Abstract
Aims
Loop diuretics (LD) relieve symptoms and signs of congestion due to heart failure (HF), but many patients prescribed LD do not have such a diagnosis. We studied the relationship between HF diagnosis, use of LD, and outcomes in patients with type 2 diabetes mellitus (T2DM) enrolled in the EMPA‐REG OUTCOME trial.
Methods and results
The relationship between HF diagnosis, use of LD, and outcomes was evaluated in four patient subgroups with T2DM: (i) investigator‐reported HF on LD, (ii) investigator‐reported HF not on LD, (iii) no HF on LD, and (iv) no HF and not on LD, and we assessed their risk of cardiovascular events. Of 7020 participants, 706 (10%) had a diagnosis of HF at baseline, of whom 334 were prescribed LD. However, 755 (11%) patients who did not have a diagnosis of HF were prescribed LD. Compared to those with neither HF nor prescribed LD (reference group; placebo), those with both HF and receiving LD had the highest rates for all‐cause [hazard ratio (HR) (95% confidence interval) 3.19 (2.03–5.01)] and cardiovascular mortality [3.83 [(2.28–6.44)], and HF hospitalizations [9.51 (5.61–16.14)]. Patients without HF but prescribed LD had higher rates for all three outcomes [1.62 (1.10–2.39); 1.97 (1.26–3.08); 3.20 (1.90–5.39)], which were similar to patients with HF who were not receiving LD [1.42 (0.78–2.57); 1.56 (0.78–3.11); 3.00 (1.40–6.40)]. Empagliflozin had similar benefits regardless of subgroup (P for interaction >0.1 for all outcomes).
Conclusion
Patients with T2DM prescribed LD are at greater risk of cardiovascular events even if they are not reported to have HF; this might reflect under‐diagnosis. Empagliflozin was similarly effective in all subgroups investigated.
Keywords: Diuretics, Outcome, Type 2 diabetes mellitus, EMPA‐REG OUTCOME
Cumulative incidence curves for the composite endpoint of heart failure (HF) hospitalization or cardiovascular (CV) mortality, and all‐cause mortality in patients with and without HF, who were prescribed or not a loop diuretic (LD). BL, baseline.
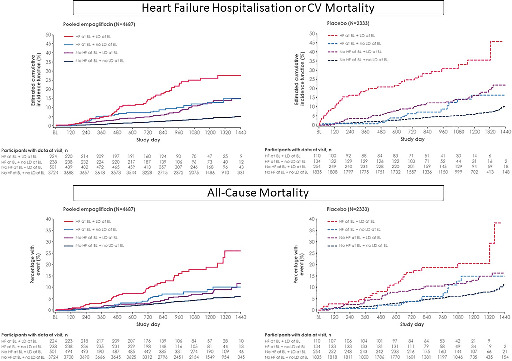
Background
Diuretics, especially loop diuretics (LD), are a mainstay of treatment for symptoms and signs of congestion for patients with heart failure (HF). Epidemiological studies indicate that the prevalence of HF in the adult population is around 1.5%, 1 but many more people are prescribed LD to treat symptoms, such as breathlessness, and signs, such as ankle swelling, without further investigation to exclude important diagnoses such as HF. 2 , 3 If LD mask the symptoms and signs of congestion, then the patient may not receive appropriate investigation for cardiac dysfunction, leading to a substantial under‐diagnosis of HF, particularly when left ventricular ejection fraction is preserved (HFpEF). 4
Patients with type 2 diabetes mellitus (T2DM) are at increased risk of developing HF. In the EMPA‐REG OUTCOME trial, which included patients with T2DM and atherosclerotic cardiovascular (CV) disease, only 10% had an investigator‐reported diagnosis of HF, but many others were prescribed LD. 5 We investigated the association between CV outcomes and a diagnosis of HF or prescription of LD at baseline in patients with T2DM in the EMPA‐REG OUTCOME trial and their interaction with the effects of empagliflozin.
Methods
Study design
The design of the EMPA‐REG OUTCOME trial is described in detail elsewhere. 5 , 6 The main inclusion criteria of the trial were a diagnosis of T2DM and atherosclerotic CV disease, age ≥ 18 years, glycated haemoglobin 7–10% (53–86 mmol/mol), body mass index (BMI) ≤45 kg/m2, and an estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2. In total, 7020 individuals received at least one dose of double‐blind treatment with the sodium–glucose co‐transporter 2 inhibitor (SGLT2i) empagliflozin 10 mg, empagliflozin 25 mg, or placebo, once daily. Investigators were encouraged to follow local guidelines for achieving glycaemic control by adjusting background glucose‐lowering therapy as needed (after the initial 12 weeks of treatment where glucose‐lowering treatment was to be kept unchanged), as well as guidelines for treating other CV risk factors. The trial was conducted in compliance with the Declaration of Helsinki and was approved by local authorities. All patients provided written informed consent prior to taking part in the trial. This post hoc analysis included all trial participants treated with at least one dose of empagliflozin or placebo. A diagnosis of HF at baseline was reported by the investigators based on the narrow standardized Medical Dictionary for Regulatory Activities (MedDRA) query (SMQ) ‘cardiac failure’, which included the following terms: acute pulmonary oedema; cardiac failure; cardiac failure, acute; cardiac failure, chronic; cardiac failure, congestive; cardiogenic shock; cardiopulmonary failure; left ventricular failure; pulmonary oedema; right ventricular failure. For the current analysis, we defined four subgroups at baseline: patients (i) with neither a diagnosis of HF nor prescribed LD, (ii) prescribed LD who did not have a diagnosis of HF, (iii) prescribed LD who had investigator‐reported HF, and (iv) with an investigator‐reported diagnosis of HF but not prescribed LD. We subsequently also explored the outcome of the small subgroup of patients (n = 109) who did not have a diagnosis of HF but were receiving both loop and thiazide diuretics, a powerful diuretic combination.
Outcomes
We explored time to CV death, hospitalization for HF (HHF), HHF or CV death (excluding fatal stroke), HHF or HF death, and all‐cause mortality. All CV, HF and mortality outcomes were prospectively adjudicated by an independent expert committee. In addition, we explored the outcome ‘investigator‐reported HF’ based on the narrow standardized MedDRA query adverse event definition of ‘cardiac failure’, as detailed above.
Statistical analyses
Participant characteristics are given as median (interquartile range) for continuous variables and number and proportion (%) for categorical variables. Baseline characteristics between the four subgroups were compared using Chi‐square test for categorical variables and ANOVA for continuous variables. We used Cox regression models to analyse the risk of the above time to event outcomes. We assessed the risk between subgroups of baseline HF status and use of LD within treatment groups using a model with terms for age, sex, baseline BMI, baseline glycated haemoglobin, baseline eGFR, geographical region, treatment, subgroups at baseline and an interaction of treatment by subgroup at baseline. These variables were selected a priori, and in accordance with the primary analysis of this trial, as defined in the statistical analysis plan and clinical trial protocol. The same Cox regression model was used to assess the treatment effect of empagliflozin across the subgroups. In addition, we calculated absolute risk reductions defined as incidence rate differences and number needed to treat (NNT). NNTs were derived as the reciprocal of the difference between the control and treatment groups in the proportion of patients who experienced a CV event within 3 years of treatment with empagliflozin, assuming exponential distribution of time to events. Poisson regression models were used to calculate the absolute risk reduction, including treatment with a log‐link applied by each subgroup. In the model log (days at risk) for the time to first event, censoring was used as offset. Interaction P‐values were calculated by t‐tests, using the estimated interaction effect and variance of the interaction, as determined from the delta method following Poisson regression. In order to assess whether our findings were specific for the use of LD only, we performed sensitivity analyses studying the association of use of thiazide diuretics at baseline with outcome as well as studying the subgroup of patients using the combination of loop and thiazide diuretics. We calculated change in serum sodium and potassium concentrations from baseline to end of treatment.
All analyses are post hoc and not adjusted for multiplicity. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Of 7020 participants, 706 (10%) had a diagnosis of HF at baseline, of whom 334 (47%) were prescribed a LD and 372 (53%) were not, 755 (11%) had no HF but were receiving a prescription for a LD, and 5559 (79%) had no HF and were not prescribed a LD, which was used as a reference group (Table 1 ). Furosemide was the most widely used LD (n = 981, for HF and no HF combined), followed by torasemide (n = 81) and, more rarely, bumetanide (n = 31) or ethacrynic acid (n = 2). Patients with a diagnosis of HF receiving a LD or receiving a LD but without a diagnosis of HF had similar characteristics and therapy, including the proportion on angiotensin‐converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) and beta‐blocker. They differed in many respects from patients who neither had HF nor were prescribed a LD, who were younger, had a shorter duration of T2DM, were less likely to have coronary artery disease (CAD) or macroalbuminuria, had a lower BMI, higher eGFR, and were less likely to be treated with beta‐blockers, ACEi or ARBs and insulin, but more likely to receive a thiazide (Table 1 ). The prevalence of atrial fibrillation increased from 3.7% in the reference group, to 8.7% in those without HF but prescribed a LD, to 16.9% in those with HF (11.6% and 22.8% for those not receiving or receiving a LD, respectively).
Table 1.
Baseline characteristics in pooled treatment groups by subgroups according to baseline heart failure status and use of loop diuretic
| Variable | No diagnosis of heart failure | Diagnosis of heart failure | P‐value (comparison across 4 groups) | ||
|---|---|---|---|---|---|
| No loop diuretic (n = 5559) | Using loop diuretic (n = 755) | No loop diuretic (n = 372) | Using loop diuretic (n = 334) | ||
| Demographics | |||||
| Age, years | 63 (57–69) | 66 (60–71) | 64 (58–69) | 66 (59–72) | <0.0001 |
| Female sex | 1562 (28.1) | 231 (30.6) | 130 (34.9) | 81 (24.3) | 0.0056 |
| History of stroke | 1318 (23.7) | 154 (20.4) | 101 (27.2) | 64 (19.2) | 0.016 |
| CAD | 4065 (73.1) | 649 (86.0) | 292 (78.5) | 302 (90.4) | <0.0001 |
| History of MI | 2411 (43.4) | 406 (53.8) | 242 (65.1) | 214 (64.1) | <0.0001 |
| PAD | 1145 (20.6) | 166 (22.0) | 78 (21.0) | 72 (21.6) | 0.82 |
| Atrial fibrillation | 204 (3.7) | 66 (8.7) | 43 (11.6) | 76 (22.8) | <0.0001 |
| Region | <0.0001 | ||||
| Europe | 2198 (39.5) | 325 (43.0) | 244 (65.6) | 118 (35.3) | |
| North America | 1029 (18.5) | 195 (25.8) | 51 (13.7) | 119 (35.6) | |
| Latin America | 909 (16.4) | 100 (13.2) | 35 (9.4) | 37 (11.1) | |
| Africa | 232 (4.2) | 47 (6.2) | 8 (2.2) | 26 (7.8) | |
| Asia | 1191 (21.4) | 88 (11.7) | 34 (9.1) | 34 (10.2) | |
| BMI, kg/m2 | 29.7 (26.4–33.3) | 32.5 (28.7–36.5) | 30.9 (27.4–34.5) | 32.6 (28.4–37.3) | <0.0001 |
| Systolic BP, mmHg | 134 (125–145) | 134 (124–147) | 136 (125–147) | 131 (118–142) | <0.0001 |
| Diastolic BP, mmHg | 77 (70–83) | 74 (67–81) | 80 (71–86) | 74 (67–81) | <0.0001 |
| Time since T2DM diagnosis, years | <0.0001 | ||||
| ≤1 | 135 (2.4) | 14 (1.9) | 19 (5.1) | 12 (3.6) | |
| >1 to 5 | 904 (16.3) | 73 (9.7) | 62 (16.7) | 44 (13.2) | |
| >5 to 10 | 1407 (25.3) | 161 (21.3) | 103 (27.7) | 75 (22.5) | |
| >10 | 3113 (56.0) | 507 (67.2) | 188 (50.5) | 203 (60.8) | |
| Blood and urine tests | |||||
| eGFR, mL/min/1.73 m2 | 74.7 (61.9–89.2) | 62.0 (47.9–76.6) | 71.9 (58.4–85.4) | 61.3 (48.8–75.7) | <0.0001 |
| ≥90, mL/min/1.73 m2 | 1335 (24.0) | 93 (12.3) | 77 (20.7) | 33 (9.9) | <0.0001 |
| 60 to <90, mL/min/1.73 m2 | 3011 (54.2) | 317 (42.0) | 189 (50.8) | 144 (43.1) | |
| 30 to <60, mL/min/1.73 m2 | 1198 (21.6) | 335 (44.4) | 104 (28.0) | 155 (46.4) | |
| <30, mL/min/1.73 m2 | 13 (0.2) | 10 (1.3) | 2 (0.5) | 2 (0.6) | |
| Missing | 2 (<0.1) | 0 | 0 (0) | 0 (0) | |
| HbA1c, % | 7.9 (7.4–8.6) | 8.0 (7.5–8.8) | 8.0 (7.4–8.6) | 7.9 (7.5–8.7) | 0.22 |
| LDL‐cholesterol, mg/dL | 79 (60–104) | 76 (59–99) | 92 (66–118) | 75 (58–99) | <0.0001 |
| UACR | <0.0001 | ||||
| Normal | 3364 (60.5) | 395 (52.3) | 221 (59.4) | 191 (57.2) | |
| Micro | 1583 (28.5) | 228 (30.2) | 107 (28.8) | 95 (28.4) | |
| Macro | 555 (10.0) | 124 (16.4) | 44 (11.8) | 46 (13.8) | |
| Missing | 57 (1.0) | 8 (1.1) | 0 (0) | 2 (0.6) | |
| Treatments, n (%) | |||||
| Metformin | 4256 (76.6) | 491 (65.0) | 247 (66.4) | 199 (59.6) | <0.0001 |
| Insulin | 2498 (44.9) | 495 (65.6) | 185 (49.7) | 209 (62.6) | <0.0001 |
| Beta‐blocker | 3427 (61.6) | 568 (75.2) | 285 (76.6) | 274 (82.0) | <0.0001 |
| ACEi/ARB | 4417 (79.5) | 637 (84.4) | 315 (84.7) | 297 (88.9) | <0.0001 |
| Statin | 4238 (76.2) | 622 (82.4) | 266 (71.5) | 277 (82.9) | <0.0001 |
| Vitamin K antagonists | 221 (4.0) | 97 (12.8) | 28 (7.5) | 76 (22.8) | <0.0001 |
| Loop diuretics, thiazides, n (%) | Not done | ||||
| Furosemide | NA | 680 (90.1) | NA | 301 (90.1) | |
| Torasemide | NA | 55 (7.3) | NA | 26 (7.8) | |
| Bumetanide | NA | 21 (2.8) | NA | 10 (3.0) | |
| Ethacrynic acid | NA | 1 (0.1) | NA | 1 (0.3) | |
| Hydrochlorothiazide | 1239 (22.3) | 105 (13.9) | 71 (19.1) | 23 (6.9) | |
| Chlortalidone | 51 (0.9) | 3 (0.4) | 4 (1.1) | 0 (0) | |
Data given as n (%) or median (interquartile range).
ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; MI, myocardial infarction; NA, not available; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus; UACR, urine albumin‐to‐creatinine ratio.
Outcomes according to diagnosis of heart failure and loop diuretic use
Most patients (89.6%) receiving LD at baseline continued them until the end of treatment period. At the end of treatment, LD had been stopped in 26 (7.8%) patients with HF (of whom 6 (5.5%) and 20 (8.9%) had been randomized to placebo and empagliflozin, respectively). Of those without HF, LD had been stopped in 87 (11.5%) patients by end of treatment period (23 (9.1%) and 64 (12.8%) of those randomized to placebo and empagliflozin, respectively).
In the placebo group, compared to those without HF and not receiving a LD (reference group), those with HF not receiving a LD and those without HF but receiving a LD had a similarly greater risk for all CV outcomes, with a 40–60% higher all‐cause mortality and about a threefold greater risk for HHF (Table 2 ). Those with HF receiving a LD had the greatest risk. Similar associations were found in those assigned to empagliflozin (Graphical Abstract). However, empagliflozin reduced the risk of CV events across all four subgroups compared to placebo (P for interaction >0.1 for all, Figure 1 ), also when assessed on the absolute scale (online supplementary Table S1 ).
Table 2.
Associations between subgroups and cardiovascular and heart failure outcomes in the treatment groups separately
| Events | No diagnosis of HF | Diagnosis of HF | ||||||
|---|---|---|---|---|---|---|---|---|
| No loop diuretic | Using loop diuretic | No loop diuretic | Using loop diuretic | |||||
| IR/1000 py | HR | IR/1000 py | HR (95% CI) | IR/1000 py | HR (95% CI) | IR/1000 py | HR (95% CI) | |
| Placebo | ||||||||
| CV death | 15.5 | Reference | 35.3 | 1.97 (1.26–3.08) | 25.3 | 1.56 (0.78–3.11) | 64.8 | 3.83 (2.28–6.44) |
| HHF | 8.1 | Reference | 31.7 | 3.20 (1.90–5.39) | 23.7 | 3.00 (1.40–6.40) | 93.7 | 9.51 (5.61–16.14) |
| CV death or HHF a | 20.6 | Reference | 57.6 | 2.35 (1.63–3.40) | 41.4 | 2.00 (1.15–3.51) | 149.1 | 6.29 (4.26–9.29) |
| HHF or HF death | 8.9 | Reference | 31.7 | 2.90 (1.73–4.85) | 29.6 | 3.39 (1.71–6.74) | 106.5 | 9.81 (5.95–16.19) |
| All‐cause mortality | 23.2 | Reference | 44.8 | 1.62 (1.10–2.39) | 33.7 | 1.42 (0.78–2.57) | 82.8 | 3.19 (2.03–5.01) |
| Investigator‐reported HF | 13.5 | Reference | 44.1 | 2.62 (1.70–4.03) | 38.8 | 2.78 (1.53–5.04) | 129.1 | 7.48 (4.81–11.64) |
| Empagliflozin | ||||||||
| CV death | 9.4 | Reference | 19.7 | 1.86 (1.22–2.83) | 21.3 | 2.16 (1.23–3.79) | 40.7 | 3.88 (2.46–6.10) |
| HHF | 4.2 | Reference | 22.9 | 4.51 (2.85–7.15) | 23.9 | 5.51 (3.06–9.92) | 59.9 | 11.71 (7.38–18.59) |
| CV death or HHF a | 12.4 | Reference | 39.3 | 2.72 (1.98–3.75) | 41.4 | 3.27 (2.14–4.99) | 89.0 | 6.11 (4.36–8.56) |
| HHF or HF death | 4.4 | Reference | 22.9 | 4.29 (2.72–6.78) | 23.9 | 5.25 (2.92–9.43) | 61.8 | 11.48 (7.29–18.06) |
| All‐cause mortality | 15.6 | Reference | 27.1 | 1.52 (1.07–2.16) | 28.9 | 1.80 (1.12–2.90) | 62.7 | 3.47 (2.41–5.00) |
| Investigator‐reported HF | 8.4 | Reference | 31.0 | 2.99 (2.07–4.33) | 43.8 | 5.00 (3.24–7.71) | 80.9 | 7.72 (5.31–11.22) |
The Cox model includes age, sex, region, baseline glycated haemoglobin, baseline estimated glomerular filtration rate, baseline body mass index, subgroups by HF status and loop diuretic use, treatment, and treatment by subgroup interaction.
CI, confidence interval; CV, cardiovascular; HF, heart failure; HHF, heart failure hospitalization; HR, hazard ratio; IR, incidence rate; py, patients/year.
Excludes fatal stroke.
Figure 1.
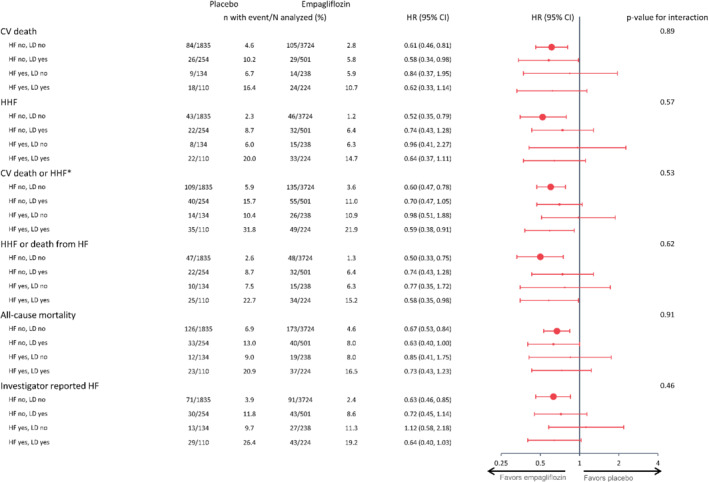
Treatment effect of empagliflozin vs. placebo across subgroups by baseline heart failure (HF) status and use of loop diuretic (LD). The Cox model includes age, sex, region, baseline glycated haemoglobin, baseline estimated glomerular filtration rate, baseline body mass index, subgroups by HF status and LD use, treatment, and treatment by subgroup interaction. CI, confidence interval; CV, cardiovascular; HHF, hospitalization for heart failure; HR, hazard ratio. *Excluding fatal stroke.
Sensitivity analysis
Of those without HF, 1386 were receiving a thiazide, and 4928 were not. Patient characteristics of these two groups were broadly similar (online supplementary Table S2 ). Rates for CV outcomes were similar for those who were or were not receiving thiazides (online supplementary Table S3 ). Empagliflozin reduced the risk of all outcomes whether or not patients received thiazides at baseline (P for interaction>0.1; online supplementary Figure S1 ). The outcome of the small subgroup of patients who were not reported to have HF but were receiving loop and thiazide diuretics in combination was similar to that for patients with HF (online supplementary Table S4 ).
Safety
Rates of adverse events and serious adverse events, especially those related to renal function, were greater in patients receiving a LD with or without HF compared to the other patients (Table 3 ). Mean serum sodium and potassium concentrations were similar at the beginning and end of the treatment regardless of a HF diagnosis or LD use for patients assigned to placebo or empagliflozin (online supplementary Table S5 ).
Table 3.
Adverse events reported during the trial by subgroups and treatment
| No diagnosis of HF | ||||||||
|---|---|---|---|---|---|---|---|---|
| No loop diuretic | Using loop diuretic | |||||||
| Placebo (n = 1835) | Empagliflozin (n = 3724) | Placebo (n = 254) | Empagliflozin (n = 501) | |||||
| n (%) | IR/100 py | n (%) | IR/100 py | n (%) | IR/100 py | n (%) | IR/100 py | |
| Any AE | 1666 (90.8) | 167.20 | 3358 (90.2) | 146.72 | 243 (95.7) | 249.18 | 459 (91.6) | 174.91 |
| Any SAE | 726 (39.6) | 20.06 | 1341 (36.0) | 17.14 | 136 (53.5) | 31.28 | 246 (49.1) | 28.67 |
| Confirmed hypoglycaemic event | 495 (27.0) | 13.32 | 1023 (27.5) | 13.52 | 87 (34.3) | 19.03 | 165 (32.9) | 18.92 |
| Hypoglycaemic event requiring assistance | 28 (1.5) | 0.61 | 47 (1.3) | 0.48 | 7 (2.8) | 1.13 | 11 (2.2) | 0.92 |
| Urinary tract infection | 337 (18.4) | 8.21 | 657 (17.6) | 7.59 | 43 (16.9) | 7.54 | 103 (20.6) | 9.87 |
| Genital infection | 33 (1.8) | 0.72 | 245 (6.6) | 2.62 | 4 (1.6) | 0.64 | 29 (5.8) | 2.49 |
| Decreased renal function | 98 (5.3) | 2.17 | 159 (4.3) | 1.66 | 36 (14.2) | 6.25 | 47 (9.4) | 4.08 |
| Volume depletion | 79 (4.3) | 1.75 | 157 (4.2) | 1.65 | 19 (7.5) | 3.21 | 43 (8.6) | 3.75 |
| Hyperkalaemia a | 52 (2.8) | 1.14 | 62 (1.7) | 0.64 | 14 (5.5) | 2.31 | 15 (3.0) | 1.26 |
| Diagnosis of HF | ||||||||
|---|---|---|---|---|---|---|---|---|
| No loop diuretic | Using loop diuretic | |||||||
| Placebo (n = 134) | Empagliflozin (n = 238) | Placebo (n = 110) | Empagliflozin (n = 224) | |||||
| n (%) | IR/100 py | n (%) | IR/100 py | n (%) | IR/100 py | n (%) | IR/100 py | |
| Any AE | 121 (90.3) | 162.05 | 200 (84.0) | 96.20 | 109 (99.1) | 381.27 | 213 (95.1) | 289.95 |
| Any SAE | 62 (46.3) | 28.4 | 80 (33.6) | 16.42 | 64 (58.2) | 42.63 | 122 (54.5) | 34.66 |
| Confirmed hypoglycaemic event | 30 (22.4) | 12.51 | 49 (20.6) | 9.85 | 38 (34.5) | 21.35 | 66 (29.5) | 17.11 |
| Hypoglycaemic event requiring assistance | 1 (0.7) | 0.33 | 1 (0.4) | 0.17 | 0 (0) | 0 | 4 (1.8) | 0.81 |
| Urinary tract infection | 21 (15.7) | 7.53 | 37 (15.5) | 6.85 | 22 (20.0) | 11.0 | 45 (20.1) | 10.27 |
| Genital infection | 2 (1.5) | 0.67 | 11 (4.6) | 1.94 | 3 (2.7) | 1.31 | 16 (7.1) | 3.39 |
| Decreased renal function | 5 (3.7) | 1.70 | 16 (6.7) | 2.84 | 16 (14.5) | 7.4 | 24 (10.7) | 5.17 |
| Volume depletion | 6 (4.5) | 2.05 | 7 (2.9) | 1.22 | 11 (10.0) | 4.99 | 32 (14.3) | 6.97 |
| Hyperkalaemia a | 6 (4.5) | 2.02 | 5 (2.1) | 0.86 | 6 (5.5) | 2.69 | 11 (4.9) | 2.27 |
Data are for patients who had one or more event and who had received at least one dose of study drug. All events occurred within 7 days after the last receipt of the study drug.
AE, adverse event; HF, heart failure; IR, incidence rate; py, patients/year; SAE, serious adverse event.
Hyperkalaemia defined by the MedDRA terms hyperkalaemia and blood potassium increase.
Discussion
In the EMPA‐REG OUTCOME trial, patients treated with LD but who were not reported to have a diagnosis of HF had an adverse prognosis and rather similar characteristics to patients with HF. This was not the case for treatment with thiazide diuretics. Empagliflozin improved outcome overall and there was no evidence of an interaction according to a diagnosis of HF or treatment with LD. To the best of our knowledge, this is the first report that suggests a relationship between LD use and adverse CV outcomes in patients with T2DM without HF but supports a previous observation in a large trial investigating an anticoagulant for patients with atrial fibrillation. 2
A key question is whether patients treated with LD in the absence of a reported diagnosis of HF in EMPA‐REG OUTCOME actually had underlying but unrecognized cardiac dysfunction and HF. The history of myocardial infarction and atrial fibrillation, worse renal function, and greater use of insulin (a sodium‐retaining hormone) at baseline support this hypothesis. However, some patients may have received LD for hypertension, ankle swelling due to venous insufficiency, or for breathlessness due to causes other than HF. These patients might not have an increased risk of CV events, diluting the prognostic contribution of those with unrecognized HF and accounting for the more favourable overall outcome of this group compared to those who had both a diagnosis of HF and receiving a LD.
Patients with a diagnosis of HF who were not receiving LD had a similar prognosis to patients who did not have a diagnosis of HF but who were receiving LD. This could reflect diagnostic inaccuracy in both groups or indicate that when cardiac dysfunction does not lead to congestion and LD prescription, the prognosis is much better than for those who do develop congestion. 7 Other clinical trials of HF also show that patients who are not treated with LD are at lower risk of events, suggesting that lack of congestion is associated with a good outcome. 8 , 9 Guidelines from the European Society of Cardiology state: ‘The aim of diuretic therapy is to achieve and maintain euvolaemia with the lowest achievable dose. … In selected asymptomatic euvolaemic/hypovolaemic patients, the use of a diuretic drug might be (temporarily) discontinued.’ 10 Randomized trials of withdrawing LD from patients with established HF have met with mixed success. 11 , 12 Treating congestion with LD may control the immediate problem, and even be life‐saving but might subsequently accelerate the progression of disease by activation of neuroendocrine systems and unfavourable effects on kidney function and metabolism. However, being sure that a patient is euvolaemic and that LD can be safely reduced or withdrawn is not easy and might expose the patient to the risk of recurrent congestion. Managing diuretics well may be one of the most difficult things a HF specialist is required to do. 13
Interestingly, the proportions of patients prescribed an ACEi and beta‐blocker was similar for patients prescribed LD whether or not they had a diagnosis of HF. These treatments may have been for hypertension or CAD but it is also possible that patients had been diagnosed with HF but this had simply not been recorded. Prescription of a LD should alert clinicians, investigators and trial monitors to a possible diagnosis of HF and the need for further scrutiny of medical records or further investigations. Objective evidence of cardiac dysfunction to support or refute a diagnosis of HF at baseline was not available in EMPA‐REG OUTCOME.
Several studies report an association between diuretic use and adverse outcomes. A post‐hoc analysis of 759 patients enrolled in the Diabetic Retinopathy Study showed that prescription of any type of diuretic to treat hypertension was associated with a more than threefold increase in mortality compared to those who had untreated hypertension. 14 More recently, the Heart Outcomes Prevention Evaluation (HOPE) study evaluated whether ramipril prevented the development of CV complications, including HF, in 9297 high‐risk patients with or without T2DM. In this trial, 15% of participants were receiving a diuretic, although no distinction was made between loop and thiazide diuretic. Diuretic use at baseline (risk ratio 1.76), as well as a diagnosis of diabetes, independently predicted the development of HF. 15 In a Norwegian study of 307 patients (mean age 65 years, of whom 13% had T2DM) with suspected CAD without HF or renal impairment (eGFR <60 mL/min/1.73 m2), those (n = 109) treated with a LD had a higher mortality [HR 1.82 (95% CI 1.20–2.76)] compared to matched controls over the following decade. 16 Cleland et al. 2 analysed data from the SPORTIF trials, and reported that annual mortality increased from 2.1% for patients neither prescribed a LD nor with left ventricular dysfunction (LVD), to 2.5% for those with LVD alone, 4.2% for those prescribed LD who did not have LVD and 6.6% for those with LVD prescribed LD; for death or worsening HF events rates were 3.8%, 7.6%, 8.3% and 16.1%, respectively. Using electronic primary care health records in Sweden, Carlsson and colleagues identified nearly 6000 patients with hypertension and atrial fibrillation. Prescription of LD (n = 2935) was much more common than a diagnosis of HF (n = 894) and was associated with a 39% increase in mortality. 17 Similarly, Friday and colleagues have reported that LD use is common in primary care in the United Kingdom and associated with mortality rates only slightly less than those with a secondary‐care diagnosis of HF. 18 Our data, along with these studies, point to the possibility that cardiac dysfunction and subclinical HF may be under‐recognized in a variety of patient groups, including those with T2DM.
Side effects of LD include activation of the renin–angiotensin–aldosterone and sympathetic nervous systems, 19 , 20 hypokalaemia, calciuria, hypovolaemia, falls and worsening renal function, which might contribute to an increase in morbidity and mortality. Our analyses demonstrate that patients with T2DM treated with a LD had higher rates of adverse events consistent with volume depletion and decreased renal function, regardless of HF status. However, LD are a pharmaco‐epidemiological signature of symptoms and signs of congestion, often due to cardiac dysfunction, and it is the congestion rather than the adverse effects of LD that is driving prognosis. 7
We did not find an association between use of thiazide diuretics alone and outcomes in patients with T2DM, which possibly reflects their different indication. Although thiazides might impair glucose metabolism, they are often used to treat hypertension in T2DM and have been shown to reduce CV events in this setting. For example, in the SHEP trial, chlortalidone reduced mortality in older patients with T2DM despite worsening glycaemia compared to placebo. 21 , 22 However, patients receiving both LD, which block sodium retention in the loop of Henle, and thiazide diuretics, which act on the distal convoluted tubule (sequential nephron blockade) had similar rates of CV events to patients with HF. This is a powerful diuretic combination that is usually reserved by HF specialists for the treatment of advanced HF. It is unclear whether other physician groups are aware of how potent this combination is and its potential to treat, inadvertently, congestion and its symptoms and signs, thereby concealing a diagnosis of HF.
Empagliflozin reduced CV events regardless of HF or LD classification. Several hypotheses have sought to explain the CV benefits of empagliflozin, but the precise underlying mechanisms remain unknown. 23 An exploratory analysis from EMPA‐REG OUTCOME found that the increase in haematocrit reported after SGLT2 inhibition mediated approximately 50% of the reduction in CV death. 24 Increases in haematocrit have also been identified as mediators of the beneficial effects of canagliflozin on HF events in the CANVAS trial. 25 One possible explanation for the rise in haematocrit is a reduction in plasma volume, which might also explain lower rates of initiating and higher rates of stopping LD in those assigned to empagliflozin. 26 Similarly, in a study of claims data in patients with HF and T2DM from the United States, those on SGLT2i were less likely to be initiated on LD in the following 12 months compared to those who received other oral anti‐glycaemic medications (22.7% vs. 34.0%, P = 0.001). 27 There may be a synergistic natriuretic/diuretic effect between LD and SGLT2i 28 and several mechanisms to explain why a SGLT2i might cause or improve diuresis, including an osmotic effect from glucosuria, modest natriuresis, or inhibition of renal sodium–hydrogen exchanger‐3 (NHE3). 29 It should be pointed out, however, that an alternative explanation for the rise in haematocrit is an increase in red blood cell mass due to increased production of erythropoietin. 30 More mechanistic studies will be required to provide further insights into the causes of increased haematocrit from SGLT2i as well as into the interactions between these agents and LD at the level of the kidney in HF. 31
Limitations
These analyses were developed post‐hoc. We did not differentiate amongst LD as there were too few patients receiving agents other than furosemide. We did not account for changes in prescription, and doses, of LD during the trial but relatively few were reported to have stopped or initiated a LD. 26 Neither plasma concentrations of natriuretic peptides nor echocardiograms were recorded at baseline or follow‐up and therefore we are unable to verify the presence of cardiac dysfunction nor differentiate amongst left ventricular phenotypes in those with a diagnosis of HF.
Conclusion
In EMPA‐REG OUTCOME, patients receiving LD had a worse outcome whether or not they were reported to have HF. Amongst patients with T2DM who did not have a diagnosis of HF, the risk of CV events, including death, was greater for those who were prescribed LD, particularly when combined with a thiazide diuretic. In the absence of a diagnosis of HF, the prescription of LD should prompt investigators and clinicians to consider undetected cardiac dysfunction and HF. Conversely, patients with a diagnosis of HF who are not treated with LD have a better prognosis than those with HF treated with LD. Whether this reflects inaccurate diagnosis of less severe cardiac dysfunction should be considered. The reduction in morbidity and mortality with empagliflozin is similar both in relative and absolute terms for patients with T2DM whether or not they have a diagnosis of HF or are prescribed LD.
Supporting information
Figure S1. Sensitivity analysis of incidence rates and risk of outcomes within the empagliflozin and placebo groups across subgroups by baseline heart failure status and use of thiazide diuretic.
Table S1. Absolute treatment effect of empagliflozin vs. placebo across subgroups by heart failure status and use of loop diuretic.
Table S2. Baseline characteristics by subgroups according to baseline heart failure status and use of thiazide diuretic.
Table S3. Sensitivity analysis showing associations between subgroups (by HF status and use of thiazide diuretics at baseline) and cardiovascular/heart failure outcomes in the treatment groups separately.
Table S4. Associations between subgroups and cardiovascular/heart failure outcomes in the treatment groups separately.
Table S5. Change in sodium and potassium from baseline to end of trial (last value on treatment) by treatment and subgroups by heart failure status and use of loop diuretic at baseline.
Acknowledgements
P.P. and J.G.F.C. are supported by the British Heart Foundation Centre of Research Excellence (RE/18/6134217). P.P. has received a research grant (Scotland Grant) from Heart Research UK.
Funding
Conflict of interest: P.P. has received travel support from Boehringer Ingelheim and consulting honoraria from Novartis. D.F. reports CME honoraria and consultation fees from Boehringer Ingelheim, Lilly, Sanofi, AstraZeneca, and Amgen, and DSMB honoraria from NovoNordisk. M.N.K. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, personal fees from Sanofi, Amgen, Novo Nordisk, Merck (Diabetes and Cardiovascular), Janssen, Bayer, Applied Therapeutics, Esperion, Eli Lilly, and Vifor Pharma, outside the submitted work. B.Z. has received consulting honoraria from NovoNordisk, Boehringer Ingelheim, Eli Lilly, Merck, AstraZeneca, Sanofi and Janssen. C.W. reports significant honoraria from Boehringer Ingelheim and modest honoraria from AstraZeneca, Bayer, Eli Lilly, Mitsubishi, and MSD. S.E.I. has consulted and/or served on Clinical Trial Steering/Executive/Publications Committees for Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Merck, Esperion and Abbott. J.M.T. reports personal fees from Reprieve Medical, AstraZeneca, Novartis, Cardionomic, Bayer, MagentaMed, W.L. Gore, Windtree therapeutics, Lexicon, grants and personal fees from BMS, 3ive Labs, Boehringer Ingelheim, Sanofi, FIRE1, grants from Otsuka, Abbott, Merck outside the submitted work; in addition, Dr Testani has a patent to treating diuretic resistance issued. J.G.F.C. reports personal fees from Abbott, grants and personal fees from Amgen, Bayer, Novartis, Pharmacosmos, Vifor, BMS, Servier, personal fees and non‐financial support from Medtronic, outside the submitted work. A.P.O, L.S., I.Z. and J.G. are employees of Boehringer Ingelheim.
References
- 1. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cleland JG, Shelton R, Nikitin N, Ford S, Frison L, Grind M. Prevalence of markers of heart failure in patients with atrial fibrillation and the effects of ximelagatran compared to warfarin on the incidence of morbid and fatal events: a report from the SPORTIF III and V trials. Eur J Heart Fail 2007;9:730–739. [DOI] [PubMed] [Google Scholar]
- 3. van Kraaij DJ, Jansen RW, Gribnau FW, Hoefnagels WH. Loop diuretics in patients aged 75 years or older: general practitioners' assessment of indications and possibilities for withdrawal. Eur J Clin Pharmacol 1998;54:323–327. [DOI] [PubMed] [Google Scholar]
- 4. Boonman‐de Winter LJ, Rutten FH, Cramer MJM, Landman MJ, Liem AH, Rutten GEHM, Hoes AW. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012;55:2154–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, Bluhmki E, Hantel S, Kempthorne‐Rawson J, Newman J, Johansen O, Woerle HJ, Broedl UC. Rationale, design, and baseline characteristics of a randomized, placebo‐controlled cardiovascular outcome trial of empagliflozin (EMPA‐REG OUTCOME™). Cardiovasc Diabetol 2014;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pellicori P, Cleland JG, Zhang J, Kallvikbacka‐Bennett A, Urbinati A, Shah P, Kazmi S, Clark AL. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther 2016;30:599–609. [DOI] [PubMed] [Google Scholar]
- 8. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail 2007;9:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006;27:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 11. Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland JGF. The effects of short‐term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail 2017;19:643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rohde LE, Rover MM, Figueiredo Neto JA, Danzmann LC, Bertoldi EG, Simões MV, Silvestre OM, Ribeiro ALP, Moura LZ, Beck‐da‐Silva L, Prado D, Sant'Anna RT, Bridi LH, Zimerman A, Raupp da Rosa P, Biolo A. Short‐term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double‐blind, multicentre, randomized trial. Eur Heart J 2019;40:3605–3612. [DOI] [PubMed] [Google Scholar]
- 13. Shah MR, Stevenson LW. Searching for evidence: refractory questions in advanced heart failure. J Card Fail 2004;10:210–218. [DOI] [PubMed] [Google Scholar]
- 14. Warram JH, Laffel LMB, Valsania P, Christlieb AR, Krolewski AS. Excess mortality associated with diuretic therapy in diabetes mellitus. Arch Intern Med 1991;151:1350–1356. [PubMed] [Google Scholar]
- 15. Arnold JM, Yusuf S, Young J, Mathew J, Johnstone D, Avezum A, Lonn E, Pogue J, Bosch J; HOPE Investigators . Prevention of heart failure in patients in the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation 2003;107:1284–1290. [DOI] [PubMed] [Google Scholar]
- 16. Schartum‐Hansen H, Løland KH, Svingen GF, Seifert R, Pedersen ER, Nordrehaug JE, Bleie Ø, Ebbing M, Berge C, Nilsen DW, Nygård O. Use of loop diuretics is associated with increased mortality in patients with suspected coronary artery disease, but without systolic heart failure or renal impairment: an observational study using propensity score matching. PLoS One 2015;10:e0124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlsson AC, Wändell P, Sundquist K, Johansson SE, Sundquist J. Effects of prescribed antihypertensives and other cardiovascular drugs on mortality in patients with atrial fibrillation and hypertension: a cohort study from Sweden. Hypertens Res 2014;37:553–559. [DOI] [PubMed] [Google Scholar]
- 18. Friday J, Pellicori P, Papworth R, Wolters M, McAllister D, Kean S, Cleland JGF. 82 people prescribed loop diuretics have a poor outcome even without a diagnostic label of heart failure. Heart 2019;105(Suppl 6):A69 (abstr). [Google Scholar]
- 19. Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med 1985;103:1–6. [DOI] [PubMed] [Google Scholar]
- 20. Bayliss J, Norell M, Canepa‐Anson R, Sutton G, Poole‐Wilson P. Untreated heart failure clinical and neuroendocrine effects of introducing diuretics. Br Heart J 1987;57:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savage PJ, Pressel SL, Curb JD, Schron EB, Applegate WB, Black HR, Cohen J, Davis BR, Frost P, Smith W, Gonzalez N, Guthrie GP, Oberman A, Rutan G, Probstfield JL, Stamler J. Influence of long‐term, low‐dose, diuretic‐based, antihypertensive therapy on glucose, lipid, uric acid, and potassium levels in older men and women with isolated systolic hypertension: the Systolic Hypertension in the Elderly Program. SHEP Cooperative Research Group. Arch Intern Med 1998;158:741–751. [DOI] [PubMed] [Google Scholar]
- 22. Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR; SHEP Collaborative Research Group . Long‐term effect of diuretic‐based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol 2005;95:29–35. [DOI] [PubMed] [Google Scholar]
- 23. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia 2018;61:2108–2117. [DOI] [PubMed] [Google Scholar]
- 24. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care 2018;41:356–363. [DOI] [PubMed] [Google Scholar]
- 25. Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, Neal B. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 2020;8:57–66. [DOI] [PubMed] [Google Scholar]
- 26. Januzzi J, Ferreira JP, Böhm M, Kaul S, Wanner C, Brueckmann M, Petrie MC, Ofstad AP, Zeller C, George J, Fitchett D, Zannad F. Empagliflozin reduces the risk of a broad spectrum of heart failure outcomes regardless of heart failure status at baseline. Eur J Heart Fail 2019;21:386–388. [DOI] [PubMed] [Google Scholar]
- 27. Weeda ER, Cassarly C, Brinton DL, Shirley DW, Simpson KN. Loop diuretic use among patients with heart failure and type 2 diabetes treated with sodium glucose cotransporter‐2 inhibitors. J Diabetes Complications 2019;33:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Griffin M, Rao VS, Ivey‐Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium‐glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2017;2:1025–1029. [DOI] [PubMed] [Google Scholar]
- 30. Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation 2020;141:704–707. [DOI] [PubMed] [Google Scholar]
- 31. Mordi NA, Mordi IR, Singh JS, Baig F, Choy AM, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of sodium‐glucose cotransporter 2 (SGLT2) inhibition in combination with loop diuretics in diabetic patients with chronic heart failure (RECEDE‐CHF): protocol for a randomised controlled double‐blind cross‐over trial. BMJ Open 2017;7:e018097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sensitivity analysis of incidence rates and risk of outcomes within the empagliflozin and placebo groups across subgroups by baseline heart failure status and use of thiazide diuretic.
Table S1. Absolute treatment effect of empagliflozin vs. placebo across subgroups by heart failure status and use of loop diuretic.
Table S2. Baseline characteristics by subgroups according to baseline heart failure status and use of thiazide diuretic.
Table S3. Sensitivity analysis showing associations between subgroups (by HF status and use of thiazide diuretics at baseline) and cardiovascular/heart failure outcomes in the treatment groups separately.
Table S4. Associations between subgroups and cardiovascular/heart failure outcomes in the treatment groups separately.
Table S5. Change in sodium and potassium from baseline to end of trial (last value on treatment) by treatment and subgroups by heart failure status and use of loop diuretic at baseline.


