Abstract
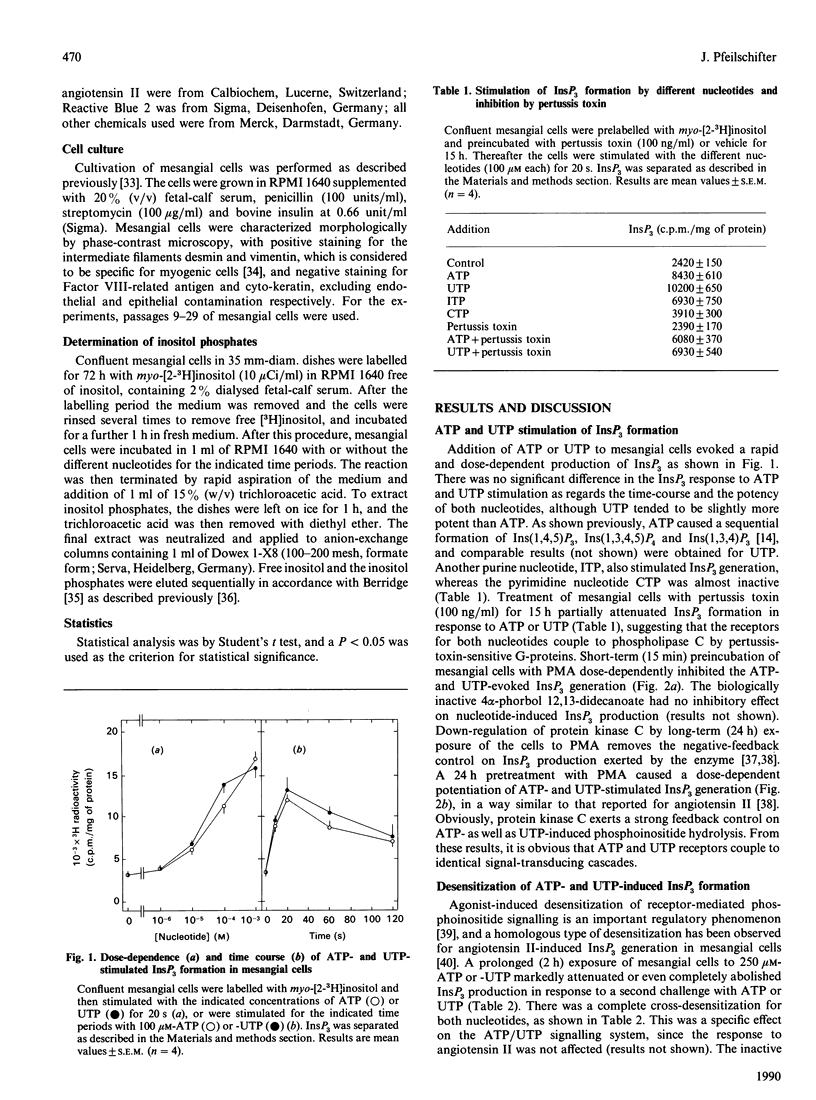
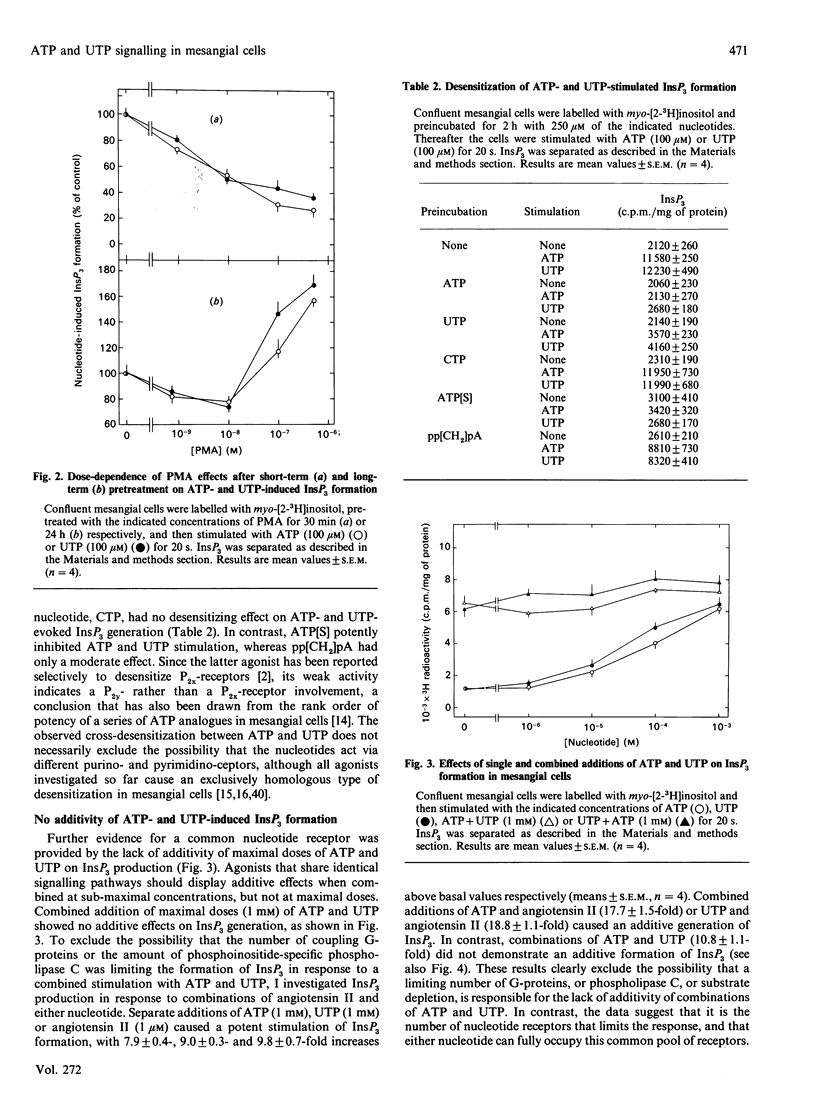
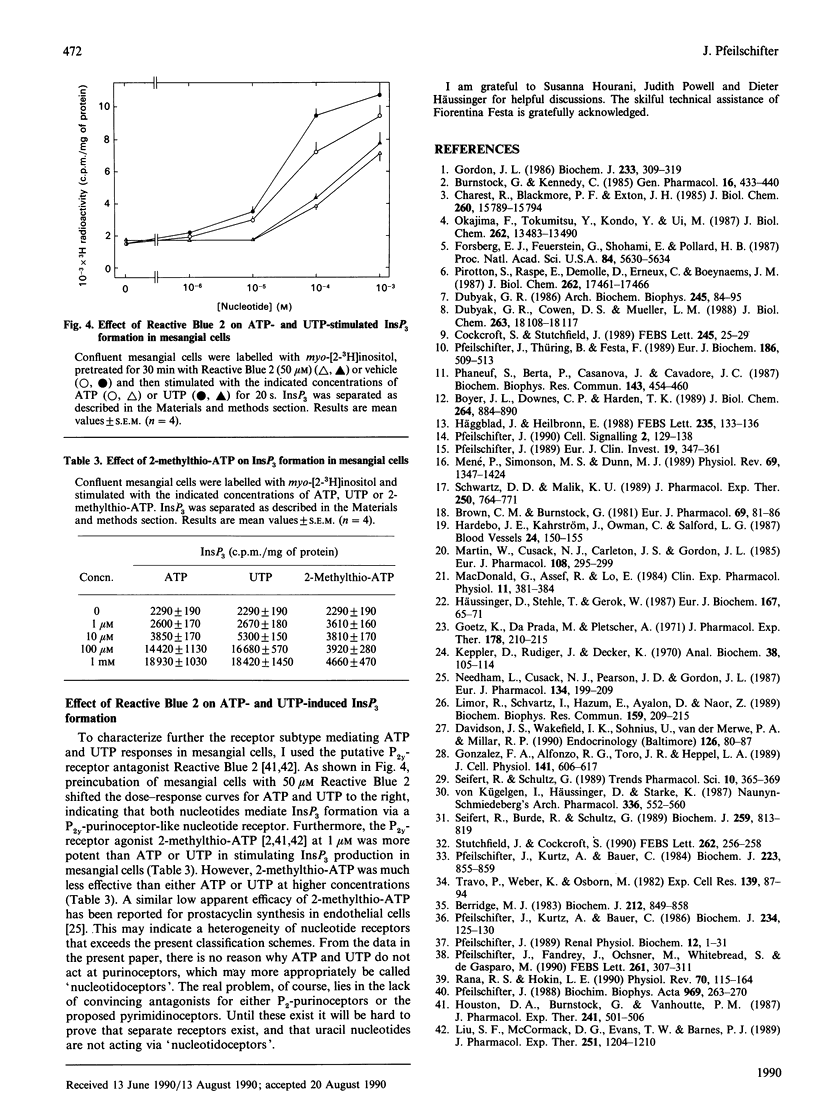
Extracellular ATP and UTP caused a rapid formation of InsP3, with similar kinetics and dose-dependences. ITP also displayed strong agonistic properties in terms of InsP3 production, whereas CTP was almost inactive. Pretreatment of the cells with pertussis toxin attenuated ATP- and UTP-stimulated InsP3 generation to a comparable extent, indicating that both nucleotides couple to phospholipase C by a pertussis-toxin-sensitive G-protein. Short-term (15 min) treatment of the cells with phorbol 12-myristate 13-acetate (PMA) produced a dose-dependent inhibition of ATP- and UTP-induced InsP3 formation. Furthermore, down-regulation of protein kinase C by long-term (24 h) exposure of the cells to PMA resulted in a comparable potentiation of phosphoinositide hydrolysis by both nucleotides. Preincubation of mesangial cells with ATP or UTP caused a pronounced cross-desensitization of subsequent nucleotide-stimulated InsP3 production. ATP and UTP displayed no additivity in terms of InsP3 formation, when used at maximally effective concentrations. In contrast, the peptide hormone angiotensin II interacted in an additive manner with either nucleotide in stimulating phosphoinositide hydrolysis. Reactive Blue 2, a putative P2y-purinoceptor antagonist, caused a rightward shift of both the ATP and UTP dose-response curves. However, since 2-methylthio-ATP was only a partial agonist in stimulating InsP3 formation, the mesangial-cell ATP receptor appears to be different from a classic P2y-receptor. In summary, these results provide no evidence for separate purino- and pyrimidino-ceptors on mesangial cells. In contrast, ATP and UTP may use a common nucleotide receptor for transducing their signals in mesangial cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L., Downes C. P., Harden T. K. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989 Jan 15;264(2):884–890. [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. The structural conformation of the polyphosphate chain of the ATP molecule is critical for its promotion of prostaglandin biosynthesis. Eur J Pharmacol. 1981 Jan 5;69(1):81–86. doi: 10.1016/0014-2999(81)90604-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. ATP stimulates secretion in human neutrophils and HL60 cells via a pertussis toxin-sensitive guanine nucleotide-binding protein coupled to phospholipase C. FEBS Lett. 1989 Mar 13;245(1-2):25–29. doi: 10.1016/0014-5793(89)80184-x. [DOI] [PubMed] [Google Scholar]
- Davidson J. S., Wakefield I. K., Sohnius U., van der Merwe P. A., Millar R. P. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology. 1990 Jan;126(1):80–87. doi: 10.1210/endo-126-1-80. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Cowen D. S., Meuller L. M. Activation of inositol phospholipid breakdown in HL60 cells by P2-purinergic receptors for extracellular ATP. Evidence for mediation by both pertussis toxin-sensitive and pertussis toxin-insensitive mechanisms. J Biol Chem. 1988 Dec 5;263(34):18108–18117. [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz U., Da Prada M., Pletscher A. Adenine-, guanine- and uridine-5'-phosphonucleotides in blood platelets and storage organelles of various species. J Pharmacol Exp Ther. 1971 Jul;178(1):210–215. [PubMed] [Google Scholar]
- Gonzalez F. A., Alfonzo R. G., Toro J. R., Heppel L. A. Receptor specific for certain nucleotides stimulates inositol phosphate metabolism and Ca2+ fluxes in A431 cells. J Cell Physiol. 1989 Dec;141(3):606–617. doi: 10.1002/jcp.1041410320. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardebo J. E., Kåhrström J., Owman C., Salford L. G. Endothelium-dependent relaxation by uridine tri- and diphosphate in isolated human pial vessels. Blood Vessels. 1987;24(3):150–155. doi: 10.1159/000158690. [DOI] [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Häggblad J., Heilbronn E. P2-purinoceptor-stimulated phosphoinositide turnover in chick myotubes. Calcium mobilization and the role of guanyl nucleotide-binding proteins. FEBS Lett. 1988 Aug 1;235(1-2):133–136. doi: 10.1016/0014-5793(88)81248-1. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stehle T., Gerok W. Actions of extracellular UTP and ATP in perfused rat liver. A comparative study. Eur J Biochem. 1987 Aug 17;167(1):65–71. doi: 10.1111/j.1432-1033.1987.tb13304.x. [DOI] [PubMed] [Google Scholar]
- Keppler D., Rudigier J., Decker K. Enzymic determination of uracil nucleotides in tissues. Anal Biochem. 1970 Nov;38(1):105–114. doi: 10.1016/0003-2697(70)90160-0. [DOI] [PubMed] [Google Scholar]
- Limor R., Schvartz I., Hazum E., Ayalon D., Naor Z. Effect of guanine nucleotides on phospholipase C activity in permeabilized pituitary cells: possible involvement of an inhibitory GTP-binding protein. Biochem Biophys Res Commun. 1989 Feb 28;159(1):209–215. doi: 10.1016/0006-291x(89)92424-8. [DOI] [PubMed] [Google Scholar]
- Liu S. F., McCormack D. G., Evans T. W., Barnes P. J. Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J Pharmacol Exp Ther. 1989 Dec;251(3):1204–1210. [PubMed] [Google Scholar]
- Macdonald G., Assef R., Guiffre A., Lo E. Vasoconstrictor effects of uridine and its nucleotides and their inhibition by adenosine. Clin Exp Pharmacol Physiol. 1984 Jul-Aug;11(4):381–384. doi: 10.1111/j.1440-1681.1984.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Martin W., Cusack N. J., Carleton J. S., Gordon J. L. Specificity of P2-purinoceptor that mediates endothelium-dependent relaxation of the pig aorta. Eur J Pharmacol. 1985 Feb 5;108(3):295–299. doi: 10.1016/0014-2999(85)90452-2. [DOI] [PubMed] [Google Scholar]
- Mené P., Simonson M. S., Dunn M. J. Physiology of the mesangial cell. Physiol Rev. 1989 Oct;69(4):1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Pfeilschifter J. M. Cellular signalling in the kidney: the role of inositol lipids. Ren Physiol Biochem. 1989 Jan-Feb;12(1):1–31. doi: 10.1159/000173176. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Cross-talk between transmembrane signalling systems: a prerequisite for the delicate regulation of glomerular haemodynamics by mesangial cells. Eur J Clin Invest. 1989 Aug;19(4):347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J. Extracellular ATP stimulates polyphosphoinositide hydrolysis and prostaglandin synthesis in rat renal mesangial cells. Involvement of a pertussis toxin-sensitive guanine nucleotide binding protein and feedback inhibition by protein kinase C. Cell Signal. 1990;2(2):129–138. doi: 10.1016/0898-6568(90)90016-4. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Fandrey J., Ochsner M., Whitebread S., de Gasparo M. Potentiation of angiotensin II-stimulated phosphoinositide hydrolysis, calcium mobilization and contraction of renal mesangial cells upon down-regulation of protein kinase C. FEBS Lett. 1990 Feb 26;261(2):307–311. doi: 10.1016/0014-5793(90)80578-7. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Kurtz A., Bauer C. Activation of phospholipase C and prostaglandin synthesis by [arginine]vasopressin in cultures. Biochem J. 1984 Nov 1;223(3):855–859. doi: 10.1042/bj2230855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Kurtz A., Bauer C. Role of phospholipase C and protein kinase C in vasoconstrictor-induced prostaglandin synthesis in cultured rat renal mesangial cells. Biochem J. 1986 Feb 15;234(1):125–130. doi: 10.1042/bj2340125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J. Protein kinase C from rat renal mesangial cells: its role in homologous desensitization of angiotensin II-induced polyphosphoinositide hydrolysis. Biochim Biophys Acta. 1988 May 13;969(3):263–270. doi: 10.1016/0167-4889(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Thüring B., Festa F. Extracellular ATP stimulates poly(inositol phospholipid) hydrolysis and eicosanoid synthesis in mouse peritoneal macrophages in culture. Eur J Biochem. 1989 Dec 22;186(3):509–513. doi: 10.1111/j.1432-1033.1989.tb15236.x. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Schwartz D. D., Malik K. U. Renal periarterial nerve stimulation-induced vasoconstriction at low frequencies is primarily due to release of a purinergic transmitter in the rat. J Pharmacol Exp Ther. 1989 Sep;250(3):764–771. [PubMed] [Google Scholar]
- Seifert R., Burde R., Schultz G. Activation of NADPH oxidase by purine and pyrimidine nucleotides involves G proteins and is potentiated by chemotactic peptides. Biochem J. 1989 May 1;259(3):813–819. doi: 10.1042/bj2590813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R., Schultz G. Involvement of pyrimidinoceptors in the regulation of cell functions by uridine and by uracil nucleotides. Trends Pharmacol Sci. 1989 Sep;10(9):365–369. doi: 10.1016/0165-6147(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Undifferentiated HL60 cells respond to extracellular ATP and UTP by stimulating phospholipase C activation and exocytosis. FEBS Lett. 1990 Mar 26;262(2):256–258. doi: 10.1016/0014-5793(90)80204-v. [DOI] [PubMed] [Google Scholar]
- Travo P., Weber K., Osborn M. Co-existence of vimentin and desmin type intermediate filaments in a subpopulation of adult rat vascular smooth muscle cells growing in primary culture. Exp Cell Res. 1982 May;139(1):87–94. doi: 10.1016/0014-4827(82)90321-4. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Häussinger D., Starke K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1987 Nov;336(5):556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]


