Abstract
Background
Asthma is an inflammatory disease where the balance between Th1/Th2 and Th17/Treg plays a crucial role in its pathogenesis. Shikonin is used to treat a variety of autoimmune diseases due to its good anti-inflammatory activity. However, the effect and mechanism of shikonin on asthma remain unknown.
Method
Mice were sensitized with ovalbumin (OVA)/house dust mite (HDM) and treated with shikonin. Lung inflammation was assessed histologically and via flow cytometry. Bronchoalveolar lavage fluid (BALF) was analyzed for cell counts and cytokines. Shikonin’s impact on p-STAT3 was studied in vivo and in vitro.
Results
Shikonin inhibited OVA or HDM-induced inflammation and airway hyperresponsiveness. Upon treatment, a restoration of the Th1/Th2 and Th17/Treg balance was observed, evidenced by a reduction in IL-4 and IL-17A levels in BALF, alongside an elevation in interferon-gamma and IL-10. Furthermore, shikonin impeded the infiltration of eosinophils, neutrophils, macrophages, and lymphocytes into lung tissue. The observed decrease in STAT3 phosphorylation and diminished nuclear translocation of p-STAT3 confirmed that shikonin promotes the balance of Th1/Th2 and Th17/Treg by regulating airway epithelial STAT3.
Conclusion
Shikonin mitigates asthma symptoms through a STAT3-dependent mechanism, indicating its potential as an anti-asthmatic therapeutic agent.
Keywords: shikonin, asthma, STAT3, Th1/Th2, Th17/Treg
1. Introduction
As a common chronic respiratory disease asthma with high morbidity and mortality is seriously affecting the health of people of all ages [1]. It is characterized by reversible airway obstruction, mucus hypersecretion, airway inflammation, and airway hyperresponsiveness, which often lead to wheezing, chest tightness, and breathlessness in asthmatic patients [2]. There is no gold standard for the diagnosis of asthma, because it is a heterogeneous disease with complex pathogenesis, and the clinical manifestations, treatment response, and prognosis of patients are different [3]. At present, the goal of treating asthma is to control the occurrence of asthma as much as possible, restore the lung function of patients, and reduce the risk of death [4]. In recent years, although glucocorticoids, bronchodilators, and some targeted drugs have been widely used in clinical practice, some patients cannot achieve complete control, and the incidence of asthma is still increasing [5]. Therefore, it is urgent to elucidate the pathogenesis of asthma and find effective therapeutic drugs.
Asthma is an inflammatory disease with an abnormal immune response, which is closely related to the activation of CD4+ T helper (Th) cells in the lung. Excessive immune response in asthmatic patients increases vascular permeability and causes airway inflammation, which is the result of the interaction between epithelial cells and immune cells [6]. In the process of immune response, there is an imbalance between Th1 and Th2. The highly expressed Th2 cells can stimulate type 2 immunity by secreting a large number of cytokines such as IL-4, IL-5, IL-9, and IL-13, which will lead to the accumulation of mast cells, macrophages, and eosinophils [7]. Increased airway responsiveness and airway inflammation are considered to be one of the pathogenesis of asthma [8]. IL-4 is a characteristic cytokine of Th2 cells as well as a major cytokine leading to asthma. On the one hand, by inducing the expression of mucin genes, IL-4 can lead to the excessive secretion of mucus by goblet cells within the airways [9]. On the other hand, IL-4 is also a factor secreted by T cells themselves, which can induce the occurrence of asthma by promoting the transformation of T cells into Th2 cells [10]. On the contrary, Th1 cells can release IL-2, IL-12, and interferon-gamma (IFN-γ) [11], among which the characteristic cytokine IFN-γ plays an inhibitory role in asthma by hindering the aggregation of eosinophils and reducing the expression of immunoglobulin [12]. In addition to Th1/Th2, Th17/Treg is important for the pathogenesis of asthma [13]. Immunosuppression can be mediated by Treg via secreting anti-inflammatory cytokines such as IL-10 and transforming growth factor-β1 [14]. However, inflammatory response can be induced by Th17 via generating IL-17A to promote the mobilization, recruitment, and activation of neutrophilic granulocyte [15]. Therefore, there is an imbalance between Th1/Th2 and Th17/Treg in asthma. The severity of asthma can be assessed by detecting the balance of Th1/Th2 and Th17/Treg.
Shikonin, the main bioactive component of lithospermum erythrorhizon roots has various biological functions such as anti-cancer, anti-inflammation, and promoting wound healing [16]. Studies have shown that shikonin can inhibit the inflammatory process of osteoarthritis by activating the mitogen-activated protein kinases (MAPK) and inhibiting the phosphorylation of STAT3 to down-regulate IL-1β-induced inflammatory factors in chondrocytes [17]. In rheumatoid arthritis, the inhibitory effect of lipopolysaccharide (LPS) on IL-10 expression in fibroblast-like synoviocytes can be significantly reversed by shikonin due to the inactivation of the PKC-NF-κB pathway, thereby reducing the production of tumor necrosis factor-α (TNF-α) [18]. Treatment of LPS-induced human primary nucleus pulposus cells with shikonin could reverse the expression levels of inflammatory cytokines (TNF-α and IL-1β) and apoptosis-related molecules (Bax, Bcl-2, and cleaved caspase 3), because shikonin can inhibit the expression of p-p65 and the nuclear translocation of p65. This study demonstrates that the anti-inflammatory and anti-apoptotic effects of shikonin on human intervertebral disc degeneration are achieved through the NF-κB pathway [19]. In addition, shikonin can reduce the secretion of pro-inflammatory factors such as TNF-α, IL-1, and IL-6 by inhibiting the polarization of M1 macrophages induced by LPS- and Th1-derived cytokines (such as IFN-γ and IL-1β), thereby alleviating collagen-induced arthritis in mice [20]. However, only a few studies have shown the effect of shikonin on inflammation in asthma mice. Wang et al. found that intraperitoneal injection of shikonin can reduce pathological airway remodeling, inflammatory cell infiltration, and collagen deposition in ovalbumin (OVA)-induced asthma mice. Further mechanism studies have shown that shikonin can inactivate NF-κB by blocking the activation of IκBα, which inhibits the proliferation and migration of primary airway smooth muscle cells and the activity of matrix metalloproteinases (MMP2 and MMP9), thereby reducing allergic airway remodeling [21]. Although the effect of shikonin on asthma has been reported, the mechanism by which shikonin inhibits inflammation in asthma mice has not been determined.
Asthma is mainly caused by airway inflammation, and STAT3 involved in inflammatory and immune response plays an important role in the pathogenesis of asthma [22]. A study in mice showed that lung inflammation can be caused by STAT3 via accelerating the development of Th17 cells and promoting the production of cytokines by Th2 and Th17 cells, which will lead to the occurrence of asthma [23]. Airway epithelial cells play an extremely important role in the pathogenesis of asthma. The specific knockout of STAT3 expressed in airway epithelial cells can significantly down-regulate house dust mite (HDM)-induced airway inflammation in asthma mice [24,25]. However, whether shikonin can inhibit asthma through this mechanism remains to be explored.
To evaluate the mechanism by which shikonin alleviates the phenotype of asthma mice, we used OVA- and HDM-induced asthma mice to observe the effects of shikonin on the expression of Th1-, Th2-, Treg-, and Th17-related cytokine profiles, airway responsiveness, and airway inflammation in vivo. In addition, we evaluated the effects of shikonin on the expression and localization of p-STAT3 in airway epithelial cells. In vitro, we analyzed whether the STAT3 activator colivelin could reverse the inhibitory effect of shikonin on asthma and detected the expression of p-STAT3 and its downstream factors.
2. Materials and methods
2.1. Drug and reagents
Shikonin (S7576, purity ≥98%) was purchased from Sigma (St. Louis, MO, USA). OVA (100 μg/mL, Sigma) was mixed with an equal volume of 10% (w/v) alum (Sigma) in distilled water. HDM (50,000 BU/mL) was obtained from Allergopharma (Reinbeck, Germany). STAT3 agonist (Colivelin) was acquired from Tocris (Bristol, Britain).
2.2. Mouse model of asthma
Female BALB/c mice (4 weeks old) were purchased from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China) and raised in a constant temperature SPF environment. Mice were randomly divided into five groups: control, OVA model, HDM model, OVA + shikonin (4 mg/kg), and HDM + shikonin (4 mg/kg), with three mice in each group. For the OVA model group, mice were sensitized with an intraperitoneal injection of 50 μg OVA mixed with 10% aluminum hydroxide solution on days 1, 14, and 28. From the 21st day, the mice were placed in a closed container and atomized with 8 mL of OVA (1% in PBS) solution for 30 min, twice a week for 8 weeks. For the HDM model group, mice were sensitized through intraperitoneal injection of 20 μg of HDM combined with 1 mg of aluminum hydroxide constituted in 0.1 mL of saline on days 0 and 14. The mice were further challenged with intranasal instillation of 10 μg of HDM in 30 μL of saline three times per week from weeks 3 to 8. For OVA + shikonin (4 mg/kg) and HDM + shikonin (4 mg/kg) groups, mice in each group were treated with shikonin (4 mg/kg) by intratracheal instillation half an hour before each challenge. The control group was treated with normal saline instead of OVA, HDM, and shikonin. All animal procedures were performed with the approval of the Animal Care and Use Committee of Xi’an International Medical Center Hospital (Approval No. GJYX-KY-2023-006).
2.3. Cell culture and stimulation
The mouse lung epithelial-12 (MLE-12) cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM/F12 (Gibco, USA) containing 2% fetal calf serum. The cells were incubated with TNF-α (20 ng/mL) for 2 h to induce the in vitro model of asthma. To investigate the effect and mechanism of shikonin, shikonin (5 μg/mL) and colivelin (25 μg/mL) were used to treat cells prior to TNF-α stimulation.
2.4. Airway hyperresponsiveness
After the last challenge, the mice in each group were inhaled with 300 μL of 50 mg/mL methacholine (Mech), and the atomization was monitored for 3 min each time. The Penh values reflecting airway responsiveness were recorded using a Buxco non-invasive pulmonary function detector (Wilmington, NC, USA).
2.5. Bronchoalveolar lavage fluid (BALF) cell count and serum analysis
BALF was collected by flushing the lungs of mice with 0.9 mL of cold PBS containing 2 mM ethylene diamine tetraacetic acid and 2% fetal bovine serum. After BALF was centrifuged at 1,500 × g for 10 min at 4°C, the cell precipitate was resuspended. Then, the total number of BALF cells was calculated using a hemocytometer, and the number of differential cells (eosinophils, neutrophils, macrophages, and lymphocytes) was determined by Wright-Giemsa staining. The enzyme linked immunosorbent assay (ELISA) kits (R&D systems, Minneapolis, MN, USA) were used to detect the levels of IFN-γ, IL-4, IL-17A, and IL-10 in BALF supernatant.
2.6. Lung histopathology
The mice were euthanized with isoflurane, and the left lung was fixed with 4% paraformaldehyde. After dehydration, embedding, and sectioning, the specimens were stained with hematoxylin/eosin (HE). The degree of inflammation was scored according to the infiltration of inflammatory cells around the airway: 0, no infiltrates; 1, few inflammatory cells; 2, one layer of inflammatory cells; 3, 2–4 layers of inflammatory cells; and 4, more than four layers of inflammatory cells.
2.7. Flow cytometry
The left lobe of the lung was digested to obtain single-cell suspension. Cells were stained with fluorescence-labeled antibodies (Abcam, Cambridge, UK) specific for APC-IFN-γ, PE-IL-4, PE-IL-17A, and PE-Foxp3. A CytoFLEX flow cytometer (Beckman Coulter, Villepinte, France) was used to detect the percentage of Th1 (CD4+ IFN-γ+), Treg (CD25+ FOXP3+), Th2 (CD4+ IL-4+), and Th17 (CD4+ IL-17+) cells.
2.8. Western blot analysis
Total protein was extracted with radio immunoprecipitation assay lysis buffer from airway epithelial cells or MLE-12 cells, and the concentration of these proteins was determined by bicinchoninic acid reagent. These proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Then, these membranes were blocked with 5% skim milk for 1 h and incubated with primary antibodies (Abcam) at 4°C overnight. The primary antibodies used in this study were GAPDH, anti-STAT3, anti-phospho (p)-STAT3, anti-IL-1β, and anti-IFN-γ. The next day, these membranes were washed three times with tris-borate-sodium tween and incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h. Finally, the enhanced chemiluminescence substrate was used to display the bands, which were semi-quantitatively analyzed by the ImageJ software.
2.9. Immunofluorescence analysis
Airway epithelial cells or MLE-12 cells were seeded on glass slides, fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 5% skim milk powder solution. At 4°C, these cells were incubated with primary antibodies against p-STAT3 (Abcam) overnight. On the second day, these cells were incubated with the secondary antibody in a dark room for 1 h. The nucleus was labeled with DAPI, and the fluorescence was observed under a fluorescence microscope.
2.10. ELISA
IL-1β kit (Abcam) and IFN-γ kit (Abcam) were used to detect the concentration of these factors in the culture supernatant of MLE-12 cells.
2.11. Statistical analysis
All experiments in this study were repeated three times, and the data were analyzed using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as mean ± standard error of the mean. One-way analysis of variance was used to compare the differences between groups. A P value of less than 0.05 is considered to be statistically significant.
3. Results
3.1. Shikonin alleviates the phenotypes of OVA- and HDM-induced asthma mice
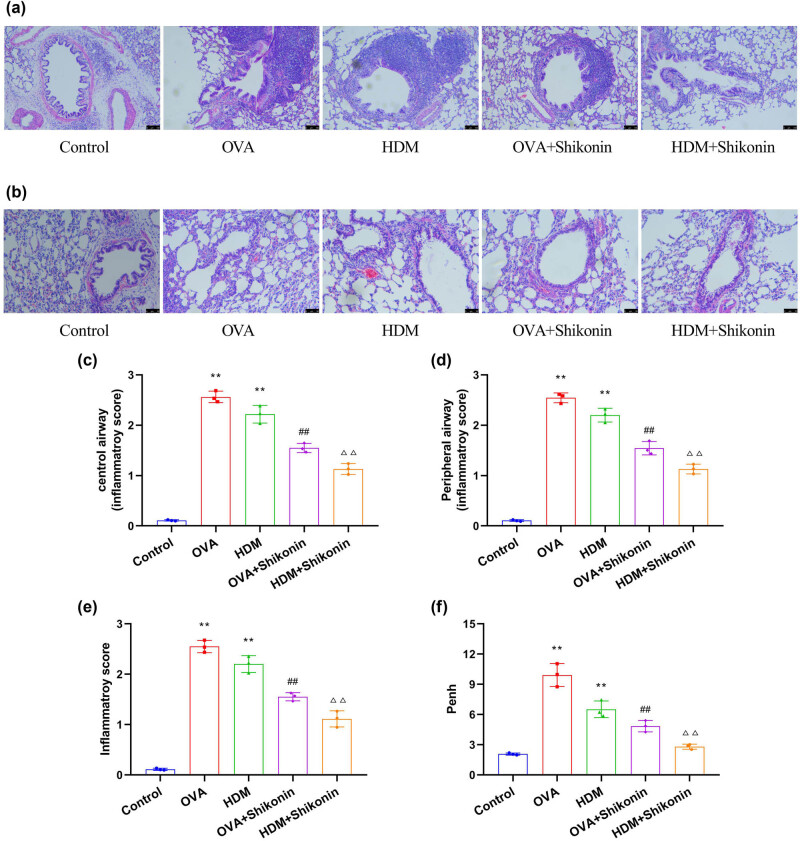
To investigate the effect of shikonin on the phenotypes of asthma mice, we examined airway inflammation and airway responsiveness of mice. First, we used OVA and HDM to sensitize and challenge 4-week-old BALB/c mice. At 7 weeks of age, asthma mice were treated by intratracheal instillation of shikonin (4 mg/kg) for 28 days. Then, HE staining was used to observe the inflammatory cell infiltration of the central and peripheral airway in the lung sections of mice. As shown in Figure 1a–e, the structure of alveolar tissue in the control group was normal, and no inflammatory cell infiltration was observed in the central and peripheral airway. The alveolar wall of OVA- and HDM-induced mice was significantly thickened, and the lumen was narrowed. The central and peripheral airways were infiltrated by a large number of inflammatory cells (lymphocytes, eosinophils, neutrophils, macrophages). After treatment with shikonin, the inflammation of lung tissue was improved. In addition, HE staining did not show significant goblet cell proliferation and staining of positive epithelial cells in the control group. In the OVA group and the HDM group, the necrosis of airway epithelial cells and the hyperplasia of goblet cells were obvious, and the function of airway mucus secretion was hyperactive. After treatment with shikonin, the positive cells of airway epithelium in the OVA + shikonin group and the HDM + shikonin group were less than those in the OVA and HDM groups by HE staining, and the mucus secretion of airway epithelium was significantly reduced. The airway responsiveness of the OVA group and the HDM group was higher than in the control group, while shikonin could decrease the airway responsiveness of asthma mice (Figure 1f). The above results collectively indicate that shikonin can reduce inflammation and airway responsiveness, thereby alleviating the phenotypes of asthma mice induced by OVA and HDM.
Figure 1.
Shikonin alleviates the phenotypes of OVA- and HDM-induced asthma mice. (a) HE staining was used to detect the inflammatory infiltration of the central airway in the lung sections of mice. (b) HE staining was used to detect peripheral airway inflammatory infiltration in the lung sections of mice. (c) Central airway inflammation score. (d) Peripheral airway inflammation score. (e) Semi-quantitative score of inflammatory infiltration in the lung sections of mice. (f) The effect of shikonin on airway reactivity in asthma mice. Compared with the control group, **p < 0.01; compared with the OVA group, ## p < 0.01; compared with the HDM group, △△ p < 0.01, n = 3.
3.2. Shikonin induces CD4+ IFN-γ+ Th1 cells and CD25+ FOXP3+ Treg cells, and inhibits CD4+ IL-4+ Th2 cells and CD4+ IL-17+ Th17 cells
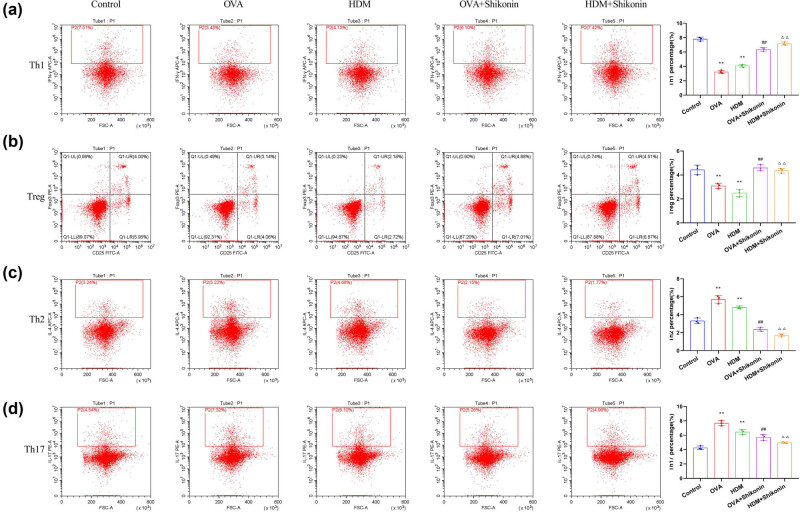
To investigate the effect of shikonin on T-cell populations in asthma mice, we evaluated the phenotypes of CD4+ cells in lung tissues by analyzing the expression of IFN-γ, FOXP3, IL-4, and IL-17 via flow cytometry. Compared with the control group, OVA or HDM sensitization and challenge decreased the proportion of CD4+ IFN-γ+ Th1 cells and CD25+ FOXP3+ Treg cells (Figure 2a and d) and increased the proportion of CD4+ IL-4+ Th2 cells and CD4+ IL-17+ Th17 cells (Figure 2b and c). After treatment with shikonin, the proportion of Th1 and Treg cells was up-regulated (Figure 2a and d), and the number of Th2 and Th17 cells was down-regulated (Figure 2a and d). These data suggest that shikonin can alleviate the imbalance of Th1/Th2 and Th17/Treg by inducing CD4+ IFN-γ+ Th1 cells and CD25+ FOXP3+ Treg cells, and inhibiting CD4+ IL-4+ Th2 cells and CD4+ IL-17+ Th17 cells, thereby improving the inflammatory responses caused by OVA or HDM.
Figure 2.
Shikonin alleviates the imbalance of Th1/Th2 and Th17/Treg in OVA- and HDM-induced asthma mice. Flow cytometry was used to evaluate the percentages of CD4+ IFN-γ+ Th1 cells (a), CD25+ FOXP3+ Treg cells (b), CD4+ IL-4+ Th2 cells (c), and CD4+ IL-17+ Th17 cells (d) in lung tissues. Compared with the control group, ** p < 0.01; compared with the OVA group, ## p < 0.01; compared with the HDM group, △△ p < 0.01, n = 3.
3.3. Shikonin inhibits the infiltration of immune cells and regulates the expression of Th-cell-related cytokines
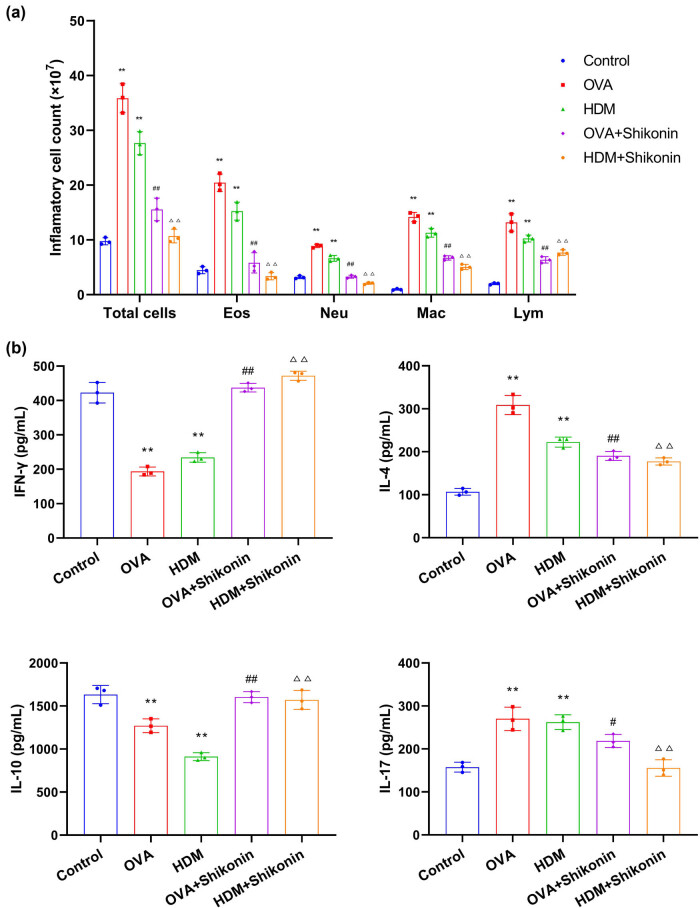
Next, we evaluated the infiltration of inflammatory cells and the expression of Th-cell-related cytokines in the lung tissue of mice. We collected the BALF from mice and counted the total number of white blood cells (total cells), eosinophils (Eos), neutrophils (Neu), macrophages (Mac), and lymphocytes (Lym). As shown in Figure 3a, the number of total cells, Eos, Neu, Mac, and Lym in the OVA and HDM groups was considerably higher than that in the control group. Compared with the OVA or HDM groups, the number of these cells in the OVA + shikonin and HDM + shikonin groups was statistically reduced. Also, Th cell-related inflammatory factors in BALF were determined by ELISA. Compared with the control group, the expression of Th1-related cytokine IFN-γ in the OVA or HDM group was decreased, while the expression levels of pro-inflammatory cytokines IL-4 and IL-17 induced by Th2 cells and Th17 were increased. In addition, the expression of Treg-related cytokine IL-10 was declined. These results indicate that Th1/Th2 and Th17/Treg were imbalanced in asthma mice induced by OVA or HDM (Figure 3b). After shikonin treatment, the level of IFN-γ was increased, and Th2-related cytokine IL-4 and Th17-related cytokine IL-17 were down-regulated. In addition, shikonin up-regulated the level of IL-10 (Figure 3b). Consequently, shikonin can regulate the balance of Th1/Th2 and Th17/Treg in asthma mice. In summary, shikonin inhibits inflammation and relieves asthma by inhibiting lung inflammatory cell infiltration and regulating the expression of Th cell-related cytokines.
Figure 3.
Shikonin alleviates immune cell infiltration and regulates the expression of Th cell-related cytokines. (a) The number of inflammatory cells, eosinophils (Eos), neutrophils (Neu), macrophages (Mac), and lymphocytes (Lym) in bronchoalveolar lavage fluid (BALF). (b) The concentration of Th cell-related inflammatory factors in BALF was detected by ELISA. Compared with the control group, ** p < 0.01; compared with the OVA group, # p < 0.05; compared with the OVA group, ## p < 0.01; compared with the HDM group, △△ p < 0.01, n = 3.
3.4. Effect of shikonin on the expression and localization of p-STAT3 in airway epithelial cells
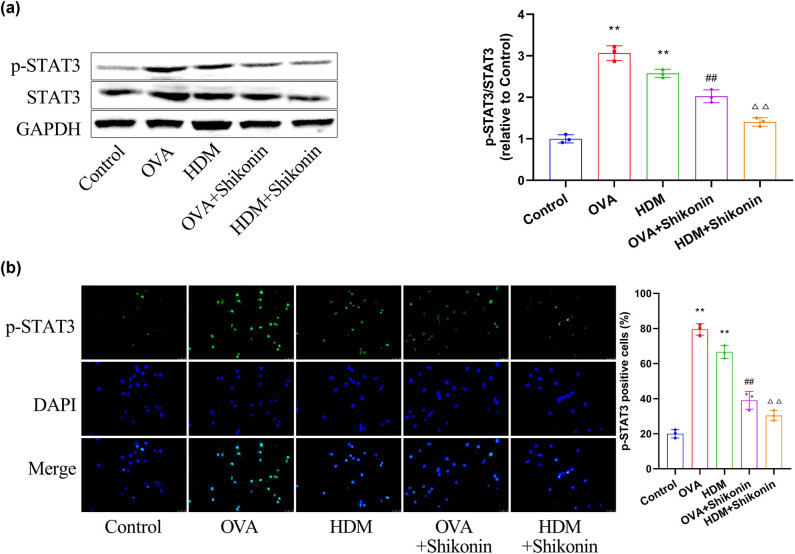
STAT3 plays an essential role in the production of inflammatory cytokines and is one of the important mechanisms leading to airway inflammation in asthma [26]. To explore whether shikonin alleviates asthma by regulating STAT3, we examined the activation of STAT3 and the nuclear translocation of p-STAT3. First, we compared the phosphorylation level of STAT3 in airway epithelial cells of mice in each group. Western blot results showed that the expression of p-STAT3 in the OVA or HDM group was notably higher than that in the control group. Protein levels of p-STAT3 were reduced in the OVA + shikonin and HDM + shikonin groups compared to the OVA or HDM group (Figure 4a). Next, we detected the expression pattern of p-STAT3 in airway epithelial cells by immunofluorescence staining. As shown in Figure 4b, the fluorescence intensity of p-STAT3 protein was significantly enhanced in the OVA and HDM groups, and the number of p-STAT3 positive staining cells was increased. However, treatment with shikonin decreased the number of p-STAT3-positive cells. These results indicate that shikonin can reduce the expression level of p-STAT3 and inhibit the transfer of p-STAT3 into the nucleus.
Figure 4.
Shikonin suppresses the expression and localization of p-STAT3 in airway epithelial cells. (a) Western blot analysis was conducted to examine the phosphorylation level of STAT3 in mouse airway epithelial cells. (b) The expression pattern of p-STAT3 in airway epithelial cells was detected by immunofluorescence staining. Compared with the control group, ** p < 0.01; compared with the OVA group, ## p < 0.01; compared with the HDM group, △△ p < 0.01, n = 3.
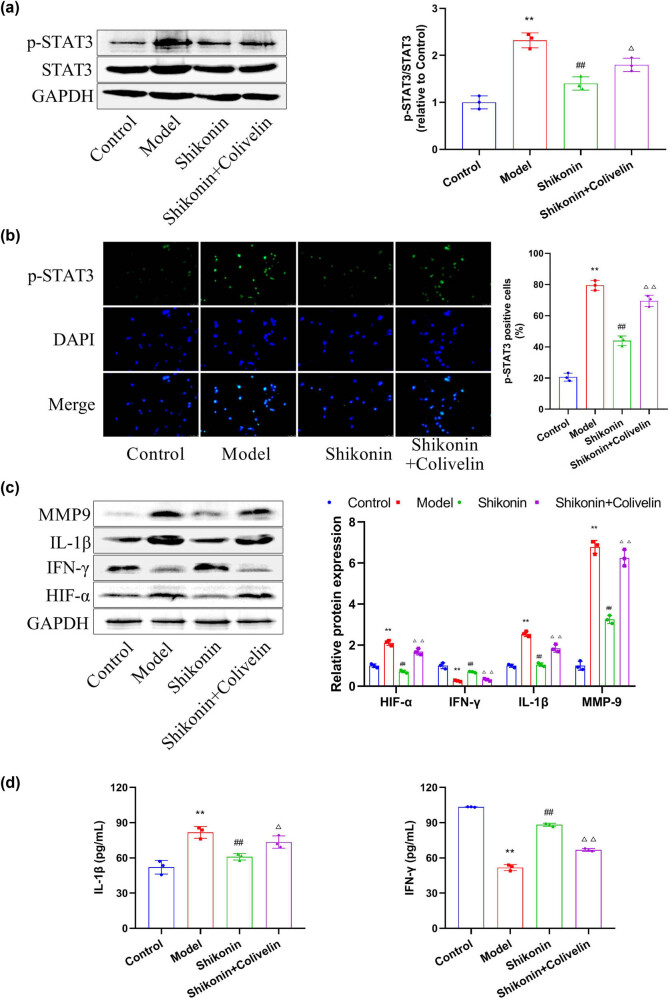
3.5. Activation of STAT3 reverses the inhibitory effect of shikonin on the function of MLE-12 cells
To further confirm the inference that shikonin can alleviate asthma by regulating STAT3, we observed the effect of shikonin on the expression and localization of p-STAT3 in TNF-α-induced mouse epithelial cell line MLE-12. In addition, MLE-12 cells were given 25 μg/mL colivelin (a STAT3 activator) in the presence of shikonin to observe whether activation of STAT3 could reverse the inhibitory effect of shikonin on asthma. As shown in Figure 5a, the expression of p-STAT3 was markedly increased under the action of TNF-α alone, which was inhibited by shikonin. Colivelin could reverse the inhibitory effect of shikonin on TNF-α-induced p-STAT3. Immunofluorescence staining showed that TNF-α promoted the transfer of p-STAT3 into the nucleus, while shikonin inhibited this phenomenon. Administration of colivelin in MLE-12 cells could antagonize this effect of shikonin (Figure 5b). Then, the expression of p-STAT3 downstream factors was detected by western blot in MLE-12 cells. Under the action of TNF-α, the expressions of the pro-inflammatory factor IL-1β were increased, the anti-inflammatory factor IFN-γ was decreased, and the levels of MMP9 and HIF-α were increased. After treatment with shikonin, the levels of IL-1β, MMP9, and HIF-α were inhibited and IFN-γ was up-regulated. However, the effects of shikonin on these factors were abated by colivelin (Figure 5c). The concentration of inflammatory factors (IL-1β and IFN-γ) in MLE-12 cell culture supernatant was detected by ELISA. Similar results were obtained (Figure 5d). In summary, activation of STAT3 can reverse the inhibitory effect of shikonin on the function of MLE-12 cells, confirming that the inhibitory effect of shikonin on asthma is dependent on STAT3.
Figure 5.
Activation of STAT3 can reverse the inhibitory effect of shikonin on the function of MLE-12 cells. (a) Western blot analysis was performed to measure the expression levels of STAT3 and p-STAT3 in mouse epithelial cell line MLE-12. (b) The localization of p-STAT3 in mouse bronchial epithelial cell line MLE-12 was detected by immunofluorescence staining. (c) Western blot was applied to measure the expression levels of HIF-α, IFN-γ, IL-1β, and MMP9. (d) ELISA was used to detect the concentrations of inflammatory factors (IL-1β and IFN-γ) in the MLE-12 cell medium. Compared with the control group, ** p < 0.01; compared with the model group, ## p < 0.01; compared with the shikonin group, △ p < 0.05; compared with the shikonin group, △△ p < 0.01, n = 3.
4. Discussion
Recent studies have identified the therapeutic role of shikonin in a variety of autoimmune diseases such as systemic lupus erythematosus, psoriasis, inflammatory bowel disease, and rheumatoid arthritis [27]. Most importantly, intratracheal instillation of shikonin can down-regulate the release of IL-4, IL-5, IL-13, and TNF-α in BALF and reduce pulmonary eosinophils and airway hyperresponsiveness [28]. Based on the findings of this research, shikonin has emerged as a novel and promising therapeutic agent for the treatment of obstructive pulmonary diseases, including asthma. To further substantiate its potential use as an anti-asthmatic drug, we investigated the inhibitory effect of shikonin on asthma in vivo and in vitro and its underlying mechanism. The results demonstrate that shikonin effectively alleviates asthma symptoms in both experimental models and cell cultures. Mechanistically, the inhibitory effect of shikonin on asthma may be related to inhibiting the expression level of STAT3 in airway epithelium.
The prevalence of asthma characterized by chronic airway inflammation and airway hyperresponsiveness is still increasing [29]. Allergens play a pivotal role as external triggers for asthma [30]. HDM is an important interference factor affecting asthma treatment in indoor living environment, which can directly stimulate bronchial and airway epithelial cells to release pro-inflammatory cytokines and chemokines and induce direct, non-allergic inflammation, accompanied by elevated serum TH2-related immunoglobulin and cytokine levels [31,32,33,34]. OVA can induce both rapid and delayed allergic reactions, easily sensitizing individuals and causing airway inflammation, which is commonly used to study acute asthma triggered by food allergies [35]. To comprehensively investigate the therapeutic effect of shikonin on asthma, we utilized OVA and HDM to establish mouse models with different allergen-induced asthma phenotypes. The results demonstrated that shikonin effectively suppressed airway inflammation and hyperresponsiveness in mice with OVA or HDM-induced asthma.
Asthma is an inflammatory disease closely associated with immune imbalance-induced abnormal secretion of inflammatory factors involving Th1/Th2 and Th17/Treg [36,37,38]. Numerous studies have reported that the anti-inflammatory effects of shikonin primarily involve modulation of signaling pathways including MAPK, NF-κB, PKM2/STAT3, PI3K/AKT/mTOR, and Wnt/β-catenin [19,39,40,41,42]. Compared with previous reports, this study focuses on whether shikonin can restore the Th1/Th2 and Th17/Treg balance and regulate the production of inflammatory factors. Our results showed that shikonin reduced inflammatory cell infiltration. On the other hand, shikonin alleviated the imbalance of Th1/Th2 and Th17/Treg by regulating IFN-γ/FOXP3 and IL-4/IL-17A in asthma mice. Shikonin also exerted a regulatory effect on inflammatory factors such as IFN-γ, IL-4, IL-17A, and IL-10.
Airway epithelial STAT3 plays a key role in the occurrence of asthma, and its activation is associated with asthma symptoms such as airway hyperresponsiveness and lung and airway inflammation [24]. By studying whether shikonin affects asthma phenotype through STAT3, it was found that shikonin can reduce the expression level of p-STAT3 and inhibit the transfer of p-STAT3 into the nucleus in asthmatic mice. Moreover, shikonin lowered the TNF-α-induced increase of p-STAT3 in MLE-12 cells. An activator of STAT3, colivelin, reversed the TNF-α-induced increase of p-STAT3 and abrogated the effects of TNF-α on inflammatory factors in MLE-12 cells. This finding is consistent with our previous findings in lung adenocarcinoma cells [43]. In addition, disruption of Th17/Treg balance caused by activation of STAT3/RORγt-STAT5/Foxp3 signaling pathway aggravated WSI-TRPM2.5 and Oe-TRPM2.5-induced asthma exacerbation further supported our conclusion [44]. Importantly, targeting STAT3 for the treatment of asthma has achieved certain research findings. Direct targeting of the 3’UTR region of STAT3 by exosome miR-301a-3p reduced the release of inflammatory cytokines TNF-α, IL-1β, and IL-6, which can inhibit OVA-induced asthma [45]. α-Asarone inhibited the phosphorylation level of STAT3, thus inhibiting mast cell activation, inflammatory cell infiltration, and the release of IL-5 and IL-13 in lung tissue, and finally effectively alleviating allergic asthma [46]. However, how shikonin regulates the expression of p-STAT3 remains unclear. Zhang et al. have reported that in esophageal squamous cell carcinoma, down-regulation of p-STAT3 by shikonin is mediated by inhibition of p-PKM2 [47]. p-PKM2, the phosphorylated pyruvate kinase M2, is a key enzyme in cell metabolism and is involved in the regulation of glycolysis. Phosphorylated PKM2 can promote the activation of downstream NLRP3 and NF-κB, thereby leading to oxidative stress and inflammatory response [48]. However, whether p-PKM2 plays a role in asthma is unclear, and whether shikonin reduces p-STAT3’s abundance in airway epithelial cells through p-PKM2 needs further investigation, which will be the direction of our future research.
To sum up, our results show that shikonin alleviates the imbalance of Th1/Th2 and Th17/Treg in the airway epithelium of asthma mice and affects the release of inflammatory factors in MLE-12 cells through a STAT3-dependent mechanism. Our study provides a new evidence for shikonin as a potential anti-asthmatic drug.
Abbreviations
- OVA
ovalbumin
- HDM
house dust mite
- IL
interleukin
- BALF
bronchoalveolar lavage fluid
- Eos
eosinophils
- Neu
neutrophils
- Mac
macrophages
- Lym
lymphocytes
- STAT3
signal transducer and activator of transcription 3
- TNF-α
tumor necrosis factor-α
- IFN-γ
interferon-gamma
- MAPK
mitogen-activated protein kinases
- LPS
lipopolysaccharide
- HE
hematoxylin/eosin
Footnotes
Funding information: This study was granted by Qin Chuang Yuan Introduced High-level Innovation and Entrepreneurship Talent Projects of Department of Science and Technology of Shaanxi Province (QCYRCXM-2022-178), Xi’an Health Healthy Committee General Research Project (2020yb58), Natural Science Foundation of Shanxi province (2015jm8465), and General Research Projects in Xi’an International Medical Center Hospital (2022ms13).
Author contributions: Yao Zhang is responsible for conceptualization, methodology, and draft writing. Lizhan Chen manages chart construction, data interpretation, and statistical analysis. Yao Zhang, Lizhan Chen, and Haifeng Ouyang performed the experiments and examined the results. All authors have read and agreed to the final manuscript version.
Conflict of interest: No conflicts of interest are to be declared for all authors.
Data availability statement: The data are available from the corresponding author upon reasonable request.
References
- [1].Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med. 2022;205(1):17–35. [DOI] [PMC free article] [PubMed]
- [2].Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission: what is it and how can it be achieved? Eur Respir J. 2022;60(5):2102583. [DOI] [PMC free article] [PubMed]
- [3].Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391(10122):783–800. [DOI] [PubMed]
- [4].Lommatzsch M, Buhl R, Korn S. The treatment of mild and moderate asthma in adults. Dtsch Arztebl Int. 2020;117(25):434–44. [DOI] [PMC free article] [PubMed]
- [5].Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(9):2521–2. [DOI] [PubMed]
- [6].Ji NF, Xie YC, Zhang MS, Zhao X, Cheng H, Wang H, et al. Ligustrazine corrects Th1/Th2 and Treg/Th17 imbalance in a mouse asthma model. Int Immunopharmacol. 2014;21(1):76–81. [DOI] [PubMed]
- [7].Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. [DOI] [PMC free article] [PubMed]
- [8].Jeong J, Lee HK. The role of CD4( +) T cells and microbiota in the pathogenesis of asthma. Int J Mol Sci. 2021;22(21):11822. [DOI] [PMC free article] [PubMed]
- [9].Massey O, Suphioglu C. Recent advances in the inhibition of the IL-4 cytokine pathway for the treatment of allergen-induced asthma. Int J Mol Sci. 2021;22(24):13655. [DOI] [PMC free article] [PubMed]
- [10].Ptáčková P, Musil J, Štach M, Lesný P, Němečková Š, Král V, et al. A new approach to CAR T-cell gene engineering and cultivation using piggyBac transposon in the presence of IL-4, IL-7 and IL-21. Cytotherapy. 2018;20(4):507–20. [DOI] [PubMed]
- [11].Liang P, Peng S, Zhang M, Ma Y, Zhen X, Li H. Huai Qi Huang corrects the balance of Th1/Th2 and Treg/Th17 in an ovalbumin-induced asthma mouse model. Biosci Rep. 2017;37(6):BSR20171071. [DOI] [PMC free article] [PubMed]
- [12].Li P, Tsang MS, Kan LL, Hou T, Hon SS, Chan BC, et al. The immuno-modulatory activities of pentaherbs formula on ovalbumin-induced allergic rhinitis mice via the activation of Th1 and Treg cells and inhibition of Th2 and Th17 cells. Molecules. 2021;27(1):239. [DOI] [PMC free article] [PubMed]
- [13].Zou XL, Chen ZG, Zhang TT, Feng DY, Li HT, Yang HL. Th17/Treg homeostasis, but not Th1/Th2 homeostasis, is implicated in exacerbation of human bronchial asthma. Ther Clin Risk Manag. 2018;14:1627–36. [DOI] [PMC free article] [PubMed]
- [14].Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine. 2015;72(2):146–53. [DOI] [PubMed]
- [15].Guzmán-Flores JM, Portales-Pérez DP. [Mechanisms of suppression of regulatory T-cells (Treg)]. Gac Med Mex. 2013;149(6):630–8. [PubMed]
- [16].Guo C, He J, Song X, Tan L, Wang M, Jiang P, et al. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 2019;149:104463. [DOI] [PubMed]
- [17].Lohberger B, Kaltenegger H, Eck N, Glänzer D, Sadoghi P, Leithner A, et al. Shikonin Derivatives Inhibit Inflammation Processes and Modulate MAPK Signaling in Human Healthy and Osteoarthritis Chondrocytes. Int J Mol Sci. 2022;23(6):3396. [DOI] [PMC free article] [PubMed]
- [18].Sun WX, Liu Y, Zhou W, Li HW, Yang J, Chen ZB. Shikonin inhibits TNF-α production through suppressing PKC-NF-κB-dependent decrease of IL-10 in rheumatoid arthritis-like cell model. J Nat Med. 2017;71(2):349–56. [DOI] [PubMed]
- [19].Liu Y, Zheng J, Chen Y, Wang F, Ye H, Wang M, et al. Shikonin protects against lipopolysaccharide-induced inflammation and apoptosis in human nucleus pulposus cells through the nuclear factor-kappa B pathway. Food Sci Nutr. 2021;9(10):5583–9. [DOI] [PMC free article] [PubMed]
- [20].Lianhua HE, Huijie L, Qingxia Q, Juan HE, Jian C, Yiping HU, et al. Shikonin alleviates collagen-induced arthritis mice by inhibiting M1 macrophage polarization. J Tradit Chin Med. 2022;42(6):932–9. [DOI] [PMC free article] [PubMed]
- [21].Wang TY, Zhou QL, Li M, Shang YX. Shikonin alleviates allergic airway remodeling by inhibiting the ERK-NF-κB signaling pathway. Int Immunopharmacol. 2017;48:169–79. [DOI] [PubMed]
- [22].Nikolskii AA, Shilovskiy IP, Barvinskaia ED, Korneev AV, Sundukova MS, Khaitov MR. Role of STAT3 Transcription Factor in Pathogenesis of Bronchial Asthma. Biochem (Mosc). 2021;86(11):1489–501. [DOI] [PubMed]
- [23].Gavino AC, Nahmod K, Bharadwaj U, Makedonas G, Tweardy DJ. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy. 2016;71(12):1684–92. [DOI] [PubMed]
- [24].Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, et al. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol. 2007;178(10):6191–9. [DOI] [PubMed]
- [25].Nishimura N, Yokota M, Kurihara S, Iwata A, Kageyama T, Ito T, et al. Airway epithelial STAT3 inhibits allergic inflammation via upregulation of stearoyl-CoA desaturase 1. Allergol Int. 2022;71(4):520–7. [DOI] [PubMed]
- [26].Huang N, Liu K, Liu J, Gao X, Zeng Z, Zhang Y, et al. Interleukin-37 alleviates airway inflammation and remodeling in asthma via inhibiting the activation of NF-κB and STAT3 signalings. Int Immunopharmacol. 2018;55:198–204. [DOI] [PubMed]
- [27].Guo Y, Zhou M, Mu Z, Guo J, Hou Y, Xu Y, et al. Recent advances in shikonin for the treatment of immune-related diseases: Anti-inflammatory and immunomodulatory mechanisms. Biomed Pharmacother. 2023;165:115138. [DOI] [PubMed]
- [28].Lee CC, Wang CN, Lai YT, Kang JJ, Liao JW, Chiang BL, et al. Shikonin inhibits maturation of bone marrow-derived dendritic cells and suppresses allergic airway inflammation in a murine model of asthma. Br J Pharmacol. 2010;161(7):1496–511. [DOI] [PMC free article] [PubMed]
- [29].Schleich F, Bougard N, Moermans C, Sabbe M, Louis R. Cytokine-targeted therapies for asthma and COPD. Eur Respir Rev. 2023;32(168):220193. [DOI] [PMC free article] [PubMed]
- [30].Bahna SL. The impact of modernization on allergy and asthma development. Allergy Asthma Proc. 2023;44(1):15–23. [DOI] [PubMed]
- [31].Lan F, Liu K, Zhang J, Qi Y, Li K, Lin P. Th17 response is augmented in OVA-induced asthmatic mice exposed to HDM. Med Sci Monit. 2011;17(5):Br132–8. [DOI] [PMC free article] [PubMed]
- [32].Wang Q, Guo L, Zeng Z, Huang Y, Tang H, Hu H, et al. Neferine attenuates HDM-induced allergic inflammation by inhibiting the activation of dendritic cell. Inflammation. 2023;46(6):2433–48. [DOI] [PubMed]
- [33].Park SJ, Lee K, Kang MA, Kim TH, Jang HJ, Ryu HW, et al. Tilianin attenuates HDM-induced allergic asthma by suppressing Th2-immune responses via downregulation of IRF4 in dendritic cells. Phytomedicine. 2021;80:153392. [DOI] [PubMed]
- [34].Abu Khweek A, Kim E, Joldrichsen MR, Amer AO, Boyaka PN. Insights into mucosal innate immune responses in house dust mite-mediated allergic asthma. Front Immunol. 2020;11:534501. [DOI] [PMC free article] [PubMed]
- [35].Kim HJ, Dinh DTT, Yang J, Herath K, Seo SH, Son YO, et al. High sucrose intake exacerbates airway inflammation through pathogenic Th2 and Th17 response in ovalbumin (OVA)-induced acute allergic asthma in C57BL/6 mice. J Nutr Biochem. 2024;124:109504. [DOI] [PubMed]
- [36].Alobaidi AH, Alsamarai AM, Alsamarai MA. Inflammation in asthma pathogenesis: role of t cells, macrophages, epithelial cells and type 2 inflammation. Antiinflammatory Antiallergy Agents Med Chem. 2021;20(4):317–32. [DOI] [PubMed]
- [37].Ji T, Li H. T-helper cells and their cytokines in pathogenesis and treatment of asthma. Front Immunol. 2023;14:1149203. [DOI] [PMC free article] [PubMed]
- [38].Zhang B, Zeng M, Zhang Q, Wang R, Jia J, Cao B, et al. Ephedrae Herba polysaccharides inhibit the inflammation of ovalbumin induced asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance. Mol Immunol. 2022;152:14–26. [DOI] [PubMed]
- [39].Liu FY, Wang MQ, Liu MM, Li T, Wang XH, Jiang F, et al. Therapeutic effects of shikonin on adjuvant-induced arthritis in rats and cellular inflammation, migration and invasion of rheumatoid fibroblast-like synoviocytes via blocking the activation of Wnt/β-catenin pathway. Phytomedicine. 2023;116:154857. [DOI] [PubMed]
- [40].Liu C, He L, Wang J, Wang Q, Sun C, Li Y, et al. Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol. 2020;260:113039. [DOI] [PubMed]
- [41].Huang B, Wang Q, Jiang L, Lu S, Li C, Xu C, et al. Shikonin ameliorated mice colitis by inhibiting dimerization and tetramerization of PKM2 in macrophages. Front Pharmacol. 2022;13:926945. [DOI] [PMC free article] [PubMed]
- [42].Lee CC, Kang JJ, Chiang BL, Wang CN, Cheng YW. Shikonin inhibited mitogen-activated IL-4 and IL-5 production on EL-4 cells through downregulation of GATA-3 and c-Maf induction. Life Sci. 2011;89(11–12):364–70. [DOI] [PubMed]
- [43].Pan T, Zhang F, Li F, Gao X, Li Z, Li X, et al. Shikonin blocks human lung adenocarcinoma cell migration and invasion in the inflammatory microenvironment via the IL‑6/STAT3 signaling pathway. Oncol Rep. 2020;44(3):1049–63. [DOI] [PMC free article] [PubMed]
- [44].Wang C, Wang D, Zhao H, Wang J, Liu N, Shi H, et al. Traffic-related PM(2.5) and diverse constituents disturb the balance of Th17/Treg cells by STAT3/RORγt-STAT5/Foxp3 signaling pathway in a rat model of asthma. Int Immunopharmacol. 2021;96:107788. [DOI] [PubMed]
- [45].Feng CY, Bai SY, Li ML, Zhao JY, Sun JM, Bao HJ, et al. Adipose-derived mesenchymal stem cell-derived exosomal miR-301a-3p regulates airway smooth muscle cells during asthma by targeting STAT3. J Asthma Allergy. 2022;15:99–110. [DOI] [PMC free article] [PubMed]
- [46].Bai H, Xue Z, Zhang W, Feng C, Zhou Z, Hu S, et al. α-Asarone alleviates allergic asthma by stabilizing mast cells through inhibition of ERK/JAK2-STAT3 pathway. Biofactors. 2023;49(1):140–52. [DOI] [PubMed]
- [47].Zhang Q, Liu Q, Zheng S, Liu T, Yang L, Han X, et al. Shikonin inhibits tumor growth of ESCC by suppressing PKM2 mediated aerobic glycolysis and STAT3 phosphorylation. J Cancer. 2021;12(16):4830–40. [DOI] [PMC free article] [PubMed]
- [48].Yang Y, Sheng J, Sheng Y, Wang J, Zhou X, Li W, et al. Lapachol treats non-alcoholic fatty liver disease by modulating the M1 polarization of Kupffer cells via PKM2. Int Immunopharmacol. 2023;120:110380. [DOI] [PubMed]