table 4. Overall, there was a significant main effect of intervention on total hippocampal volume with a significant positive effect for exercise relative to controls, Hedges’ g [CI] = 0.13, [0.02, 0.24], p = .026 (Figure 2). When removing each study in turn with a leave-one-out cross-validation analysis, all Hedges’ g’s > 0.103, p’s < .079. Removal of each study except for the Erickson et al. (2011) and Teixeira et al. (2018) studies showed significant overall Hedges’ g’s, all p’s < .05. Post-hoc tests performed separately for exercise and control groups revealed that this overall effect was driven by a significant decrease in hippocampal volume (−0.72%) in the control group, Hedges’ g = −0.077, p = .030, whereas the increase in hippocampal volume (1.2%) across treatment groups was not significant, Hedges’ g = 0.042, p = .300.
Table 4:
Summary of Hedges’ g’s for effects of exercise on hippocampal volume. Significant effects are denoted in bold font.
| Total | Left | Right | |
|---|---|---|---|
| Overall | 0.13 | 0.09 | 0.1 |
| <65 | 0.07 | ||
| ≥ 65 | .15 | ||
| Healthy | 0.12 | ||
| Patient | 0.14 | ||
| ≤ 24 weeks | 0.07 | ||
| > 24 weeks | .16 | ||
| < 150 mins | .18 | ||
| ≥150 mins | 0.1 |
Figure 2.
Forest plot showing individual study and pooled effects of exercise on total hippocampal volume. CI = confidence interval. Positive values of Hedges’ g reflect increases in hippocampal volume among those in the exercise group relative to the control group. Random effects model statistics are reported in the last row. The CI does not include zero, indicating significance.
When analyzing each hemisphere separately in the 20 interventions with available left and right hippocampal data, we found that neither individual hemisphere reached significance (Supplementary Materials): Left hemisphere, Hedges’ g [CI] = 0.09 [−0.03, 0.21], p = .127. Right hippocampus, Hedges’ g [CI] = 0.10 [−0.01, 0.22], p = .083. A leave-one-out analysis showed no changes in the non-significance of these effects in either hemisphere, left p’s = .097-.244, and right p’s = .065-.166.
For total hippocampus, there was some evidence of publication bias. A funnel plot of precision by Hedges’ g suggested two potential outliers to the right of the mean (Supplemental Material). A trim and fill analysis for total hippocampus suggested there were no studies missing to the left of the mean (studies showing a negative effect) and two studies were missing to the right of the mean (studies showing a small positive effect), with a significant adjusted Hedges’ g = 0.14, [CI] = [0.04 – 0.25]. Kendall’s tau analysis showed evidence of publication bias (tau = 0.32, p = .032), but Egger’s regression did not detect this bias (t(21) = 1.15, p = .262). A fail-safe n analysis revealed that 11 additional missing studies would need to be added for the p-value for the detected effect to surpass an alpha level of .05.
There was no evidence of heterogeneity (I2 = 0%, tau squared = 0, standard error = 0.023). However, due to the diversity of samples and characteristics across included studies, we proceeded to conduct our planned stratification analyses.
3.1.4. Stratification Analyses
We first conducted an analysis stratified by age (< 65 or ≥ 65 years of age) across studies. Analysis of these subgroups revealed a significant effect of exercise on total hippocampal volume in studies with average age greater than or equal to 65 (n = 15), Hedges’ g [CI] = 0.15, [0.02, 0.28], p = .027, but a non-significant effect in studies with average age less than 65 (n = 8), Hedges’ g [CI] = 0.07 [−0.14, 0.28], p = .509.
We next stratified analyses by patient status (healthy or patients). This analysis revealed a marginal effect in patients (n = 13), Hedges’ g [CI] = 0.14, [−0.01, 0.29], p = .076, and a non-significant effect in healthy samples (n = 10), Hedges’ g [CI] = 0.12 [−0.05, 0.28] , p = .175.
Stratifying the interventions by the number of weeks of exercise revealed a significant effect for studies with interventions lasting longer than 24 weeks (n = 14), Hedges’ g [CI] = 0.16 [0.02, 0.31) p = .024, but no significant effect with interventions lasting less than or equal to 24 weeks (n = 9), Hedges’ g [CI] = 0.07 [−0.12, 0.25) p = .475.
Analyses stratified by minutes of exercise per week revealed a significant effect on hippocampal volume for interventions providing less than 150 minutes of exercise per week (n = 10), Hedges’ g [CI] = 0.18, [0.004, 0.35], p = .046. The effect was not significant for interventions providing 150 minutes per week or more (n = 13), Hedges’ g = 0.10, [−.06, 0.24], p = .244.
4.1. Discussion
The present meta-analysis demonstrates that exercise has a significant positive effect on total hippocampal volume. This finding was significant when collapsed across a variety of populations and methods used. Follow-up analyses demonstrated a significant decrease of 0.72% in volume in the controls and a non-significant increase of 1.2% in volume in the exercise group, suggesting that exercise may prevent hippocampal atrophy. Differences in hippocampal volume in this range likely have behavioral and clinical relevance: Similar degrees of volume changes are associated with moderate to large effect sizes of improved memory and executive function in many of the interventions examined here (Callisaya et al., 2017; Erickson et al., 2011; Jonasson et al., 2017; Krogh et al., 2014; Lin et al., 2015; Maass et al., 2015; Riggs et al., 2017), as well as other exercise intervention studies assessing cognition (Colcombe & Kramer, 2003; Fabre, Chamari, Mucci, Masse-Biron, & Prefaut, 2002; Oberlin et al., 2017). Further, volume changes of this degree are within the range of typical annual age-related hippocampal atrophy (0.8%−1.7% (Jack et al., 2000; Jack et al., 1997; Raz et al., 2005)) and disease-related hippocampal volume loss (0.4% (Arnold et al., 2015; Nelson et al., 1998)). These details are critical since it suggests that even brief (i.e., less than 1 year) exercise interventions might be capable of preventing annual age- or disease-related hippocampal volume loss, and these effects may have downstream cognitive consequences.
Analyses stratified by population moderators revealed that the effects on total hippocampal volume were significant for older samples. This finding demonstrates that exercise is an effective intervention to increase hippocampal volume in older populations that have a critical need for preserving hippocampal volume. Cardiorespiratory fitness may be lower in older adults. Thus, it is possible that the effect of age is influenced by change in cardiorespiratory fitness. Future work may examine whether greater changes in cardiorespiratory fitness partly mediate changes in hippocampal volume. Further, it is important to note that the average intervention duration in the studies with younger participants was 14 weeks, compared to 39 weeks in studies with participants 65 years of age or older, confounding age with intervention duration. This confound, however, does not undermine the promising finding that older populations show a significant positive effect on hippocampal volume with exercise. Longer duration interventions in younger populations are needed to elucidate the efficacy of exercise to induce hippocampal changes in this age range. Analyses stratified by patient status showed no significant effects for healthy or patient only interventions. Although the effect in patients was not statistically reliable, perhaps as a function of the heterogeneity of the pooled sample, the effect size suggests that more studies in clinical populations with hippocampal volume losses are warranted to determine the utility of reversing deficits, potentially with longer intervention durations.
Analyses stratified by intervention duration showed significant effects of exercise on hippocampal volume for interventions lasting greater than 24 weeks, but not for interventions less than or equal to 24 weeks in duration. This indicates the importance of using a longer intervention to maximize effects on hippocampal volume. What remains unclear is how the effects of exercise on hippocampal volume change across intervention durations greater than 24 weeks. This will be a future direction as the literature on exercise interventions and hippocampal volume grows. Longer-duration interventions may be more effective for several reasons. For instance, repeated triggering of the biochemical cascade associated with exercise may be required before consistent macroscale effects on hippocampal volume can be observed. Additionally, increases in hippocampal volume may depend on an increase in physical or cardiovascular fitness, which improves with longer exercise interventions (Huang, Gibson, Tran, & Osness, 2005). Improvements in fitness may increase the intensity of exercise within an individual over the course of the intervention and increase the extent of biochemical changes induced by exercise over the course of a longer intervention. Given that the effects were not significant in interventions up to 24 weeks, these findings provide much-needed, substantive guidance on the design of exercise interventions, as well as public health implications for increasing and preserving hippocampal volume.
Analyses stratified by minutes per week of exercise revealed an unexpected pattern whereby interventions providing less than 150 minutes per week of exercise (90–135 mins) were associated with significant positive effects, and interventions with 150 minutes or more (150–270 mins) were not. This counterintuitive finding could be explained by the possibility that interventions with fewer minutes per week facilitated higher compliance or higher intensity. We were not able to examine this possibility because few studies reported condition-specific compliance or intensity rates. It will be important for future studies to measure and report intervention parameters, such as compliance, adherence, and intensity, separately for intervention and control groups to examine this possibility more thoroughly. It will similarly be important for future studies to systematically compare interventions based on minutes per week of exercise, mode of exercise, and exercise intensity to identify the optimal exercise parameters for improving brain health outcomes.
A previous meta-analysis by Firth et al. (2018) assessed the effect of exercise on hippocampal volume and found a significant effect of exercise for left hippocampus, but not for total hippocampus. Further, significant effects in the right hippocampus depended on the age group. By comparison, we found a significant effect on total hippocampus, a marginally significant effect for right hippocampus, and no effect for the left hippocampus. There are a number of differences between the Firth et al. (2018) meta-analysis and the current meta-analysis. The current meta-analysis had greater statistical power, including 23 interventions for total hippocampus compared with 14 interventions for the Firth et al. (2018) meta-analysis. Additionally, the Firth et al. (2018) meta-analysis had different study inclusion/exclusion criteria compared to the current meta-analysis. Firth et al. (2018) included a multi-modal intervention study (exercise + cognitive training) (Malchow et al., 2016) and a study that did not report hippocampal volume data in the article (Burzynska et al., 2017). Firth et al. (2018) also did not include ten other studies that were eligible for the current meta-analysis, five of which were published after the Firth et al. (2018) meta-analysis (Broadhouse et al., 2020; Callisaya et al., 2017; Ehlers et al., 2017; Frederiksen et al., 2018; Kleemeyer et al., 2016; Riggs et al., 2017; Tarumi et al., 2019; Teixeira et al., 2018; Venkatraman et al., 2020; Wagner et al., 2015).
Regarding moderators of the effects of exercise, the Firth et al. (2018) meta-analysis examined effects in interventions in patients with schizophrenia (n’s = 2–4) and found that effects of exercise in schizophrenia were not significant. Effects of exercise in other patient populations were not statistically tested. Distinct from the age- and patient-related stratified analyses presented by Firth et al. (2018), we demonstrate that the overall effect on hippocampal volume is significant in older adults and is marginal in patient populations, but not significant in younger adults or healthy populations alone. The current meta-analysis further extends the scope of the Firth et al. (2018) meta-analysis by demonstrating the influence of the duration and amount of exercise in the intervention. The current meta-analysis also provides a more definitive assessment of effects driven exclusively by exercise by excluding multi-modal interventions.
The observational literature has shown that individuals with greater physical activity earlier in life have greater hippocampal volumes later in life (Erickson, Raji, et al., 2010). These results converge across diverse volumetric techniques and study designs to suggest that exercise has a sustained effect on hippocampal volume. The effects of these observational findings may occur through the same mechanisms as our current findings. The enhancing effects of exercise and physical activity on hippocampal volume are posited to occur through multiple pathways including neurogenesis, angiogenesis, and changes in dendritic morphology (Vivar et al., 2013). For example, exercise upregulates neurotrophins, such as BDNF, which protects neurons from damage, induces neurogenesis, and facilitates long-term potentiation (Kennedy, Hardman, Macpherson, Scholey, & Pipingas, 2017; Van Praag, 2008). Blocking expression of BDNF in the hippocampus following exercise inhibits cognitive improvements (Kennedy et al., 2017). Exercise may also influence hippocampal volume through reductions in vascular-, stress-, or inflammation-related neuronal loss (Kennedy et al., 2017; Vivar et al., 2013). For instance, increasing age is associated with increased arterial stiffness, which causes damage to the microvasculature, leading to microbleeds that may lead to neuronal loss (Kennedy et al., 2017). Moreover, factors such as stress and inflammation increase neuronal loss, and increasing evidence suggests that exercise is effective at reducing both chronic stress and inflammation (Kennedy et al., 2017).
4.2. Strengths and Limitations
The present study demonstrated significant effects of exercise on overall hippocampal volume. It suggests that exercise is especially useful in older populations that are vulnerable to hippocampal volume loss and provides important information that longer duration interventions with moderate amounts of exercise show significant effects. Nonetheless, there are several limitations that should be noted. To ensure that we compared effects of physical exercise independent of other non-physical exercise interventions, we excluded some relevant studies. For instance, several additional studies combined multiple types of interventions (i.e., cognitive training and meditation), which could interact with or confound the effects of exercise, and thus were not included (Malchow et al., 2016; Sehm et al., 2014; Shimada et al., 2017; Stomby et al., 2017; Tao et al., 2017). We did, however, include studies combining different forms of physical exercise (i.e. aerobic/resistance). Moreover, one study examining effects of exercise on hippocampal volume showed significant effects, but we were unable to obtain data relevant to the present analyses to include them here (Kim, Shin, Hong, & Kim, 2017). Analyses conducted separately for the left and right hippocampus included two fewer studies (representing 3 interventions) due to data being unavailable (Pajonk et al., 2010; Scheewe et al., 2013), limiting the statistical power for left and right hippocampus analyses.
The current meta-analysis did not distinguish between aerobic and resistance training interventions because several intervention programs combined both forms of exercise (five studies combined resistance and aerobic training and one compared a single control group to aerobic and resistance groups). Both of these forms of exercise may engage the cardiovascular system and increase respiration and heart rate if performed at a sufficiently high intensity (Petersen et al., 1989). Further, both forms of exercise have been shown to induce expression of neurotrophins that are thought to mediate hippocampal plasticity (Cassilhas et al., 2016; Liu-Ambrose et al., 2018; Tsai et al., 2019). Nonetheless, there is evidence that aerobic and resistance exercise have distinct preferential effects on these biochemical pathways, whereby aerobic exercise preferentially increases levels of BDNF and resistance exercise increases IGF-1 (Cassilhas et al., 2016).
It should be noted that many studies included in the analyses were inconclusive when considered in isolation (i.e., p-values > .05). This is likely because of the small sample sizes in individual studies. However, when all the data were pooled, the evidence for an exercise effect became much stronger since most effects were in the positive direction.
There is potential for a variety of sources of bias to influence the results presented here. Given the limited number of studies available to include in this meta-analysis, we included all studies regardless of potential risk of bias in the analyses. Further, there were factors relevant to methodological quality that were not reported in many of the studies. This raises the need for high quality studies with extensive methodological reporting to fully assess bias and the effects of population and intervention characteristics. We moreover acknowledge our own potential bias, given that a study by one of the current authors (Erickson et al., 2011) was included in the current analysis. The leave-one-out analysis showed that removing each study in turn did not change the significance of the overall effect., with the exception of the Erickson et al. (2011) and Teixiera et al. (2018) studies, which showed marginal overall effects on total hippocampus when left out of the analysis.
The heterogeneity of populations included in the present meta-analysis could impose other potential confounding factors, such as mechanisms of hippocampal atrophy, developmental stage, sex, comorbid health conditions, and education. For instance, Riggs et al. (2017) included a substantially younger population than the other studies. At this time, however, there are simply not enough studies evaluating the effects of exercise interventions on hippocampal volume to stratify analyses based on all these factors. The detection of a significant effect despite this heterogeneity in the study populations suggests that exercise influences hippocampal morphology across various populations.
There are also limitations in variation of the handling of missing data across studies and variation in the use of an intent-to-treat approach or a per protocol approach. For some studies, pre-intervention data were included regardless of whether post-intervention data were available. For other studies, only participants with both pre- and post-intervention data were included in analyses and reported. Thus, it is important for studies to report data consistently based on standard conservative analyses (i.e., intent-to-treat) and to provide a detailed description of how attrition and missing data are handled. This may be presented in addition to more biased approaches (i.e., per protocol) that are informative in proof of concept and efficacy studies.
4.3. Conclusions
Exercise was associated with a positive effect on total hippocampal volume relative to controls. Effect sizes were small but within the range of typical age-related rates of annual hippocampal atrophy. When compared with the average rate of annual volumetric decline, these results demonstrate that exercise could preserve or reverse age-related hippocampal atrophy. Interventions lasting >24 weeks have the greatest effects on hippocampal volume, particularly in older samples, which may provide guidelines for prescribing exercise interventions to improve brain health. In sum, moderate amounts of prolonged exercise are a low-cost, highly accessible, and highly effective approach for increasing hippocampal volume.
Supplementary Material
Acknowledgements:
K.A.W. was supported by an NIH career development award from the National Institutes of Health (NIH/NIA K01 AG049879). C.M.S. was supported by a postdoctoral training grant (NIH/NIMH T32 MH019986) and NIH/NIA R01 AG060741 to KIE. R.L.L. was supported by a training grant from the National Institute of Health (T32 HL007560). K.I.E. was supported by grants from the National Institutes of Health (R01 DK095172; P30 AG024827; R01 AG053952).
Footnotes
Conflict of Interest Statement: K.A.W. has received an honorarium from Otsuka for disease-state educational activity. All other authors have no conflicts of interest to disclose.
References
- Araki Y, Nomura M, Tanaka H, Yamamoto H, Yamamoto T, Tsukaguchi I, & Nakamura H. (1994). MRI of the brain in diabetes mellitus. Neuroradiology, 36(2), 101–103. [DOI] [PubMed] [Google Scholar]
- Arnold SJM, Ivleva EI, Gopal TA, Reddy AP, Jeon-Slaughter H, Sacco CB, . . . Tamminga CA (2015). Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer). Schizophrenia bulletin, 41(1), 233–249. doi: 10.1093/schbul/sbu009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, . . . Craft S. (2010). Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology, 67(1), 71–79. doi: 10.1001/archneurol.2009.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi VE, Locatelli V, & Rizzi L. (2017). Neurotrophic and neuroregenerative effects of GH/IGF1. International journal of molecular sciences, 18(11), 2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Rothstein D, & Cohen J. (2005). Comprehensive meta-analysis: A computer program for research synthesis [Computer software]. Englewood, NJ: Biostat. [Google Scholar]
- Broadhouse KM, Singh MF, Suo C, Gates N, Wen W, Brodaty H, . . . Singh N. (2020). Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. NeuroImage: Clinical, 25, 102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Jiao Y, Knecht AM, Fanning J, Awick EA, Chen T, . . . Kramer AF (2017). White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Frontiers in aging neuroscience, 9, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill LS, Steadman PE, Jones CE, Laliberté CL, Dazai J, Lerch JP, . . . Sled JG (2015). MRI-detectable changes in mouse brain structure induced by voluntary exercise. NeuroImage, 113, 175–183. doi: 10.1016/j.neuroimage.2015.03.036 [DOI] [PubMed] [Google Scholar]
- Callisaya M, Daly R, Sharman J, Bruce D, Davis T, Greenaway T, . . . Phan T. (2017). Feasibility of a multi-modal exercise program on cognition in older adults with Type 2 diabetes–a pilot randomised controlled trial. BMC geriatrics, 17(1), 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Lee K, Fernandes J, Oliveira M, Tufik S, Meeusen R, & De Mello M. (2012). Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience, 202, 309–317. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Tufik S, & de Mello MT (2016). Physical exercise, neuroplasticity, spatial learning and memory. Cellular and Molecular Life Sciences, 73(5), 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Sargent-Cox K, Fraser M, Sachdev P, & Anstey KJ (2015). Being overweight is associated with hippocampal atrophy: the PATH Through Life Study. International Journal Of Obesity, 39, 1509. doi:10.1038/ijo.2015.106 https://www.nature.com/articles/ijo2015106#supplementary-information [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, & Greenough WT (2002). Exercise, experience and the aging brain. Neurobiology of Aging, 23(5), 941–955. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioural sciences. In: Hillsdale, NJ: erlbaum. [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, . . . Kramer AF (2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(11), 1166–1170. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, & Kramer AF (2003). Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science, 14(2), 125–130. [DOI] [PubMed] [Google Scholar]
- Davachi L, & Wagner AD (2002). Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of neurophysiology, 88(2), 982–990. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Tiemeier H, Luijendijk HJ, van der Lijn F, Koudstaal PJ, Hofman A, & Breteler MM (2011). A study of the bidirectional association between hippocampal volume on magnetic resonance imaging and depression in the elderly. Biological psychiatry, 70(2), 191–197. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, & Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj, 315(7109), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers DK, Daugherty AM, Burzynska AZ, Fanning J, Awick EA, Chaddock-Heyman L, . . . McAuley E. (2017). Regional brain volumes moderate, but do not mediate, the effects of group-based exercise training on reductions in loneliness in older adults. Frontiers in aging neuroscience, 9, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, & Cohen NJ (1992). The hippocampus—what does it do? Behavioral and neural biology, 57(1), 2–36. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, . . . Woods JA (2010). Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. Journal of Neuroscience, 30(15), 5368–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji C, Lopez O, Becker J, Rosano C, Newman A, . . . Kuller L. (2010). Physical activity predicts gray matter volume in late adulthood The Cardiovascular Health Study. Neurology, WNL. 0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, . . . White SM (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, 108(7), 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C, Chamari K, Mucci P, Masse-Biron J, & Prefaut C. (2002). Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. International journal of sports medicine, 23(06), 415–421. [DOI] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, & Ward PB (2018). Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage, 166, 230–238. doi: 10.1016/j.neuroimage.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Fraser MA, Shaw ME, & Cherbuin N. (2015). A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. NeuroImage, 112, 364–374. [DOI] [PubMed] [Google Scholar]
- Frederiksen KS, Larsen CT, Hasselbalch SG, Christensen AN, Høgh P, Wermuth L, . . . Garde E. (2018). A 16-week aerobic exercise intervention does not affect hippocampal volume and cortical thickness in mild to moderate Alzheimer’s disease. Frontiers in aging neuroscience, 10, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, & Higgins J. (2005). Cochrane handbook for systematic reviews of interventions. In: Version. [Google Scholar]
- Gregory SM, Parker B, & Thompson PD (2012). Physical activity, cognitive function, and brain health: what is the role of exercise training in the prevention of dementia? Brain sciences, 2(4), 684–708. doi: 10.3390/brainsci2040684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health U. D. o., & Services H. (2018). 2018 Physical Activity Guidelines Advisory Committee Scientific Report. In: Office of Disease Prevention and Health Promotion, Washington, DC. [Google Scholar]
- Huang G, Gibson CA, Tran ZV, & Osness WH (2005). Controlled endurance exercise training and VO2max changes in older adults: a meta-analysis. Preventive cardiology, 8(4), 217–225. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, . . . Kokmen E. (2000). Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology, 55(4), 484–489. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10953178 https://www.ncbi.nlm.nih.gov/pmc/PMC2724764/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, O’brien PC, Tangalos EG, . . . Kokmen E. (1997). Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology, 49(3), 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, & Boraxbekk C-J (2017). Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Frontiers in aging neuroscience, 8, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy G, Hardman RJ, Macpherson H, Scholey AB, & Pipingas A. (2017). How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. Journal of Alzheimer’s Disease, 55(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Kim YS, Shin SK, Hong SB, & Kim HJ (2017). The effects of strength exercise on hippocampus volume and functional fitness of older women. Experimental Gerontology, 97, 22–28. doi: 10.1016/j.exger.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Kleemeyer MM, Kühn S, Prindle J, Bodammer NC, Brechtel L, Garthe A, . . . Lindenberger U. (2016). Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. NeuroImage, 131, 155–161. [DOI] [PubMed] [Google Scholar]
- Klein C, Schreyer S, Kohrs F, Elhamoury P, Pfeffer A, Munder T, & Steiner B. (2017). Stimulation of adult hippocampal neurogenesis by physical exercise and enriched environment is disturbed in a CADASIL mouse model. Scientific Reports, 7, 45372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh J, Rostrup E, Thomsen C, Elfving B, Videbech P, & Nordentoft M. (2014). The effect of exercise on hippocampal volume and neurotrophines in patients with major depression–A randomized clinical trial. Journal of Affective Disorders, 165, 24–30. doi: 10.1016/j.jad.2014.04.041 [DOI] [PubMed] [Google Scholar]
- Lin J, Chan SK, Lee EH, Chang WC, Tse M, Su WW, . . . Chen EY (2015). Aerobic exercise and yoga improve neurocognitive function in women with early psychosis. NPJ Schizophr, 1(0), 15047. doi: 10.1038/npjschz.2015.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T, Barha CK, & Best JR (2018). Physical activity for brain health in older adults. Applied Physiology, Nutrition, and Metabolism, 43(11), 1105–1112. [DOI] [PubMed] [Google Scholar]
- Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, . . . Braun-Dullaeus R. (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular psychiatry, 20(5), 585. [DOI] [PubMed] [Google Scholar]
- Malchow B, Keeser D, Keller K, Hasan A, Rauchmann B-S, Kimura H, . . . Falkai P. (2016). Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophrenia Research, 173(3), 182–191. doi: 10.1016/j.schres.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Malykhin NV, & Coupland NJ (2015). Hippocampal neuroplasticity in major depressive disorder. Neuroscience, 309, 200–213. doi: 10.1016/j.neuroscience.2015.04.047 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine, 151(4), 264–269. [DOI] [PubMed] [Google Scholar]
- Morris JK, Vidoni ED, Johnson DK, Van Sciver A, Mahnken JD, Honea RA, . . . Swerdlow RH (2017). Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial. PloS one, 12(2), e0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, & Riordan HJ (1998). Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Archives of general psychiatry, 55(5), 433–440. [DOI] [PubMed] [Google Scholar]
- Nieman BJ, de Guzman AE, Gazdzinski LM, Lerch JP, Chakravarty MM, Pipitone J, . . . Mabbott DJ (2015). White and Gray Matter Abnormalities After Cranial Radiation in Children and Mice. International Journal of Radiation Oncology*Biology*Physics, 93(4), 882–891. doi: 10.1016/j.ijrobp.2015.07.2293 [DOI] [PubMed] [Google Scholar]
- Niemann C, Godde B, & Voelcker-Rehage C. (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in aging neuroscience, 6, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin LE, Waiwood AM, Cumming TB, Marsland AL, Bernhardt J, & Erickson KI (2017). Effects of physical activity on poststroke cognitive function: a meta-analysis of randomized controlled trials. Stroke, 48(11), 3093–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajonk F-G, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, . . . Meyer T. (2010). Hippocampal plasticity in response to exercise in schizophrenia. Archives of general psychiatry, 67(2), 133–143. [DOI] [PubMed] [Google Scholar]
- Petersen S, Haennel R, Kappagoda C, Belcastro A, Reid D, Wenger H, & Quinney H. (1989). The influence of high-velocity circuit resistance training on VO2max and cardiac output. Canadian journal of sport sciences= Journal canadien des sciences du sport, 14(3), 158. [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, . . . Acker JD (2005). Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral Cortex, 15(11), 1676–1689. doi: 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Riggs L, Piscione J, Laughlin S, Cunningham T, Timmons BW, Courneya KS, . . . Mabbott DJ (2017). Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro-Oncology, 19(3), 440–450. doi: 10.1093/neuonc/now177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Guralnik J, Pahor M, Glynn NW, Newman AB, Ibrahim TS, . . . MacCloud RL (2017). Hippocampal response to a 24-month physical activity intervention in sedentary older adults. The American Journal of Geriatric Psychiatry, 25(3), 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. (1979). The file drawer problem and tolerance for null results. Psychological bulletin, 86(3), 638. [Google Scholar]
- Ross M, Lithgow H, Hayes L, & Florida-James G. (2019). Potential cellular and biochemical mechanisms of exercise and physical activity on the ageing process. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science (pp. 311–338): Springer. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, van Haren NE, Sarkisyan G, Schnack HG, Brouwer RM, de Glint M, . . . Cahn W. (2013). Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. European Neuropsychopharmacology, 23(7), 675–685. [DOI] [PubMed] [Google Scholar]
- Sehm B, Taubert M, Conde V, Weise D, Classen J, Dukart J, . . . Ragert P. (2014). Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol Aging, 35(1), 232–239. doi: 10.1016/j.neurobiolaging.2013.06.021 [DOI] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, & Green MJ (2012). Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neuroscience & Biobehavioral Reviews, 36(4), 1342–1356. [DOI] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, & Jiang T. (2009). Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus, 19(11), 1055–1064. [DOI] [PubMed] [Google Scholar]
- Shimada H, Makizako H, Doi T, Park H, Tsutsumimoto K, Verghese J, & Suzuki T. (2017). Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J Am Med Dir Assoc. doi: 10.1016/j.jamda.2017.09.019 [DOI] [PubMed] [Google Scholar]
- Squire LR (1981). Two forms of human amnesia: An analysis of forgetting. Journal of Neuroscience, 1(6), 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman CM, Cohen J, Lehman ME, & Erickson KI (2016). Mediators of physical activity on neurocognitive function: a review at multiple levels of analysis. Frontiers in human neuroscience, 10, 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomby A, Otten J, Ryberg M, Nyberg L, Olsson T, & Boraxbekk CJ (2017). A Paleolithic Diet with and without Combined Aerobic and Resistance Exercise Increases Functional Brain Responses and Hippocampal Volume in Subjects with Type 2 Diabetes. Front Aging Neurosci, 9, 391. doi: 10.3389/fnagi.2017.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Nee DE, & D’Esposito M. (2018). Hippocampal-targeted Theta-burst Stimulation Enhances Associative Memory Formation. Journal of cognitive neuroscience, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Liu J, Liu W, Huang J, Xue X, Chen X, . . . Kong J. (2017). Tai Chi Chuan and Baduanjin Increase Grey Matter Volume in Older Adults: A Brain Imaging Study. J Alzheimers Dis, 60(2), 389–400. doi: 10.3233/jad-170477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Rossetti H, Thomas BP, Harris T, Tseng BY, Turner M, . . . Stowe AM (2019). Exercise training in amnestic mild cognitive impairment: a one-year randomized controlled trial. Journal of Alzheimer’s Disease, 71(2), 421–433. [DOI] [PubMed] [Google Scholar]
- Teixeira CVL, de Rezende TJR, Weiler M, Magalhães TNC, Carletti-Cassani AFMK, Silva TQAC, . . . Franco MP (2018). Cognitive and structural cerebral changes in amnestic mild cognitive impairment due to Alzheimer’s disease after multicomponent training. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 4, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, & Liu-Ambrose T. (2015). Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. British Journal of Sports Medicine, 49(4), 248–254. doi: 10.1136/bjsports-2013-093184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Dennis A, Rawlings NB, Stagg CJ, Matthews L, Morris M, . . . Nichols TE (2016). Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. NeuroImage, 131, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, & Gage FH (2018). Review: adult neurogenesis contributes to hippocampal plasticity. Cell and Tissue Research, 373(3), 693–709. doi: 10.1007/s00441-017-2735-4 [DOI] [PubMed] [Google Scholar]
- Tsai C-L, Pai M-C, Ukropec J, & Ukropcová B. (2019). Distinctive effects of aerobic and resistance exercise modes on neurocognitive and biochemical changes in individuals with mild cognitive impairment. Current Alzheimer Research, 16(4), 316–332. [DOI] [PubMed] [Google Scholar]
- Van Praag H. (2008). Neurogenesis and exercise: past and future directions. Neuromolecular medicine, 10(2), 128–140. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience, 2(3), 266. [DOI] [PubMed] [Google Scholar]
- Venkatraman VK, Sanderson A, Cox KL, Ellis KA, Steward C, Phal PM, . . . Lai M. (2020). Effect of a 24-month physical activity program on brain changes in older adults at risk of Alzheimer’s disease: the AIBL active trial. Neurobiology of Aging, 89, 132–141. [DOI] [PubMed] [Google Scholar]
- Videbech P, & Ravnkilde B. (2004). Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry, 161(11), 1957–1966. [DOI] [PubMed] [Google Scholar]
- Vivar C, Potter MC, & van Praag H. (2013). All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Current topics in behavioral neurosciences, 15, 189–210. doi: 10.1007/7854_2012_220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Herbsleb M, Cruz F. d. l., Schumann A, Brünner F, Schachtzabel C, . . . Gabriel HW (2015). Hippocampal structure, metabolism, and inflammatory response after a 6-week intense aerobic exercise in healthy young adults: a controlled trial. Journal of Cerebral Blood Flow & Metabolism, 35(10), 1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Suenderhauf C, Harrisberger F, Lenz C, Smieskova R, Chung Y, . . . Vogel T. (2016). Hippocampal volume in subjects at clinical high-risk for psychosis: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 71, 680–690. doi: 10.1016/j.neubiorev.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Ward AM, Mormino EC, Huijbers W, Schultz AP, Hedden T, & Sperling RA (2015). Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiology of Aging, 36(1), 265–272. doi: 10.1016/j.neurobiolaging.2014.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Das SR, Mueller SG, Weiner MW, & Yushkevich PA (2017). Medial temporal lobe subregional morphometry using high resolution MRI in Alzheimer’s disease. Neurobiology of Aging, 49, 204–213. doi: 10.1016/j.neurobiolaging.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, . . . Csernansky JG (2009). Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiology of Disease, 35(3), 426–432. doi: 10.1016/j.nbd.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.